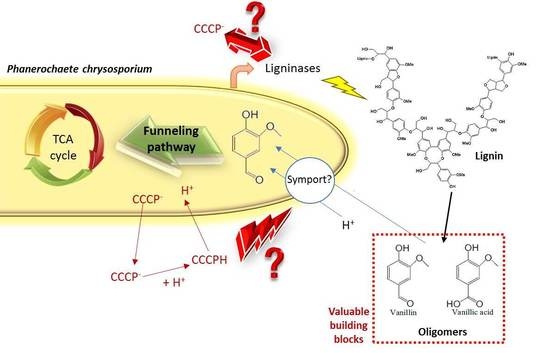
Inhibition of Phenolics Uptake by Ligninolytic Fungal Cells and Its Potential as a Tool for the Production of Lignin-Derived Aromatic Building Blocks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Phenolics Uptake Inhibition Experiments
2.3. Phenolics HPLC Quantification
2.4. Dehydrogenase Polymers Syntheses
2.5. Dehydrogenation Polymers and Purified Lignins Depolymerization Experiments
2.6. High-Performance Sized Exclusion Chromatography (HPSEC) Analyses of Lignin Oligomers
2.7. Data Processing
3. Results and Discussion
3.1. Characterization of Phenolics Cellular Uptake by Phanerochaete chrysosporium
3.2. Compatibility of Phenolics Uptake Inhibition and Ligninolytic Enzymes Production Conditions
3.3. Effect of Phenolics Uptake Inhibition on DHP Depolymerization
3.4. Applicability of the Developed Inhibition Method on Purified Lignins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, R.; Guo, M.; Zhang, X. Selective Conversion of Biorefinery Lignin into Dicarboxylic Acids. ChemSusChem 2014, 7, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Glasser, W.G.; Northey, R.A.; Schultz, T.P. Lignin: Historical, Biological, and Materials Perspectives; American Chemical Society: Washington, DC, USA, 1999. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C. Depolymerization of lignins and their applications for the preparation of polyols and rigid polyurethane foams: A review. Renew. Sustain. Energy Rev. 2016, 60, 317–329. [Google Scholar] [CrossRef]
- Reano, A.F.; Chérubin, J.; Peru, A.M.M.; Wang, Q.; Clément, T.; Domenek, S.; Allais, F. Structure–Activity Relationships and Structural Design Optimization of a Series of p-Hydroxycinnamic Acids-Based Bis- and Trisphenols as Novel Sustainable Antiradical/Antioxidant Additives. ACS Sustain. Chem. Eng. 2015, 3, 3486–3496. [Google Scholar] [CrossRef]
- Baker, L.A.; Staniforth, M.; Flourat, A.L.; Allais, F.; Stavros, V.G. Gas-Solution Phase Transient Absorption Study of the Plant Sunscreen Derivative Methyl Sinapate. ChemPhotoChem 2018, 2, 743–748. [Google Scholar] [CrossRef]
- Mention, M.M.; Flourat, A.L.; Peyrot, C.; Allais, F. Biomimetic regioselective and high-yielding Cu(i)-catalyzed dimerization of sinapate esters in green solvent CyreneTM: Towards sustainable antioxidant and anti-UV ingredients. Green Chem. 2020, 22, 2077–2085. [Google Scholar] [CrossRef]
- Pion, F.; Reano, A.F.; Oulame, M.Z.; Barbara, I.; Flourat, A.L.; Ducrot, P.-H.; Allais, F. Chemo-enzymatic synthesis, derivatizations, and polymerizations of renewable phenolic monomers derived from ferulic acid and biobased polyols: An access to sustainable copolyesters, poly(ester-urethane)s, and poly(ester-alkenamer)s. In Green Polymer Chemistry: Biobased Materials and Biocatalysis; American Chemical Society: Washington, DC, USA, 2015; Volume 1192, pp. 41–68. [Google Scholar] [CrossRef]
- Guadix-Montero, S.; Sankar, M. Review on Catalytic Cleavage of C–C Inter-unit Linkages in Lignin Model Compounds: Towards Lignin Depolymerisation. Top. Catal. 2018, 61, 183–198. [Google Scholar] [CrossRef] [Green Version]
- Upton, B.M.; Kasko, A.M. Strategies for the Conversion of Lignin to High-Value Polymeric Materials: Review and Perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 3441246843. [Google Scholar] [CrossRef]
- Bugg, T.D.H.; Ahmad, M.; Hardiman, E.M.; Rahmanpour, R. Pathways for degradation of lignin in bacteria and fungi. Nat. Prod. Rep. 2011, 28, 1883–1896. [Google Scholar] [CrossRef]
- Martínez, A.T.; Ruiz-Dueñas, F.J.; Camarero, S.; Serrano, A.; Linde, D.; Lund, H.; Vind, J.; Tovborg, M.; Herold-Majumdar, O.M.; Hofrichter, M.; et al. Oxidoreductases on their way to industrial biotransformations. Biotechnol. Adv. 2017, 35, 815–831. [Google Scholar] [CrossRef] [Green Version]
- Martínez, Á.T.; Speranza, M.; Ruiz-Dueñas, F.J.; Ferreira, P.; Camarero, S.; Guillén, F.; Martínez, M.J.; Gutiérrez Suárez, A.; del Río Andrade, J.C. Biodegradation of lignocellulosics: Microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int. Microbiol. 2005, 8, 195–204. [Google Scholar] [PubMed]
- Brink, D.P.; Ravi, K.; Lidén, G.; Gorwa-Grauslund, M.F. Mapping the diversity of microbial lignin catabolism: Experiences from the eLignin database. Appl. Microbiol. Biotechnol. 2019, 103, 3979–4002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Gautam, A.; Dutt, D. Bio-pulping: An energy saving and environment-friendly approach. Phys. Sci. Rev. 2020, 5, 20190043. [Google Scholar] [CrossRef]
- Singh, D.; Chen, S. The white-rot fungus Phanerochaete chrysosporium: Conditions for the production of lignin-degrading enzymes. Appl. Microbiol. Biotechnol. 2008, 81, 399–417. [Google Scholar] [CrossRef]
- Linger, J.G.; Vardon, D.R.; Guarnieri, M.T.; Karp, E.M.; Hunsinger, G.B.; Franden, M.A.; Johnson, C.W.; Chupka, G.; Strathmann, T.J.; Pienkos, P.T.; et al. Lignin valorization through integrated biological funneling and chemical catalysis. Proc. Natl. Acad. Sci. USA 2014, 111, 12013. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, K.E.L.; Blanchette, R.A.; Ander, P. Microbial and Enzymatic Degradation of Wood and Wood Components; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Shimizu, M.; Kobayashi, Y.; Tanaka, H.; Wariishi, H. Transportation mechanism for vanillin uptake through fungal plasma membrane. Appl. Microbiol. Biotechnol. 2005, 68, 673–679. [Google Scholar] [CrossRef]
- Fayeulle, A.; Veignie, E.; Slomianny, C.; Dewailly, E.; Munch, J.-C.; Rafin, C. Energy-dependent uptake of benzo[a]pyrene and its cytoskeleton-dependent intracellular transport by the telluric fungus Fusarium solani. Environ. Sci. Pollut. Res. 2014, 21, 3515–3523. [Google Scholar] [CrossRef]
- Quideau, S.; Ralph, J. Facile large-scale synthesis of coniferyl, sinapyl, and p-coumaryl alcohol. J. Agric. Food Chem. 1992, 40, 1108–1110. [Google Scholar] [CrossRef]
- Zhou, S.; Raouche, S.; Grisel, S.; Navarro, D.; Sigoillot, J.-C.; Herpoël-Gimbert, I. Solid-state fermentation in multi-well plates to assess pretreatment efficiency of rot fungi on lignocellulose biomass. Microb. Biotechnol. 2015, 8, 940–949. [Google Scholar] [CrossRef]
- Fodil Cherif, M.; Trache, D.; Brosse, N.; Benaliouche, F.; Tarchoun, A.F. Comparison of the Physicochemical Properties and Thermal Stability of Organosolv and Kraft Lignins from Hardwood and Softwood Biomass for Their Potential Valorization. Waste Biomass Valorization 2020, 11, 6541–6553. [Google Scholar] [CrossRef]
- Kersten, P.; Cullen, D. Extracellular oxidative systems of the lignin-degrading Basidiomycete Phanerochaete chrysosporium. Fungal Genet. Biol. 2007, 44, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Ichinose, H.; Wariishi, H. Flavin-containing monooxygenases from Phanerochaete chrysosporium responsible for fungal metabolism of phenolic compounds. Biodegradation 2012, 23, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Barnhart-Dailey, M.C.; Ye, D.; Hayes, D.C.; Maes, D.; Simoes, C.T.; Appelhans, L.; Carroll-Portillo, A.; Kent, M.S.; Timlin, J.A. Internalization and accumulation of model lignin breakdown products in bacteria and fungi. Biotechnol. Biofuels 2019, 12, 175. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.S.; Paris, A.; Codron, P.; Cassereau, J.; Procaccio, V.; Lenaers, G.; Reynier, P.; Chevrollier, A. Current mechanistic insights into the CCCP-induced cell survival response. Biochem. Pharmacol. 2018, 148, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Shary, S.; Kapich, A.N.; Panisko, E.A.; Magnuson, J.K.; Cullen, D.; Hammel, K.E. Differential expression in Phanerochaete chrysosporium of membrane-associated proteins relevant to lignin degradation. Appl. Environ. Microbiol. 2008, 74, 7252–7257. [Google Scholar] [CrossRef] [Green Version]
- Bonnarme, P.; Perez, J.; Jeffries, T.W. Regulation of ligninase production in white-rot fungi. In Enzymes in Biomass Conversion; American Chemical Society: Washington, DC, USA, 1991; Volume 460, pp. 200–206. [Google Scholar]
- Tien, M.; Kirk, T.K. Lignin peroxidase of Phanerochaete chrysosporium. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1988; Volume 161, pp. 238–249. [Google Scholar] [CrossRef]
- Huang, S.; Huang, D.; Wu, Q.; Hou, M.; Tang, X.; Zhou, J. Effect of environmental C/N ratio on activities of lignin-degrading enzymes produced by Phanerochaete chrysosporium. Pedosphere 2020, 30, 285–292. [Google Scholar] [CrossRef]
- Tribot, A.; Amer, G.; Abdou Alio, M.; de Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.-D.; Callois, J.-M.; Vial, C.; Michaud, P.; et al. Wood-lignin: Supply, extraction processes and use as bio-based material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leriche-Grandchamp, M.; Flourat, A.; Shen, H.; Picard, F.; Giordana, H.; Allais, F.; Fayeulle, A. Inhibition of Phenolics Uptake by Ligninolytic Fungal Cells and Its Potential as a Tool for the Production of Lignin-Derived Aromatic Building Blocks. J. Fungi 2020, 6, 362. https://doi.org/10.3390/jof6040362
Leriche-Grandchamp M, Flourat A, Shen H, Picard F, Giordana H, Allais F, Fayeulle A. Inhibition of Phenolics Uptake by Ligninolytic Fungal Cells and Its Potential as a Tool for the Production of Lignin-Derived Aromatic Building Blocks. Journal of Fungi. 2020; 6(4):362. https://doi.org/10.3390/jof6040362
Chicago/Turabian StyleLeriche-Grandchamp, Mathilde, Amandine Flourat, Hangchen Shen, Flavien Picard, Heloïse Giordana, Florent Allais, and Antoine Fayeulle. 2020. "Inhibition of Phenolics Uptake by Ligninolytic Fungal Cells and Its Potential as a Tool for the Production of Lignin-Derived Aromatic Building Blocks" Journal of Fungi 6, no. 4: 362. https://doi.org/10.3390/jof6040362