A One Health Perspective to Recognize Fusarium as Important in Clinical Practice
Abstract
:1. Introduction
2. The Fusarium Genus
3. Fusarium in Agriculture
Fusarium in Hospital Environment
4. Fusarium and Animal Disease
5. Fusarium in Human Diseases
5.1. Onychomycosis
5.2. Keratitis
5.3. Invasive Disease
6. Fusariosis Treatment
6.1. Localized Infection
6.2. Invasive Infection
7. Antifungal Resistance Mechanisms
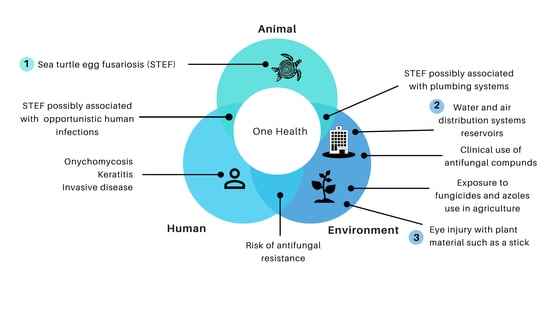
8. One Health Perspective
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- One Health Initiative One Health Initiative Will Unite Human and Veterinary Medicine. Available online: http://www.onehealthinitiative.com (accessed on 18 February 2020).
- Gauthier, G.M.; Keller, N.P. Crossover fungal pathogens: The biology and pathogenesis of fungi capable of crossing kingdoms to infect plants and humans. Fungal Genet. Biol. 2013, 61, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Gupta, V.K.; Misra, A.K.; Gaur, R.; Bajpai, V.; Issar, S. Current status of Fusarium infection in human and animal. Asian J. Anim. Vet. Adv. 2011, 6, 201–227. [Google Scholar] [CrossRef] [Green Version]
- Moretti, M.L.; Busso-Lopes, A.F.; Tararam, C.A.; Moraes, R.; Muraosa, Y.; Mikami, Y.; Gonoi, T.; Taguchi, H.; Lyra, L.; Reichert-Lima, F.; et al. Airborne transmission of invasive fusariosis in patients with hematologic malignancies. PLoS ONE 2018, 13, e0196426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garnica, M.; Nucci, M. Epidemiology of fusariosis. Curr. Fungal Infect. Rep. 2013, 7, 301–305. [Google Scholar] [CrossRef]
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal blindness: A global perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar]
- Chidambaram, J.D.; Venkatesh Prajna, N.; Srikanthi, P.; Lanjewar, S.; Shah, M.; Elakkiya, S.; Lalitha, P.; Burton, M.J. Epidemiology, risk factors, and clinical outcomes in severe microbial keratitis in South India. Ophthalmic Epidemiol. 2018, 25, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Nelson, P.E.; Dignani, M.C.; Anaissie, E.J. Taxonomy, biology, and clinical aspects of Fusarium species. Clin. Microbiol. Rev. 1994, 7, 479–504. [Google Scholar] [CrossRef]
- Guarro, J. Fusariosis, a complex infection caused by a high diversity of fungal species refractory to treatment. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1491–1500. [Google Scholar] [CrossRef]
- Van Diepeningen, A.D.; Al-Hatmi, A.M.S.; Brankovics, B.; de Hoog, G.S. Taxonomy and Clinical Spectra of Fusarium Species: Where Do We Stand in 2014? Curr. Clin. Microbiol. Rep. 2014, 1, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Al-Hatmi, A.M.S.; Sybren de Hoog, G.; Meis, J.F. Multiresistant fusarium pathogens on plants and humans: Solutions in (from) the antifungal pipeline? Infect. Drug Resist. 2019, 12, 3727–3737. [Google Scholar] [CrossRef] [Green Version]
- Lombard, L.; van der Merwe, N.A.; Groenewald, J.Z.; Crous, P.W. Generic concepts in Nectriaceae. Stud. Mycol. 2015, 80, 189–245. [Google Scholar] [CrossRef] [Green Version]
- Wiederhold, N.; Gibas, C. From the Clinical Mycology Laboratory: New Species and Changes in Fungal Taxonomy and Nomenclature. J. Fungi 2018, 4, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandoval-Denis, M.; Crous, P.W. Removing chaos from confusion: Assigning names to common human and animal pathogens in Neocosmospora. Pers. Mol. Phylogeny Evol. Fungi 2018, 41, 109–129. [Google Scholar] [CrossRef] [PubMed]
- Geiser, D.M.; Adam, G.; Alastruey-Izquierdo, A.; Alberts, J.F.; Al-Hatmi, A.M.S.; Aoki, T.; Baker, S.E.; Balmas, V.; Battacharyya, M.K.; Blomquist, C.L.; et al. A monophyletic Fusarium that includes Fusarium solani species complex is strongly supported by a 19-gene phylogenomic analysis. Phytopathology 2020. Paper under review. [Google Scholar]
- O’Donnell, K.; Al-Hatmi, A.M.; Aoki, T.; Brankovics, B.; Cano-Lira, J.F.; Coleman, J.J.; Sybren de Hoog, G.; Di Pietro, A.; Frandsen, R.J.N.; Geiser, D.M.; et al. No to Neocosmpospora: Phylogenomic and practical reasons for continued inclusions of the Fusarium solani complex in the genus Fusarium. mSphere 2020, 5, e00810-20. [Google Scholar] [CrossRef] [PubMed]
- van Diepeningen, A.D.; de Hoog, G.S. Challenges in Fusarium, a Trans-Kingdom Pathogen. Mycopathologia 2016, 181, 161–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elvers, K.T.; Leeming, K.; Moore, C.P.; Lappin-Scott, H.M. Bacterial-fungal biofilms in flowing water photo-processing tanks. J. Appl. Microbiol. 1998, 84, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; O’Donnell, K.; Sutton, D.A.; Nalim, F.A.; Summerbell, R.C.; Padhye, A.A.; Geiser, D.M. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J. Clin. Microbiol. 2006, 44, 2185–2190. [Google Scholar] [CrossRef] [Green Version]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [Green Version]
- Gullino, M.L.; Daughtrey, M.L.; Garibaldi, A.; Elmer, W.H. Fusarium wilts of ornamental crops and their management. Crop Prot. 2015, 73, 50–59. [Google Scholar] [CrossRef]
- Wu, F. Measuring the economic impacts of Fusarium toxins in animal feeds. Anim. Feed Sci. Technol. 2007, 137, 363–374. [Google Scholar] [CrossRef]
- Singh, R.P.; Singh, P.K.; Rutkoski, J.; Hodson, D.P.; He, X.; Jørgensen, L.N.; Hovmøller, M.S.; Huerta-Espino, J. Disease Impact on Wheat Yield Potential and Prospects of Genetic Control. Annu. Rev. Phytopathol. 2016, 54, 303–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, M.M.; Faria-Ramos, I.; Cruz, L.C.; Pina-Vaz, C.; Gonçalves Rodrigues, A. Genesis of Azole Antifungal Resistance from Agriculture to Clinical Settings. J. Agric. Food Chem. 2015, 63, 7463–7468. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Moreno, C.; Lavergne, R.A.; Hagen, F.; Morio, F.; Meis, J.F.; Le Pape, P. Azole-resistant Aspergillus fumigatus harboring TR 34 /L98H, TR 46 /Y121F/T289A and TR 53 mutations related to flower fields in Colombia. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Schoustra, S.E.; Debets, A.J.M.; Rijs, A.J.M.M.; Zhang, J.; Snelders, E.; Leendertse, P.C.; Melchers, W.J.G.; Rietveld, A.G.; Zwaan, B.J.; Verweij, P.E. Environmental hotspots for azole resistance selection of Aspergillus fumigatus, The Netherlands. Emerg. Infect. Dis. 2019, 25, 1347–1353. [Google Scholar] [CrossRef] [Green Version]
- Godeau, C.; Reboux, G.; Scherer, E.; Laboissiere, A.; Lechenault-Bergerot, C.; Millon, L.; Rocchi, S. Azole-resistant Aspergillus fumigatus in the hospital: Surveillance from flower beds to corridors. Am. J. Infect. Control 2019, 48, 2019–2021. [Google Scholar] [CrossRef]
- Dunne, K.; Hagen, F.; Pomeroy, N.; Meis, J.F.; Rogers, T.R. Intercountry Transfer of Triazole-Resistant Aspergillus fumigatus on Plant Bulbs. Clin. Infect. Dis. 2017, 65, 147–149. [Google Scholar] [CrossRef] [Green Version]
- Buil, J.B.; Hare, R.K.; Zwaan, B.J.; Arendrup, M.C.; Melchers, W.J.G.; Verweij, P.E. The fading boundaries between patient and environmental routes of triazole resistance selection in Aspergillus fumigatus. PLoS Pathog. 2019, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Riat, A.; Plojoux, J.; Gindro, K.; Schrenzel, J.; Sanglard, D. Azole Resistance of Environmental and Clinical Aspergillus. Antimicrob. Agents Chemother. 2018, 4, 1–7. [Google Scholar]
- Homa, M.; Galgóczy, L.; Manikandan, P.; Narendran, V.; Sinka, R.; Csernetics, á.; Vágvölgyi, C.; Kredics, L.; Papp, T. South Indian Isolates of the Fusarium solani species complex from clinical and environmental samples: Identification, antifungal susceptibilities, and virulence. Front. Microbiol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Colombia.Co En Floricultura, La Respuesta Es Colombia. Available online: https://www.colombia.co/pais-colombia/hechos/en-floricultura-la-respuesta-es-colombia-2/ (accessed on 2 March 2020).
- Alvarez-Moreno, C.; Lavergne, R.A.; Hagen, F.; Morio, F.; Meis, J.F.; Le Pape, P. Fungicide-driven alterations in azole-resistant Aspergillus fumigatus are related to vegetable crops in Colombia, South America. Mycologia 2019, 111, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Anaissie, E.J.; Kuchar, R.T.; Rex, J.H.; Francesconi, A.; Kasai, M.; Müller, F.C.; Lozano-Chiu, M.; Summerbell, R.C.; Dignani, M.C.; Chanock, S.J.; et al. Fusariosis Associated with Pathogenic Fusarium Species Colonization of a Hospital Water System: A New Paradigm for the Epidemiology of Opportunistic Mold Infections. Clin. Infect. Dis. 2002, 33, 1871–1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Short, D.P.G.; O’Donnell, K.; Zhang, N.; Juba, J.H.; Geiser, D.M. Widespread occurrence of diverse human pathogenic types of the fungus Fusarium detected in plumbing drains. J. Clin. Microbiol. 2011, 49, 4264–4272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litvinov, N.; da Silva, M.T.N.; van der Heijden, I.M.; Graça, M.G.; Marques de Oliveira, L.; Fu, L.; Giudice, M.; Zilda de Aquino, M.; Odone-Filho, V.; Marques, H.H.; et al. An outbreak of invasive fusariosis in a children’s cancer hospital. Clin. Microbiol. Infect. 2015, 21, 268.e1–268.e7. [Google Scholar] [CrossRef] [Green Version]
- Carter, C.D.; Barr, B.A. Infection control issues in construction and renovation. Infect. Control. Hosp. Epidemiol. 1997, 18, 587–596. [Google Scholar] [CrossRef]
- Nucci, M.; Anaissie, E. Fusarium infections in immunocompromised patients. Clin. Microbiol. Rev. 2007, 20, 695–704. [Google Scholar] [CrossRef] [Green Version]
- Cafarchia, C.; Paradies, R.; Figueredo, L.A.; Iatta, R.; Desantis, S.; Di Bello, A.V.F.; Zizzo, N.; van Diepeningen, A.D. Fusarium spp. in Loggerhead Sea Turtles (Caretta caretta): From Colonization to Infection. Vet. Pathol. 2020, 57, 139–146. [Google Scholar] [CrossRef]
- Garcia-Hartmann, M.; Hennequin, C.; Catteau, S.; Béatini, C.; Blanc, V. Cas groupés d’infection à Fusarium solani chez de jeunes tortues marines Caretta caretta nées en captivité. J. Mycol. Med. 2017, 27, 113–118. [Google Scholar] [CrossRef]
- Smyth, C.W.; Sarmiento-Ramírez, J.M.; Short, D.P.G.; Diéguez-Uribeondoid, J.; O’donnell, K.; Geiser, D.M. Unraveling the ecology and epidemiology of an emerging fungal disease, sea turtle egg fusariosis (STEF). PLoS Pathog. 2019, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bhupathy, S.; Saravanan, S. Status of Marine Turtles in the Gulf of Mannar, India. Chelonian Conserv. Biol. 2006, 5, 139. [Google Scholar] [CrossRef]
- Gleason, F.H.; Allerstorfer, M.; Lilje, O. Newly emerging diseases of marine turtles, especially sea turtle egg fusariosis (SEFT), caused by species in the Fusarium solani complex (FSSC). Mycology 2020, 11, 184–194. [Google Scholar] [CrossRef] [Green Version]
- Sarmiento-Ramirez, J.M.; Sim, J.; Van West, P.; Dieguez-Uribeondo, J. Isolation of fungal pathogens from eggs of the endangered sea turtle species Chelonia mydas in Ascension Island. J. Mar. Biol. Assoc. UK 2017, 97, 661–667. [Google Scholar] [CrossRef] [Green Version]
- Sarmiento-Ramírez, J.M.; Abella, E.; Martín, M.P.; Tellería, M.T.; López-Jurado, L.F.; Marco, A.; Diéguez-Uribeondo, J. Fusarium solani is responsible for mass mortalities in nests of loggerhead sea turtle, Caretta caretta, in Boavista, Cape Verde. FEMS Microbiol. Lett. 2010, 312, 192–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallik, S.K.; Shahi, N.; Joshi, N.; Pant, K.; Kala, K.; Chandra, S.; Sarma, D. The emergence of zoonotic Fusarium oxysporum infection in captive-reared fingerlings of golden mahseer, Tor putitora (Hamilton, 1822) from the central Himalayan region of India. Transbound. Emerg. Dis. 2020, 67, 555–563. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Wiederhold, N.; Robert, V.A.R.G.; Crous, P.W.; Geiser, D.M. Veterinary fusarioses within the United States. J. Clin. Microbiol. 2016, 54, 2813–2819. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, K.; Sutton, D.A.; Rinaldi, M.G.; Sarver, B.A.J.; Balajee, S.A.; Schroers, H.J.; Summerbell, R.C.; Robert, V.A.R.G.; Crous, P.W.; Zhang, N.; et al. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J. Clin. Microbiol. 2010, 48, 3708–3718. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, M.; Negi, B.; Kaushik, N.; Adhikari, A.; Al-Khedhairy, A.A.; Kaushik, N.K.; Choi, E.H. T-2 mycotoxin: Toxicological effects and decontamination strategies. Oncotarget 2017, 8, 33933–33952. [Google Scholar] [CrossRef] [Green Version]
- Lipner, S.R.; Scher, R.K. Onychomycosis: Clinical overview and diagnosis. J. Am. Acad. Derm. 2019, 80, 835–851. [Google Scholar] [CrossRef]
- Thomas, J.; Jacobson, G.A.; Narkowicz, C.K.; Peterson, G.M.; Burnet, H.; Sharpe, C. Toenail onychomycosis: An important global disease burden. J. Clin. Pharm. 2010, 35, 497–519. [Google Scholar]
- Eewski, B.E. Onychomycosis: Treatment, quality of life, and economic issues. Am. J. Clin. Derm. 2000, 1, 19–26. [Google Scholar] [CrossRef]
- Guevara-Suarez, M.; Cano-Lira, J.F.; Cepero de García, M.C.; Sopo, L.; De Bedout, C.; Cano, L.E.; García, A.M.; Motta, A.; Amézquita, A.; Cárdenas, M.; et al. Genotyping of Fusarium Isolates from Onychomycoses in Colombia: Detection of Two New Species Within the Fusarium solani Species Complex and In Vitro Antifungal Susceptibility Testing. Mycopathologia 2016, 181, 165–174. [Google Scholar] [CrossRef]
- Gupta, A.K.; Drummond-Main, C.; Cooper, E.A.; Brintnell, W.; Piraccini, B.M.; Tosti, A. Systematic review of nondermatophyte mold onychomycosis: Diagnosis, clinical types, epidemiology, and treatment. J. Am. Acad. Derm. 2012, 66, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Monod, M.; Méhul, B. Recent findings in onychomycosis and their application for appropriate treatment. J. Fungi 2019, 5, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veiga, F.F.; De Castro-Hoshino, L.V.; Sato, F.; Bombassaro, A.; Vicente, V.A.; Mendes, V.; Baesso, M.L.; Negri, M.; Svidzinski, T.I.E. Fusarium oxysporum is an onychomycosis etiopathogenic agent. Future Microbiol. 2018, 13, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Pal, S. Information transmission in microbial and fungal communication: From classical to quantum. J. Cell Commun. Signal. 2018, 12, 491–502. [Google Scholar] [CrossRef]
- Gupta, A.K.; Daigle, D.; Carviel, J.L. The role of biofilms in onychomycosis. J. Am. Acad. Derm. 2016, 74, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Ung, L.; Bispo, P.J.M.; Shanbhag, S.S.; Gilmore, M.S.; Chodosh, J. The persistent dilemma of microbial keratitis: Global burden, diagnosis, and antimicrobial resistance. Surv. Ophthalmol. 2019, 64, 255–271. [Google Scholar] [CrossRef]
- Wong, T.Y.; Ng, T.P.; Fong, K.S.; Tan, D.T.H. Risk factors and clinical outcomes between fungal and bacterial keratitis: A comparative study. CLAO J. 1997, 23, 275–281. [Google Scholar]
- Sun, S.; Lui, Q.; Han, L.; Ma, Q.; He, S.; Li, X.; Zhang, H.; Zhang, J.; Liu, X.; Wang, L. Identification and Characterization of Fusarium proliferatum, a New Species of Fungi that Cause Fungal Keratitis. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Tuli, S. Fungal keratitis. Clin. Ophthalmol. 2011, 5, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.; Sachdev, A.; Coggon, D.; Hossain, P. Geographic variations in microbial keratitis: An analysis of the peer-reviewed literature. Br. J. Ophthalmol. 2011, 95, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.K.E.; So, K.; Chung, P.H.; Tsang, H.F.; Chuang, S.K. A multi-country outbreak of fungal keratitis associated with a brand of contact lens solution: The Hong Kong experience. Int. J. Infect. Dis. 2009, 13, 443–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakhundi, S.; Siddiqui, R.; Khan, N.A. Pathogenesis of microbial keratitis. Microb. Pathog. 2017, 104, 97–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, P.A.; Kaliamurthy, J. Mycotic keratitis: Epidemiology, diagnosis and management. Clin. Microbiol. Infect. 2013, 19, 210–220. [Google Scholar] [CrossRef] [Green Version]
- Oechsler, R.A.; Feilmeier, M.R.; Miller, D.; Shi, W.; Hofling-Lima, A.L.; Alfonso, E.C. Fusarium keratitis: Genotyping, in vitro susceptibility and clinical outcomes. Cornea 2013, 32, 667–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheikhrouhou, F.; Makni, F.; Neji, S.; Trigui, A.; Sellami, H.; Trabelsi, H.; Guidara, R.; Fki, J.; Ayadi, A. Epidemiological profile of fungal keratitis in Sfax (Tunisia). J. Mycol. Med. 2014, 24, 308–312. [Google Scholar] [CrossRef]
- Muller, G.; Kara-Jose, N.; Silvestre, R. Perfil epidemiológico das ceratomicoses atendidas no HC-UNICAMP. Arq. Bras. Oftalmol. 2012, 75, 247–250. [Google Scholar] [CrossRef]
- Vanzzini Zago, V.; Manzano-Gayosso, P.; Hernández-Hernández, F.; Méndez-Tovar, L.J.; Gómez-Leal, A.; López Martínez, R. Queratomicosis en un centro de atención oftalmológica en la Ciudad de México. Rev. Iberoam. Micol. 2010, 27, 57–61. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L. Association of Tear Film Stability and Corneal Surface Temperature in Pudong Patients. Curr. Eye Res. 2017, 42, 655–660. [Google Scholar] [CrossRef]
- Jones, B.R. Principles in the management of oculomycosis. XXXI Edward Jackson Memorial Lecture. Am. J. Ophthalmol. 1975, 79, 719–751. [Google Scholar] [CrossRef]
- Raza, S.K.; Mallet, A.I.; Howell, S.A.; Thomas, P.A. An in-vitro study of the sterol content and toxin production of Fusarium isolates from mycotic keratitis. J. Med. Microbiol. 1994, 41, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Liu, X.; Ma, Z.; Yin, Y.; Sun, L.; Yang, L.; Zheng, Y. Fungal keratitis: Pathogenesis, diagnosis and prevention. Microb. Pathog. 2020, 138, 1–10. [Google Scholar] [CrossRef]
- Córdova-Alcántara, I.M.; Venegas-Cortés, D.L.; Martínez-Rivera, M.Á.; Pérez, N.O.; Rodriguez-Tovar, A.V. Biofilm characterization of Fusarium solani keratitis isolate: Increased resistance to antifungals and UV light. J. Microbiol. 2019, 57, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Calvillo-Medina, R.P.; Reyes-Grajeda, J.P.; Barba-Escoto, L.; Bautista-Hernandez, L.A.; Campos-Guillén, J.; Jones, G.H.; Bautista-de Lucio, V.M. Proteome analysis of biofilm produced by a Fusarium falciforme keratitis infectious agent. Microb. Pathog. 2019, 130, 232–241. [Google Scholar] [CrossRef]
- Nucci, M.; Marr, K.A.; Vehreschild, M.J.G.T.; de Souza, C.A.; Velasco, E.; Cappellano, P.; Carlesse, F.; Queiroz-Telles, F.; Sheppard, D.C.; Kindo, A.; et al. Improvement in the outcome of invasive fusariosis in the last decade. Clin. Microbiol. Infect. 2014, 20, 580–585. [Google Scholar] [CrossRef] [Green Version]
- Nucci, M.; Anaissie, E. Cutaneous Infection by Fusarium Species in Healthy and Immunocompromised Hosts: Implications for Diagnosis and Management. Clin. Infect. Dis. 2002, 35, 909–920. [Google Scholar] [CrossRef] [Green Version]
- Legrand, C.; Anaissie, E.; Hashem, R.; Nelson, P.; Bodey, G.P.; Ro, J. Experimental fusarial hyalohyphomycosis in a murine model. J. Infect. Dis. 1991, 164, 944–948. [Google Scholar] [CrossRef]
- Costa, M.I.; Vilugron Rodrigues, F.A.; Veiga, F.F.; Jarros, I.C.; Kischkel, B.; Negri, M.; Alexandrino Becker, T.C.; Svidzinski, T.I.E. Effects of intratracheal Fusarium solani inoculation in immunocompetent mice. Microb. Pathog. 2019, 128, 317–322. [Google Scholar] [CrossRef]
- Yang, C.; Hamel, C.; Vujanovic, V.; Gan, Y. Fungicide: Modes of Action and Possible Impact on Nontarget Microorganisms. ISRN Ecol. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Price, C.L.; Parker, J.E.; Warrilow, A.G.; Kelly, D.E.; Kelly, S.L. Azole fungicides—understanding resistance mechanisms in agricultural fungal pathogens. Pest Manag. Sci. 2015, 71, 1054–1058. [Google Scholar] [CrossRef]
- Qian, H.; Du, J.; Chi, M.; Sun, X.; Liang, W.; Huang, J.; Li, B. The Y137H mutation in the cytochrome P450 FgCYP51B protein confers reduced sensitivity to tebuconazole in Fusarium graminearum. Pest Manag. Sci. 2018, 74, 1472–1477. [Google Scholar] [CrossRef]
- Kang, Z.; Huang, L.; Krieg, U.; Mauler-Machnik, A.; Buchenauer, H. Effects of tebuconazole on morphology, structure, cell wall components and trichothecene production of Fusarium culmorum in vitro. Pest Manag. Sci. 2001, 57, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Hellin, P.; King, R.; Urban, M.; Hammond-Kosack, K.E.; Legrève, A. The adaptation of Fusarium culmorum to DMI fungicides is mediated by major transcriptome modifications in response to azole fungicide, including the overexpression of a PDR transporter (FcABC1). Front. Microbiol. 2018, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatmi, A.; Curfs-Breuker, I.; de Hoog, G.; Meis, J.; Verweij, P. Antifungal Susceptibility Testing of Fusarium: A Practical Approach. J. Fungi 2017, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Tosti, A.; Piraccini, B.M.; Lorenzi, S.; Iorizzo, M. Treatment of nondermatophyte mold and Candida onychomycosis. Derm. Clin. 2003, 21, 491–497. [Google Scholar] [CrossRef]
- Sahay, P.; Singhal, D.; Nagpal, R.; Maharana, P.K.; Farid, M.; Gelman, R.; Sinha, R.; Agarwal, T.; Titiyal, J.S.; Sharma, N. Pharmacologic therapy of mycotic keratitis. Surv. Ophthalmol. 2019, 64, 380–400. [Google Scholar] [CrossRef]
- Bunya, V.Y.; Hammersmith, K.M.; Rapuano, C.J.; Ayres, B.D.; Cohen, E.J. Topical and Oral Voriconazole in the Treatment of Fungal Keratitis. Am. J. Ophthalmol. 2007, 143, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Das, S.; Virdi, A.; Fernandes, M.; Sahu, S.K.; Koday, N.K.; Ali, M.H.; Garg, P.; Motukupally, S.R. Re-appraisal of topical 1% voriconazole and 5% natamycin in the treatment of fungal keratitis in a randomised trial. Br. J. Ophthalmol. 2015, 99, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Lainhart, W. Fusarium spp., a Genus of Common PlantPathogens That Can Cause Devastating, Opportunistic Human Disease. Clin. Microbiol. Newsl. 2018, 40, 1–5. [Google Scholar] [CrossRef]
- Lortholary, O.; Obenga, G.; Biswas, P.; Caillot, D.; Chachaty, E.; Bienvenu, A.L.; Cornet, M.; Greene, J.; Herbrecht, R.; Lacroix, C.; et al. International retrospective analysis of 73 cases of invasive fusariosis treated with voriconazole. Antimicrob. Agents Chemother. 2010, 54, 4446–4450. [Google Scholar] [CrossRef] [Green Version]
- Nucci, M.; Anaissie, E. How we treat invasive fungal diseases in patients with acute leukemia: The importance of an individualized approach. Blood 2014, 124, 3858–3870. [Google Scholar] [CrossRef] [Green Version]
- Venturini, T.P.; Al-Hatmi, A.M.S.; Rossato, L.; Azevedo, M.I.; Keller, J.T.; Weiblen, C.; Santurio, J.M.; Alves, S.H. Do antibacterial and antifungal combinations have better activity against clinically relevant fusarium species? In vitro synergism. Int. J. Antimicrob. Agents 2018, 51, 784–788. [Google Scholar] [CrossRef]
- Al-Hatmi, A.M.S.; Meis, J.F.; de Hoog, G.S. Fusarium: Molecular Diversity and Intrinsic Drug Resistance. PLoS Pathog. 2016, 12, e1005464. [Google Scholar] [CrossRef]
- White, T.C.; Marr, K.A.; Bowden, R.A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 1998, 11, 382–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, J.E.; Warrilow, A.G.S.; Price, C.L.; Mullins, J.G.L.; Kelly, D.E.; Kelly, S.L. Resistance to antifungals that target CYP51. J. Chem. Biol. 2014, 7, 143–161. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Cantero, A.; López-Fernández, L.; Guarro, J.; Capilla, J. Azole resistance mechanisms in Aspergillus: Update and recent advances. Int. J. Antimicrob. Agents 2020, 55, 1–10. [Google Scholar] [CrossRef]
- Becher, R.; Weihmann, F.; Deising, H.B.; Wirsel, S.G.R. Development of a novel multiplex DNA microarray for Fusarium graminearum and analysis of azole fungicide responses. BMC Genom. 2011, 12, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, J.E.; Lamping, E.; Santhanam, J.; Milne, T.J.; Abd Razak, M.F.; Zakaria, L.; Cannon, R.D. A 23 bp cyp51A Promoter Deletion Associated With Voriconazole Resistance in Clinical and Environmental Isolates of Neocosmospora keratoplastica. Front. Microbiol. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Turra, D.; Zhou, S.; Ayhan, D.H.; DeIulio, G.A.; Guo, L.; Broz, K.; Wiederhold, N.; Coleman, J.J.; et al. The genome of opportunistic fungal pathogen Fusarium oxysporum carries a unique set of lineage-specific chromosomes. Commun. Biol. 2020, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Castillo-Castañeda, A.; Cañas-Duarte, S.J.; Guevara-Suarez, M.; Guarro, J.; Restrepo, S.; Celis Ramírez, A.M. Transcriptional response of Fusarium oxysporum and Neocosmospora solani challenged with amphotericin B or posaconazole. Microbiology 2020, 166, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Kathuria, S.; Xu, J.; Meis, J.F. Emergence of Azole-Resistant Aspergillus fumigatus Strains due to Agricultural Azole Use Creates an Increasing Threat to Human Health. PLoS Pathog. 2013, 9, 3–7. [Google Scholar] [CrossRef]
- Brauer, V.S.; Rezende, C.P.; Pessoni, A.M.; De Paula, R.G.; Rangappa, K.S.; Nayaka, S.C.; Gupta, V.K.; Almeida, F. Antifungal agents in agriculture: Friends and foes of public health. Biomolecules 2019, 9, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez Tudela, J.L.; Cole, D.C.; Ravasi, G.; Bruisma, N.; Chiller, T.C.; Ford, N.; Denning, D.W. Integration of fungal diseases into health systems in Latin America. Lancet Infect. Dis. 2020, 20, 890–892. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sáenz, V.; Alvarez-Moreno, C.; Pape, P.L.; Restrepo, S.; Guarro, J.; Ramírez, A.M.C. A One Health Perspective to Recognize Fusarium as Important in Clinical Practice. J. Fungi 2020, 6, 235. https://doi.org/10.3390/jof6040235
Sáenz V, Alvarez-Moreno C, Pape PL, Restrepo S, Guarro J, Ramírez AMC. A One Health Perspective to Recognize Fusarium as Important in Clinical Practice. Journal of Fungi. 2020; 6(4):235. https://doi.org/10.3390/jof6040235
Chicago/Turabian StyleSáenz, Valeri, Carlos Alvarez-Moreno, Patrice Le Pape, Silvia Restrepo, Josep Guarro, and Adriana Marcela Celis Ramírez. 2020. "A One Health Perspective to Recognize Fusarium as Important in Clinical Practice" Journal of Fungi 6, no. 4: 235. https://doi.org/10.3390/jof6040235