The Fungal Endophyte Serendipita williamsii Does Not Affect Phosphorus Status But Carbon and Nitrogen Dynamics in Arbuscular Mycorrhizal Tomato Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Material and Inoculum Production
2.2. Plant Material, Inoculation, and Cultivation
2.3. Photosynthetic Performance
2.4. Harvest and Nutrient Analysis of Tomato Shoots
2.5. Detection of AMF in Colonized Roots
2.6. DNA Extraction and Detection of Serendipita spp. in Colonized Roots by Nested PCR
2.7. Statistical Analysis
3. Results
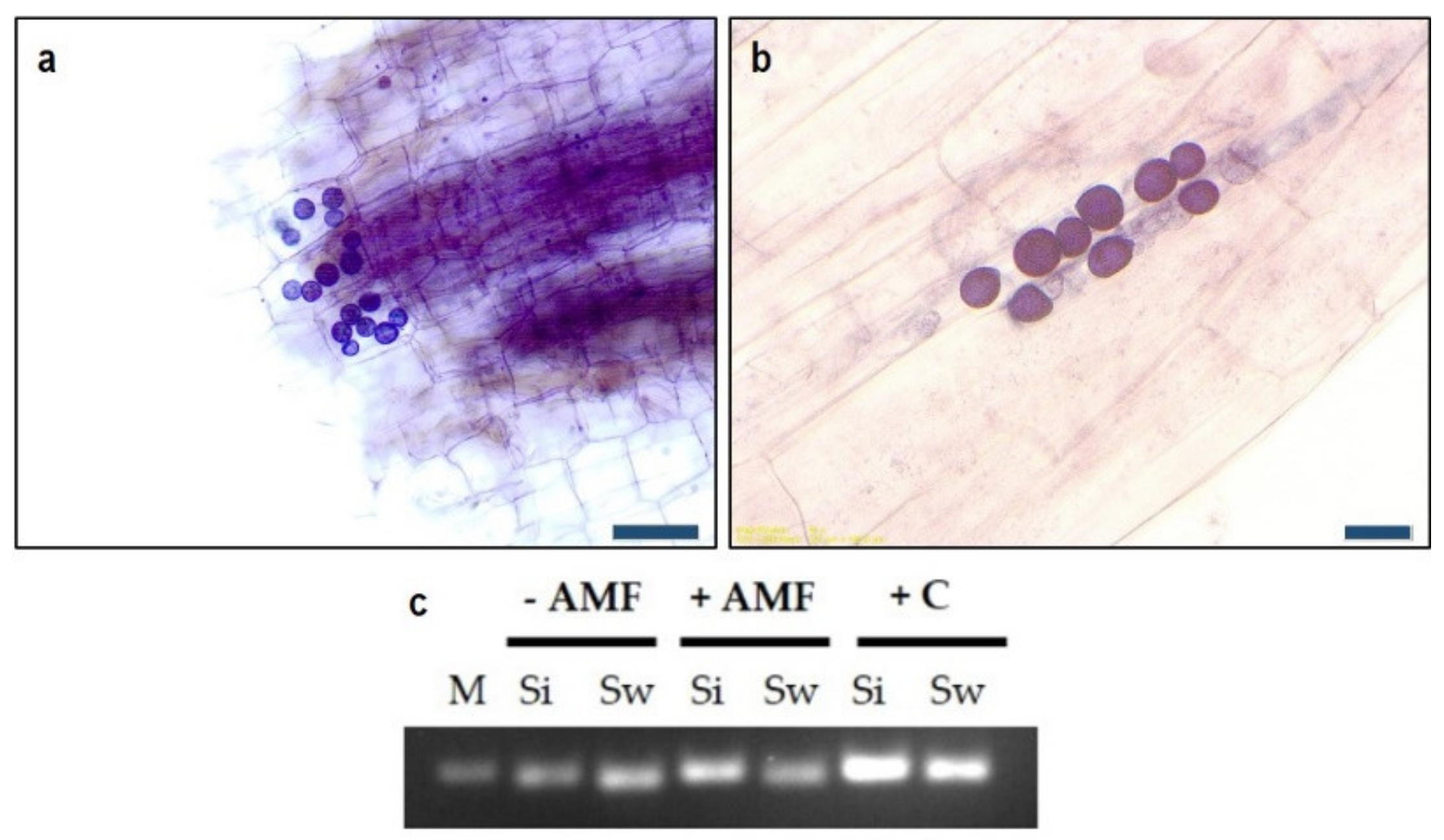
3.1. Fungal Colonization
3.1.1. Root Colonization by AMF
3.1.2. Root Colonization by Serendipita spp.
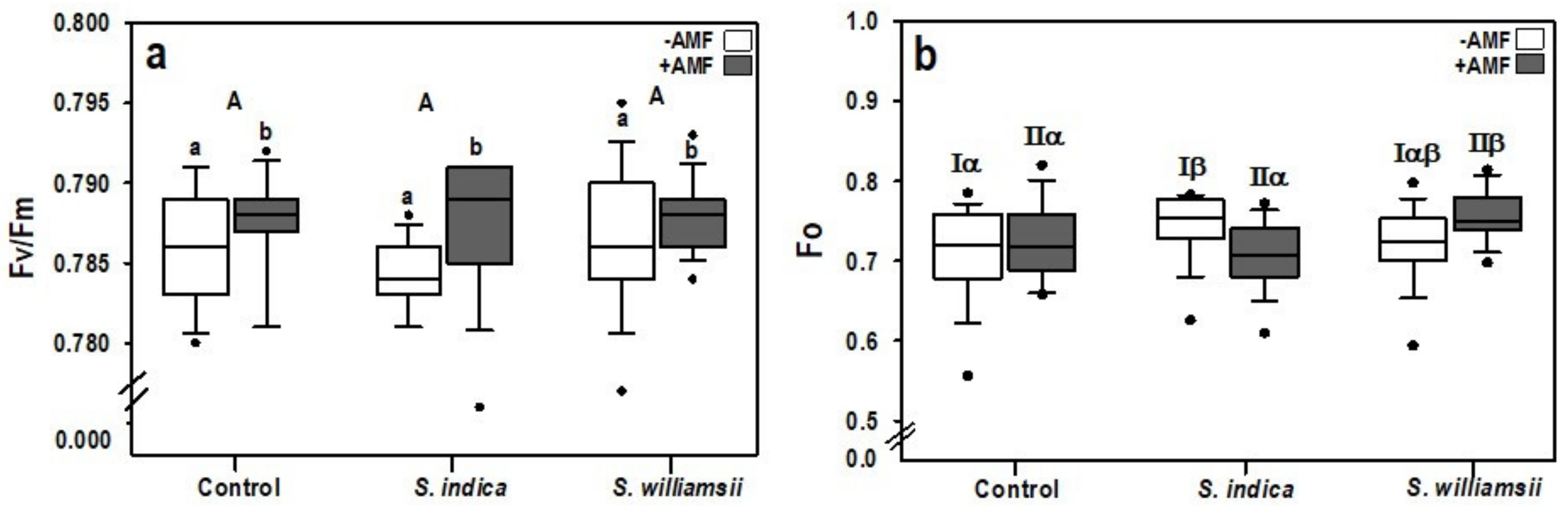
3.2. Plant Growth and Performance of Photosystem II
3.3. Nutrient Status in Tomato Shoot
3.3.1. Macronutrients
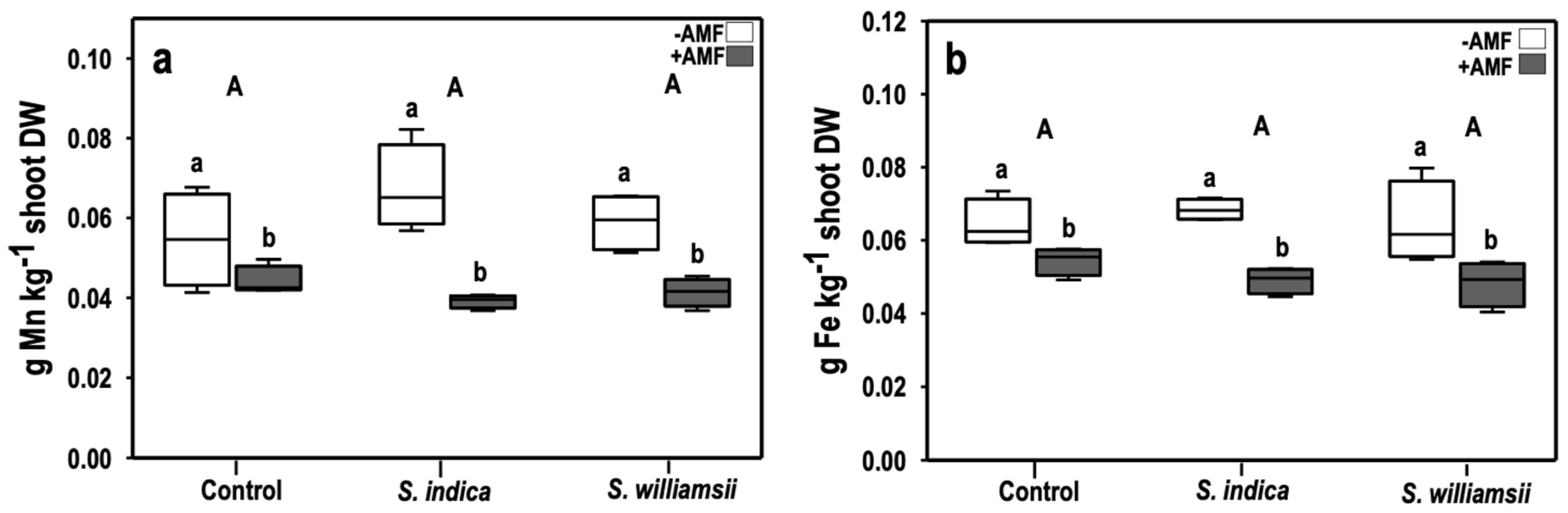
3.3.2. Micronutrients
3.4. Correlation between Nutrients and AMF Root Colonization
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Franken, P. The plant strengthening root endophyte Piriformospora indica: Potential application and the biology behind. Appl. Microbiol. Biotechnol. 2012, 96, 1455–1464. [Google Scholar] [CrossRef] [Green Version]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Whipps, J.M. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 2001, 52, 487–511. [Google Scholar] [CrossRef]
- Venneman, J.; Audenaert, K.; Verwaeren, J.; Baert, G.; Boeckx, P.; Moango, A.M.; Dhed’a, B.D.; Vereecke, D.; Haesaert, G. Congolese rhizospheric soils as a rich source of new plant growth-promoting endophytic Piriformospora isolates. Front. Microbiol. 2017, 8, 212. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; White, J., Jr.; Arnold, A.E.; Redman, A.R.A. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, R.; Huang, Y.; Gebrechristos, S.; Mikolajczyk, B.; Brown, H.; Prasad, R.; Varma, A.; Bushley, K.E. Transcriptional responses of soybean roots to colonization with the root endophytic fungus Piriformospora indica reveals altered phenylpropanoid and secondary metabolism. Sci. Rep. 2018, 8, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Blaalid, R.; Carlsen, T.O.R.; Kumar, S.; Halvorsen, R.; Ugland, K.I.; Fontana, G.; Kauserud, H. Changes in the root−associated fungal communities along a primary succession gradient analysed by 454 pyrosequencing. Mol. Ecol. 2012, 21, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Detheridge, A.P.; Brand, G.; Fychan, R.; Crotty, F.V.; Sanderson, R.; Griffith, G.W.; Marley, C.L. The legacy effect of cover crops on soil fungal populations in a cereal rotation. Agric. Ecosyst. Environ. 2016, 228, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Correa, A.; Cruz, C.; Perez-Tienda, J.; Ferrol, N. Shedding light onto nutrient responses of arbuscular mycorrhizal plants: Nutrient interactions may lead to unpredicted outcomes of the symbiosis. Plant Sci. 2014, 221, 29–41. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Bago, B.; Pfeffer, P.E.; Shachar-Hill, Y. Carbon metabolism and transport in arbuscular mycorrhizas. Plant Physiol. 2000, 124, 949–958. [Google Scholar] [CrossRef] [Green Version]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, R.á.; Zeto, S. Mineral acquisition by arbuscular mycorrhizal plants. J. Plant Nutr. 2000, 23, 867–902. [Google Scholar] [CrossRef]
- Marschner, P. Rhizosphere biology. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 369–388. [Google Scholar]
- Zitterl-Eglseer, K.; Nell, M.; Lamien-Meda, A.; Steinkellner, S.; Wawrosch, C.; Kopp, B.; Zitterl, W.; Vierheilig, H.; Novak, J. Effects of root colonization by symbiotic arbuscular mycorrhizal fungi on the yield of pharmacologically active compounds in Angelica archangelica L. Acta Physiol. Plant 2015, 37. [Google Scholar] [CrossRef]
- Cesaro, P.; Massa, N.; Cantamessa, S.; Todeschini, V.; Bona, E.; Berta, G.; Barbato, R.; Lingua, G. Tomato responses to Funneliformis mosseae during the early stages of arbuscular mycorrhizal symbiosis. Mycorrhiza 2020, 30, 601–610. [Google Scholar] [CrossRef]
- Khaosaad, T.; Garcia-Garrido, J.M.; Steinkellner, S.; Vierheilig, H. Take-all disease is systemically reduced in roots of mycorrhizal barley plants. Soil Biol. Biochem. 2007, 39, 727–734. [Google Scholar] [CrossRef]
- Steinkellner, S.; Hage-Ahmed, K.; Garcia-Garrido, J.M.; Illana, A.; Ocampo, J.A.; Vierheilig, H. A comparison of wild-type, old and modern tomato cultivars in the interaction with the arbuscular mycorrhizal fungus Glomus mosseae and the tomato pathogen Fusarium oxysporum f. sp lycopersici. Mycorrhiza 2012, 22, 189–194. [Google Scholar] [CrossRef]
- Verma, S.; Varma, A.; Rexer, K.H.; Hassel, A.; Kost, G.; Sarbhoy, A.; Bisen, P.; Butehorn, B.; Franken, P. Piriformospora indica, gen. et sp. nov., a new root-colonizing fungus. Mycologia 1998, 90, 896–903. [Google Scholar] [CrossRef]
- Varma, S.; Verma, A.; Sahay, N.; Butehorn, B.; Franken, P. Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl. Environ. Microbiol. 1999, 65, 2741–2744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, P.G. Orchidaceous rhizoctonias in pot cultures of vesicular arbuscular mycorrhizal fungi. Can. J. Bot. Rev. Can. Bot. 1985, 63, 1329–1333. [Google Scholar] [CrossRef]
- Sefloo, N.G.; Wieczorek, K.; Steinkellner, S.; Hage-Ahmed, K. Serendipita species trigger cultivar-specific responses to fusarium wilt in tomato. Agronomy 2019, 9, 595. [Google Scholar] [CrossRef] [Green Version]
- Achatz, B.; von Rueden, S.; Andrade, D.; Neumann, E.; Pons-Kuehnemann, J.; Kogel, K.-H.; Franken, P.; Waller, F. Root colonization by Piriformospora indica enhances grain yield in barley under diverse nutrient regimes by accelerating plant development. Plant Soil 2010, 333, 59–70. [Google Scholar] [CrossRef]
- Fakhro, A.; Andrade-Linares, D.R.; von Bargen, S.; Bandte, M.; Buettner, C.; Grosch, R.; Schwarz, D.; Franken, P. Impact of Piriformospora indica on tomato growth and on interaction with fungal and viral pathogens. Mycorrhiza 2010, 20, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Barazani, O.; Benderoth, M.; Groten, K.; Kuhlemeier, C.; Baldwin, I.T. Piriformospora indica and Sebacina vermifera increase growth performance at the expense of herbivore resistance in Nicotiana attenuata. Oecologia 2005, 146, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Li, D.; Qi, Q.; Sun, X.; Anue, M.R.; David, B.M.; Zhang, Y.; Hao, X.; Zhang, Z.; Lai, Z. The root endophytic fungus Serendipita indica improves resistance of banana to Fusarium oxysporum f. sp. cubense tropical race 4. Eur. J. Plant Pathol. 2020, 156, 87–100. [Google Scholar] [CrossRef]
- Cosme, M.; Lu, J.; Erb, M.; Stout, M.J.; Franken, P.; Wurst, S. A fungal endophyte helps plants to tolerate root herbivory through changes in gibberellin and jasmonate signaling. New Phytol. 2016, 211, 1065–1076. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, V.; Tuteja, N.; Johri, A.K. Antioxidant enzyme activities in maize plants colonized with Piriformospora indica. Microbiology. 2009, 155, 780–790. [Google Scholar] [CrossRef] [Green Version]
- Narayan, O.P.; Verma, N.; Singh, A.K.; Oelmueller, R.; Kumar, M.; Prasad, D.; Kapoor, R.; Dua, M.; Johri, A.K. Antioxidant enzymes in chickpea colonized by Piriformospora indica participate in defense against the pathogen Botrytis cinerea. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Rabiey, M.; Ullah, I.; Shaw, M. The endophytic fungus Piriformospora indica protects wheat from fusarium crown rot disease in simulated UK autumn conditions. Plant Pathol. 2015, 64, 1029–1040. [Google Scholar] [CrossRef]
- Dabral, S.; Yashaswee; Varma, A.; Choudhary, D.K.; Bahuguna, R.N.; Nath, M. Biopriming with Piriformospora indica ameliorates cadmium stress in rice by lowering oxidative stress and cell death in root cells. Ecotoxicol. Environ. Saf. 2019, 186. [Google Scholar] [CrossRef]
- Hosseini, F.; Mosaddeghi, M.R.; Dexter, A.R. Effect of the fungus Piriformospora indica on physiological characteristics and root morphology of wheat under combined drought and mechanical stresses. Plant Physiol. Biochem. 2017, 118, 107–120. [Google Scholar] [CrossRef]
- Sabra, M.; Aboulnasr, A.; Franken, P.; Perreca, E.; Wright, L.P.; Camehl, I. Beneficial root endophytic fungi increase growth and quality parameters of sweet basil in heavy metal contaminated soil. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Wang, J.; Xu, L.; Wang, A.; Huang, L.; Du, H.; Qiu, L.; Oelmueller, R. Drought stress responses in maize are diminished by Piriformospora indica. Plant Signal. Behav. 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- Heidarianpour, M.B.; Aliasgharzad, N.; Olsson, P.A. Positive effects of co-inoculation with Rhizophagus irregularis and Serendipita indica on tomato growth under saline conditions, and their individual colonization estimated by signature lipids. Mycorrhiza 2020, 30, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.W.; Kafer, E. Improved protocols for Aspergillus minimal medium: Trace element and minimal medium salt stock solutions. Fungal Genet. Rep. 2001, 48, 20–21. [Google Scholar] [CrossRef] [Green Version]
- Vierheilig, H.; Coughlan, A.P.; Wyss, U.; Piché, Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl. Environ. Microbiol. 1998, 64, 5004–5007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koide, R.; Elliott, G. Cost, benefit and efficiency of the vesicular-arbuscular mycorrhizal symbiosis. Funct. Ecol. 1989, 3, 252–255. [Google Scholar] [CrossRef]
- Steineck, O. Nährlösungen der Pflanzenkultur. Bodenkultur 1951, 5, 313–324. [Google Scholar]
- Dumas, J.B.A. Procédés de l’analyse organique. Ann. Chem. Phys. 1831, 47, 198–213. [Google Scholar]
- McGonigle, T.; Miller, M.; Evans, D.; Fairchild, G.; Swan, J. A new method which gives an objective measure of colonization of roots by vesicular—Arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [Green Version]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Stielow, J.B.; Lévesque, C.A.; Seifert, K.A.; Meyer, W.; Iriny, L.; Smits, D.; Renfurm, R.; Verkley, G.J.; Groenewald, M.; Chaduli, D.; et al. One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia 2015, 35, 242–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basiewicz, M.; Weiss, M.; Kogel, K.-H.; Langen, G.; Zorn, H.; Zuccaro, A. Molecular and phenotypic characterization of Sebacina vermifera strains associated with orchids, and the description of Piriformospora williamsii sp. nov. Fungal Biol. 2012, 116, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant 2016, 38, 102. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, S.; Hückelhoven, R.; Schäfer, P.; Imani, J.; Sharma, M.; Weiss, M.; Waller, F.; Kogel, K.-H. The root endophytic fungus Piriformospora indica requires host cell death for proliferation during mutualistic symbiosis with barley. Proc. Natl. Acad. Sci. USA 2006, 103, 18450–18457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douds, D.D.; Johnson, C.R.; Koch, K.E. Carbon cost of the fungal symbiont relative to net leaf P accumulation in a split-root VA mycorrhizal symbiosis. Plant Physiol. 1988, 86, 491–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rousk, J.; Bååth, E.; Göransson, H.; Fransson, A.-M. Assessing plant-microbial competition for 33P using uptake into phospholipids. Appl. Soil Ecol. 2007, 36, 233–237. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, J.; Ding, X.; He, X.; Zhang, F.; Feng, G. Hyphosphere interactions between an arbuscular mycorrhizal fungus and a phosphate solubilizing bacterium promote phytate mineralization in soil. Soil Biol. Biochem. 2014, 74, 177–183. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, N.; Li, X.; Long, J.; Sui, X.; Wu, Y.; Li, J.; Wang, J.; Zhong, H.; Sun, G.Y. Arbuscular mycorrhizal fungi (Glomus mosseae) improves growth, photosynthesis and protects photosystem II in leaves of Lolium perenne L. in cadmium contaminated soil. Front. Plant Sci. 2018, 9, 1156. [Google Scholar] [CrossRef] [Green Version]
- Hart, M.; Ehret, D.L.; Krumbein, A.; Leung, C.; Murch, S.; Turi, C.; Franken, P. Inoculation with arbuscular mycorrhizal fungi improves the nutritional value of tomatoes. Mycorrhiza 2015, 25, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Raghothama, K. Phosphate acquisition. Annu. Rev. Plant Biol. 1999, 50, 665–693. [Google Scholar] [CrossRef]
- Tinker, P.; Jones, M.; Durall, D. A functional comparison of ecto-and endomycorrhizas. In Mycorrhizas in Ecosystems; CAB International: Walingford, UK, 1992; pp. 303–310. [Google Scholar]
- Lambers, H.; Chapin, F.S., III; Pons, T.L. Plant Physiological Ecology; Springer Science & Business Media: Berlin, Germany, 2008. [Google Scholar]
- Del Barrio-Duque, A.; Ley, J.; Samad, A.; Antonielli, L.; Sessitsch, A.; Compant, S. Beneficial endophytic bacteria-Serendipita indica interaction for crop enhancement and resistance to phytopathogens. Front. Microbiol. 2019, 10, 2888. [Google Scholar] [CrossRef]
- Meena, K.K.; Mesapogu, S.; Kumar, M.; Yandigeri, M.S.; Singh, G.; Saxena, A.K. Co-inoculation of the endophytic fungus Piriformospora indica with the phosphate-solubilising bacterium Pseudomonas striata affects population dynamics and plant growth in chickpea. Biol. Fertil. Soils 2010, 46, 169–174. [Google Scholar] [CrossRef]
- Karandashov, V.; Nagy, R.; Wegmüller, S.; Amrhein, N.; Bucher, M. Evolutionary conservation of a phosphate transporter in the arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2004, 101, 6285–6290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarma, M.; Kumar, V.; Saharan, K.; Srivastava, R.; Sharma, A.; Prakash, A.; Sahai, V.; Bisaria, V. Application of inorganic carrier−based formulations of fluorescent pseudomonads and Piriformospora indica on tomato plants and evaluation of their efficacy. J. Appl. Microbiol. 2011, 111, 456–466. [Google Scholar] [CrossRef]
- Sharma, M.; Schmid, M.; Rothballer, M.; Hause, G.; Zuccaro, A.; Imani, J.; Kämpfer, P.; Domann, E.; Schäfer, P.; Hartmann, A. Detection and identification of bacteria intimately associated with fungi of the order Sebacinales. Cell. Microbiol. 2008, 10, 2235–2246. [Google Scholar] [CrossRef]
- Glaeser, S.P.; Imani, J.; Alabid, I.; Guo, H.; Kumar, N.; Kämpfer, P.; Hardt, M.; Blom, J.; Goesmann, A.; Rothballer, M. Non-pathogenic Rhizobium radiobacter F4 deploys plant beneficial activity independent of its host Piriformospora indica. ISME J. 2016, 10, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Shahollari, B.; Varma, A.; Oelmuller, R. Expression of a receptor kinase in Arabidopsis roots is stimulated by the basidiomycete Piriformospora indica and the protein accumulates in Triton X-100 insoluble plasma membrane microdomains. J. Plant Physiol. 2005, 162, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wei, Q.; Xu, L.; Li, H.; Oelmüller, R.; Zhang, W. Piriformospora indica enhances phosphorus absorption by stimulating acid phosphatase activities and organic acid accumulation in Brassica napus. Plant Soil 2018, 432, 333–344. [Google Scholar] [CrossRef]
- Yadav, V.; Kumar, M.; Deep, D.K.; Kumar, H.; Sharma, R.; Tripathi, T.; Tuteja, N.; Saxena, A.K.; Johri, A.K. A phosphate transporter from the root endophytic fungus Piriformospora indica plays a role in phosphate transport to the host plant. J. Biol. Chem. 2010, 285, 26532–26544. [Google Scholar] [CrossRef] [Green Version]
- Ngwene, B.; Boukail, S.; Soellner, L.; Franken, P.; Andrade-Linares, D.R. Phosphate utilization by the fungal root endophyte Piriformospora indica. Plant Soil 2016, 405, 231–241. [Google Scholar] [CrossRef] [Green Version]
- McNeill, A.; Unkovich, M. The nitrogen cycle in terrestrial ecosystems. In Nutrient Cycling in Terrestrial Ecosystems; Springer: Berlin, Germany, 2007; pp. 37–64. [Google Scholar] [CrossRef]
- Balestrini, R.; Brunetti, C.; Chitarra, W.; Nerva, L. Photosynthetic traits and nitrogen uptake in crops: Which is the role of arbuscular mycorrhizal fungi? Plants 2020, 9, 1105. [Google Scholar] [CrossRef] [PubMed]
- Rivero, J.; Gamir, J.; Aroca, R.; Pozo, M.J.; Flors, V. Metabolic transition in mycorrhizal tomato roots. Front Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bücking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 2005, 435, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Johansen, A. Depletion of soil mineral N by roots of Cucumis sativus L. colonized or not by arbuscular mycorrhizal fungi. Plant Soil 1999, 209, 119–127. [Google Scholar] [CrossRef]
- Nouri, E.; Breuillin-Sessoms, F.; Feller, U.; Reinhardt, D. Phosphorus and nitrogen regulate arbuscular mycorrhizal symbiosis in Petunia hybrida. PLoS ONE 2014, 9, e90841. [Google Scholar] [CrossRef]
- Nordin, A.; Schmidt, I.K.; Shaver, G.R. Nitrogen uptake by arctic soil microbes and plants in relation to soil nitrogen supply. Ecology 2004, 85, 955–962. [Google Scholar] [CrossRef]
- Alberton, O.; Kuyper, T.W.; Gorissen, A. Taking mycocentrism seriously: Mycorrhizal fungal and plant responses to elevated CO2. New Phytol. 2005, 167, 859–868. [Google Scholar] [CrossRef]
- Corrêa, A.; Gurevitch, J.; Martins−Loução, M.; Cruz, C. C allocation to the fungus is not a cost to the plant in ectomycorrhizae. Oikos 2012, 121, 449–463. [Google Scholar] [CrossRef]
- Fellbaum, C.R.; Gachomo, E.W.; Beesetty, Y.; Choudhari, S.; Strahan, G.D.; Pfeffer, P.E.; Kiers, E.T.; Bücking, H. Carbon availability triggers fungal nitrogen uptake and transport in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2012, 109, 2666–2671. [Google Scholar] [CrossRef] [Green Version]
- Zuccaro, A.; Lahrmann, U.; Gueldener, U.; Langen, G.; Pfiffi, S.; Biedenkopf, D.; Wong, P.; Samans, B.; Grimm, C.; Basiewicz, M. Endophytic life strategies decoded by genome and transcriptome analyses of the mutualistic root symbiont Piriformospora indica. PLoS Pathog. 2011, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kothari, S.; Marschner, H.; Römheld, V. Contribution of the VA mycorrhizal hyphae in acquisition of phosphorus and zinc by maize grown in a calcareous soil. Plant Soil 1991, 131, 177–185. [Google Scholar] [CrossRef]
- Turnau, K.; Kottke, I.; Oberwinkler, F. Element localization in mycorrhizal roots of Pteridium aquilinum (L.) Kuhn collected from experimental plots treated with cadmium dust. New Phytol. 1993, 123, 313–324. [Google Scholar] [CrossRef]
- Haas, H.; Eisendle, M.; Turgeon, B.G. Siderophores in fungal physiology and virulence. Annu. Rev. Phytopathol. 2008, 46, 149–187. [Google Scholar] [CrossRef]
- Bidellaoui, B.; Segarra, G.; Hakkou, A.; Trillas, M.I. Beneficial effects of Rhizophagus irregularis and Trichoderma asperellum strain T34 on growth and fusarium wilt in tomato plants. J. Plant Pathol. 2019, 101, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Rufyikiri, G.; Huysmans, L.; Wannijn, J.; Van Hees, M.; Leyval, C.; Jakobsen, I. Arbuscular mycorrhizal fungi can decrease the uptake of uranium by subterranean clover grown at high levels of uranium in soil. Environ. Pollut. 2004, 130, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Roos, P.; Borggaard, O.K.; Zhu, Y.G.; Jakobsen, I. Mycorrhiza and root hairs in barley enhance acquisition of phosphorus and uranium from phosphate rock but mycorrhiza decreases root to shoot uranium transfer. New Phytol. 2005, 165, 591–598. [Google Scholar] [CrossRef]
- Christie, P.; Li, X.; Chen, B. Arbuscular mycorrhiza can depress translocation of zinc to shoots of host plants in soils moderately polluted with zinc. Plant Soil 2004, 261, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Padash, A.; Shahabivand, S.; Behtash, F.; Aghaee, A. A practicable method for zinc enrichment in lettuce leaves by the endophyte fungus Piriformospora indica under increasing zinc supply. Sci. Hortic. 2016, 213, 367–372. [Google Scholar] [CrossRef]
- Abadi, V.A.J.M.; Sepehri, M. Effect of Piriformospora indica and Azotobacter chroococcum on mitigation of zinc deficiency stress in wheat (Triticum aestivum L.). Symbiosis 2016, 69, 9–19. [Google Scholar] [CrossRef]




| AMF Root Colonization | |||
|---|---|---|---|
| Total Colonization | Arbuscules | Vesicles | |
| Treatments | 0.739 | 0.497 | 0.759 |
| Factor | Growth | Performance of Photosystem II | |||
|---|---|---|---|---|---|
| Shoot Length | Shoot Dry Weight | Fo 1 | Fm 2 | Fv/Fm 3 | |
| Serendipita | 0.214 | 0.365 | 0.137 | 0.949 | 0.191 |
| AMF | 0.079 | 0.536 | 0.809 | 0.197 | 0.025 |
| Serendipita × AMF | 0.121 | 0.063 | 0.010 4 | 0.045 | 0.467 |
| Treatments | Growth | |
|---|---|---|
| Shoot Length (cm) | Shoot DW (g) | |
| Control | 23.09 ± 1.32 | 1.16 ± 0.25 |
| S. indica | 24.48 ± 1.86 | 1.20 ± 0.15 |
| S. williamsii | 22.81 ± 2.38 | 1.22 ± 0.22 |
| AMF | 22.91 ± 1.79 | 1.21 ± 0.11 |
| S. indica + AMF | 22.69 ± 1.69 | 1.23 ±0.13 |
| S. williamsii + AMF | 22.76 ± 1.61 | 1.07 ± 0.15 |
| Factor | Macronutrients | ||||||
|---|---|---|---|---|---|---|---|
| P | Ca | Mg | K | C | N | CN | |
| Serendipita | 0.281 | 0.094 | 0.227 | 0.511 | 0.841 | 0.461 | 0.595 |
| AMF | 0.002 1 | 0.001 | 0.144 | 0.782 | 0.475 | 0.985 | 0.894 |
| Serendipita × AMF | 0.085 | 0.012 | 0.136 | 0.061 | 0.014 | 0.001 | 0.000 |
| Treatments | Micronutrients | ||
|---|---|---|---|
| Mn | Fe | Zn | |
| Serendipita | 0.554 | 0.612 | 0.306 |
| AMF | 0.000 1 | 0.000 | 0.720 |
| Serendipita × AMF | 0.086 | 0.345 | 0.176 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hallasgo, A.M.; Spangl, B.; Steinkellner, S.; Hage-Ahmed, K. The Fungal Endophyte Serendipita williamsii Does Not Affect Phosphorus Status But Carbon and Nitrogen Dynamics in Arbuscular Mycorrhizal Tomato Plants. J. Fungi 2020, 6, 233. https://doi.org/10.3390/jof6040233
Hallasgo AM, Spangl B, Steinkellner S, Hage-Ahmed K. The Fungal Endophyte Serendipita williamsii Does Not Affect Phosphorus Status But Carbon and Nitrogen Dynamics in Arbuscular Mycorrhizal Tomato Plants. Journal of Fungi. 2020; 6(4):233. https://doi.org/10.3390/jof6040233
Chicago/Turabian StyleHallasgo, Anna M., Bernhard Spangl, Siegrid Steinkellner, and Karin Hage-Ahmed. 2020. "The Fungal Endophyte Serendipita williamsii Does Not Affect Phosphorus Status But Carbon and Nitrogen Dynamics in Arbuscular Mycorrhizal Tomato Plants" Journal of Fungi 6, no. 4: 233. https://doi.org/10.3390/jof6040233
APA StyleHallasgo, A. M., Spangl, B., Steinkellner, S., & Hage-Ahmed, K. (2020). The Fungal Endophyte Serendipita williamsii Does Not Affect Phosphorus Status But Carbon and Nitrogen Dynamics in Arbuscular Mycorrhizal Tomato Plants. Journal of Fungi, 6(4), 233. https://doi.org/10.3390/jof6040233







