Transcriptional Analysis Revealing the Improvement of ε-Poly-L-lysine Production from Intracellular ROS Elevation after Botrytis cinerea Induction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Preparation of Sample for Transcriptome Analysis
2.3. Transcriptional Analysis of Important Genes Related to ε-PL Biosynthesis
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results
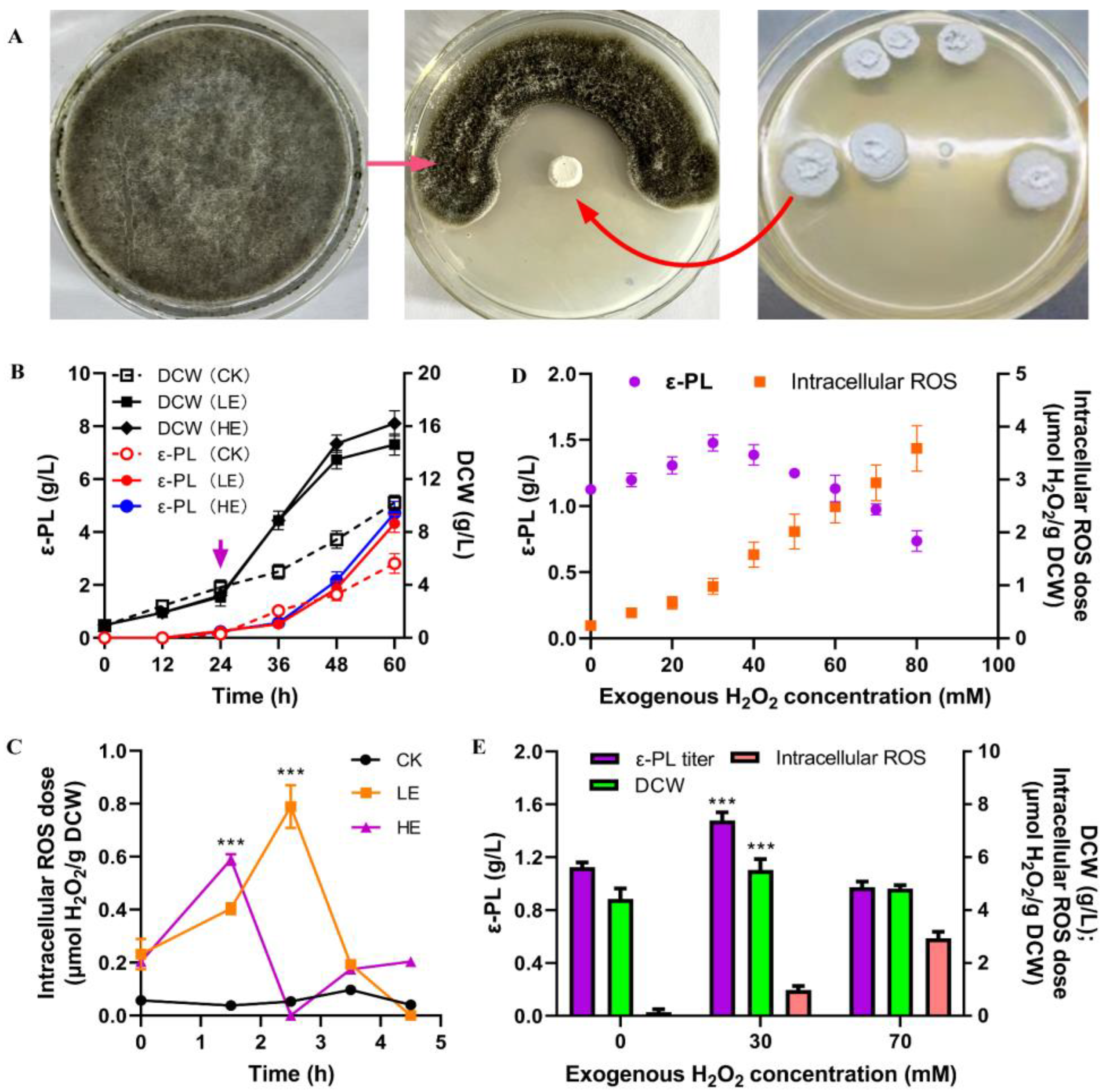
3.1. Interaction between S. albulus IFO 14147 and B. cinerea
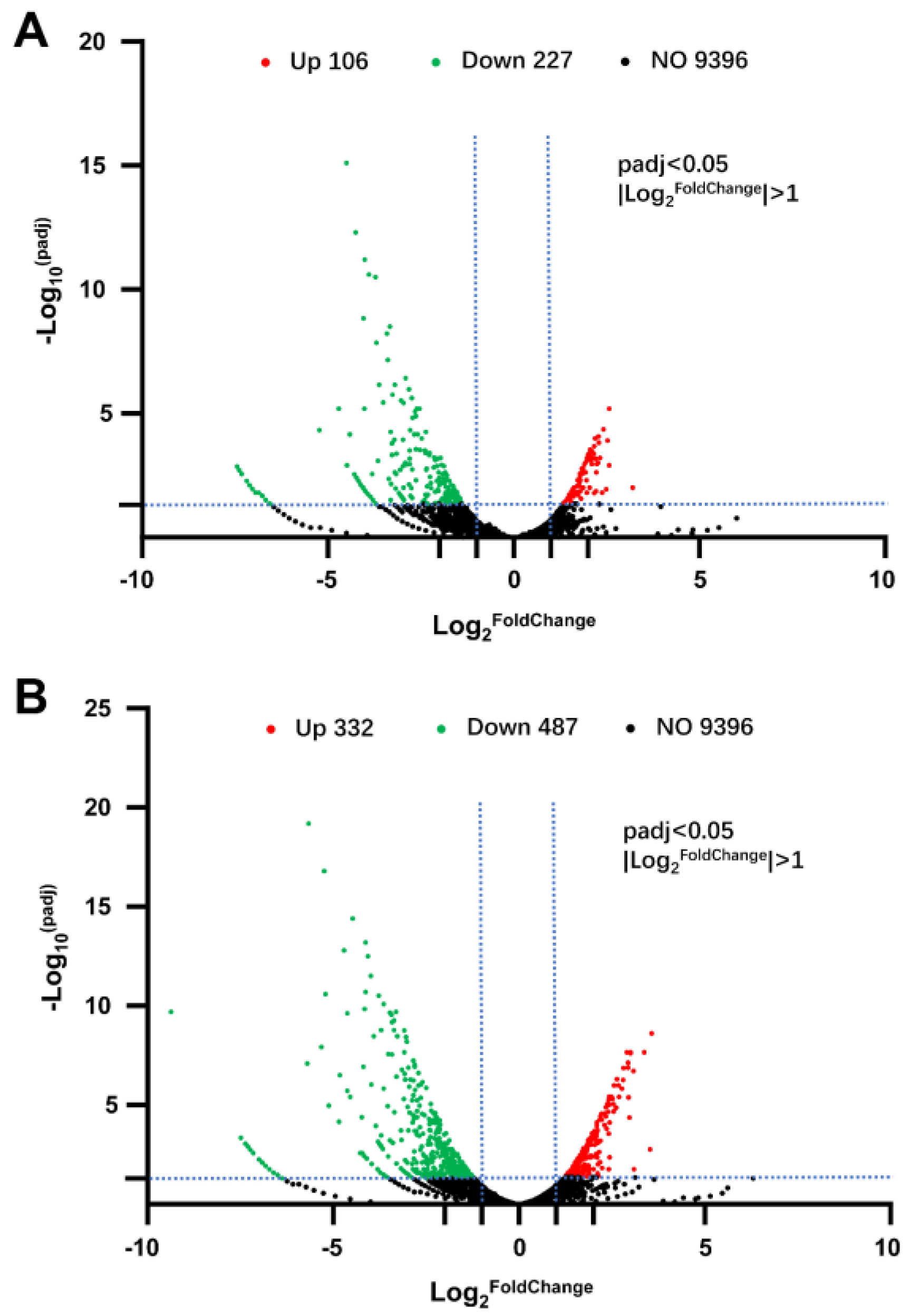
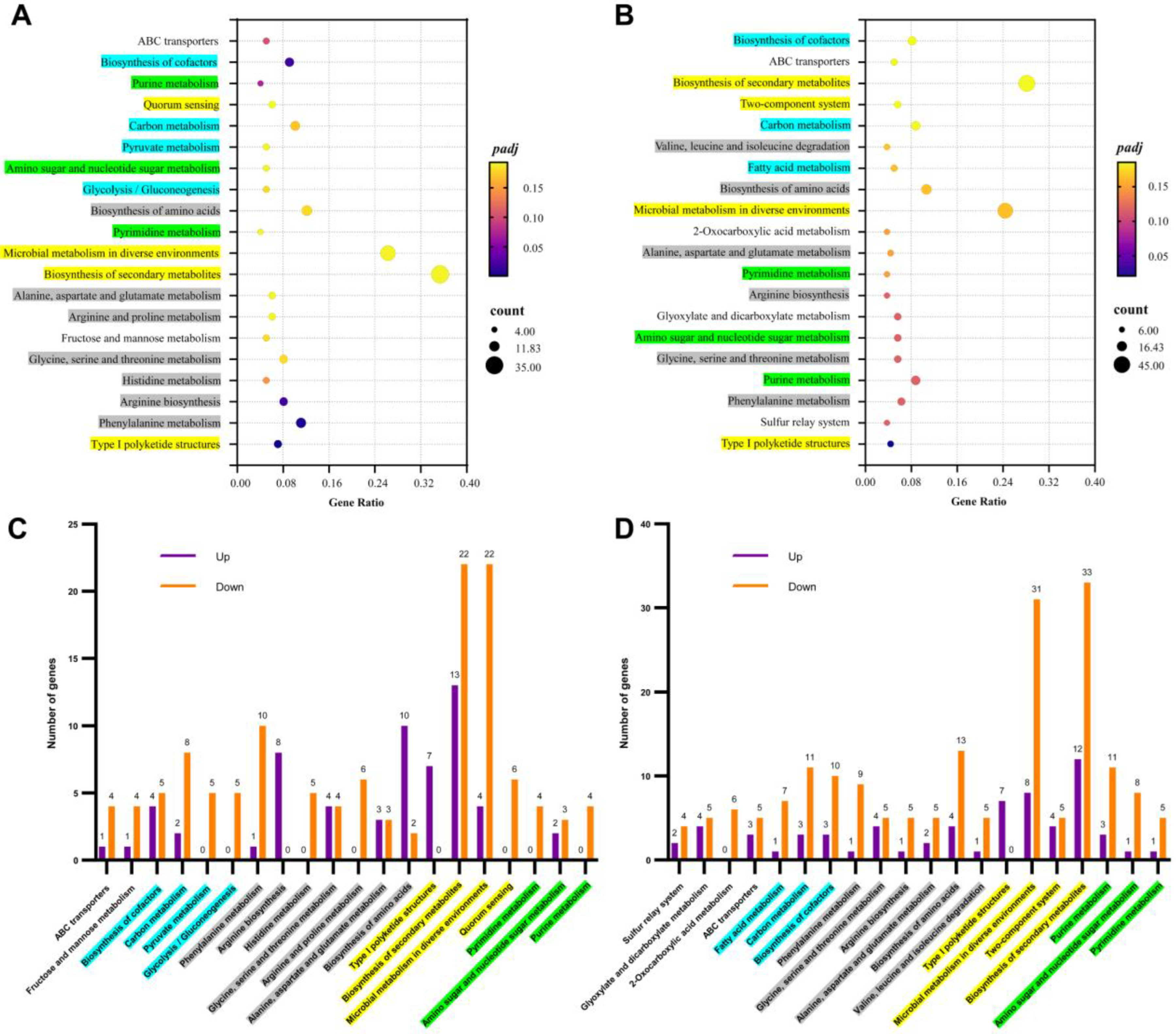
3.2. Transcriptome Analysis between Groups of LOS vs. CK and HOS vs. CK
3.3. Transcriptional Influence of Elevated Intracellular ROS on Key Genes in ε-PL Synthesis Metabolism
3.4. Transcriptional Influence of Elevated Intracellular ROS on Cell Respiration and Energy Production
3.5. Transcriptional Influence of Elevated Intracellular ROS on Genes in Transcriptional Regulators and Secondary Metabolite Biosynthesis
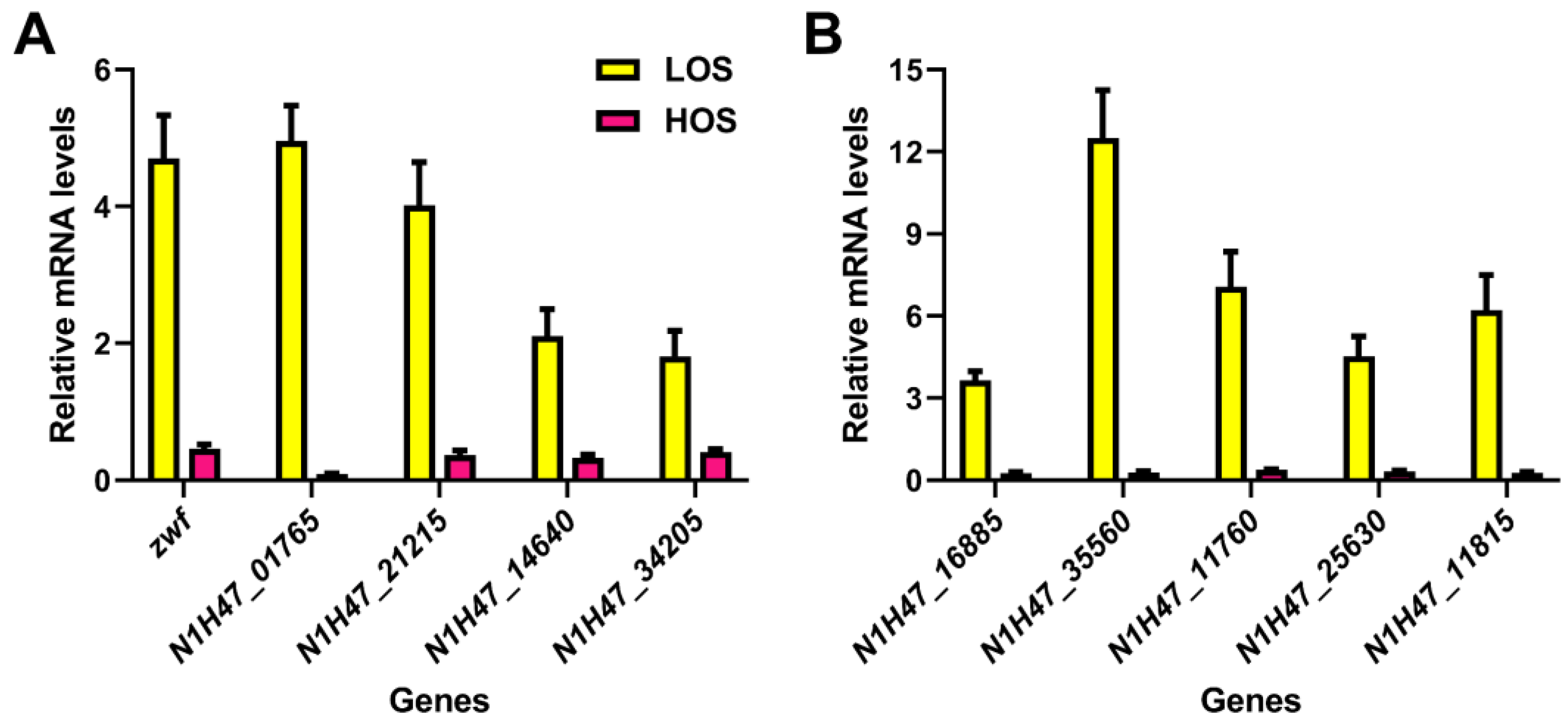
3.6. Transcriptional Verification of Key Genes in ε-PL Biosynthesis by qRT-PCR
4. Discussion
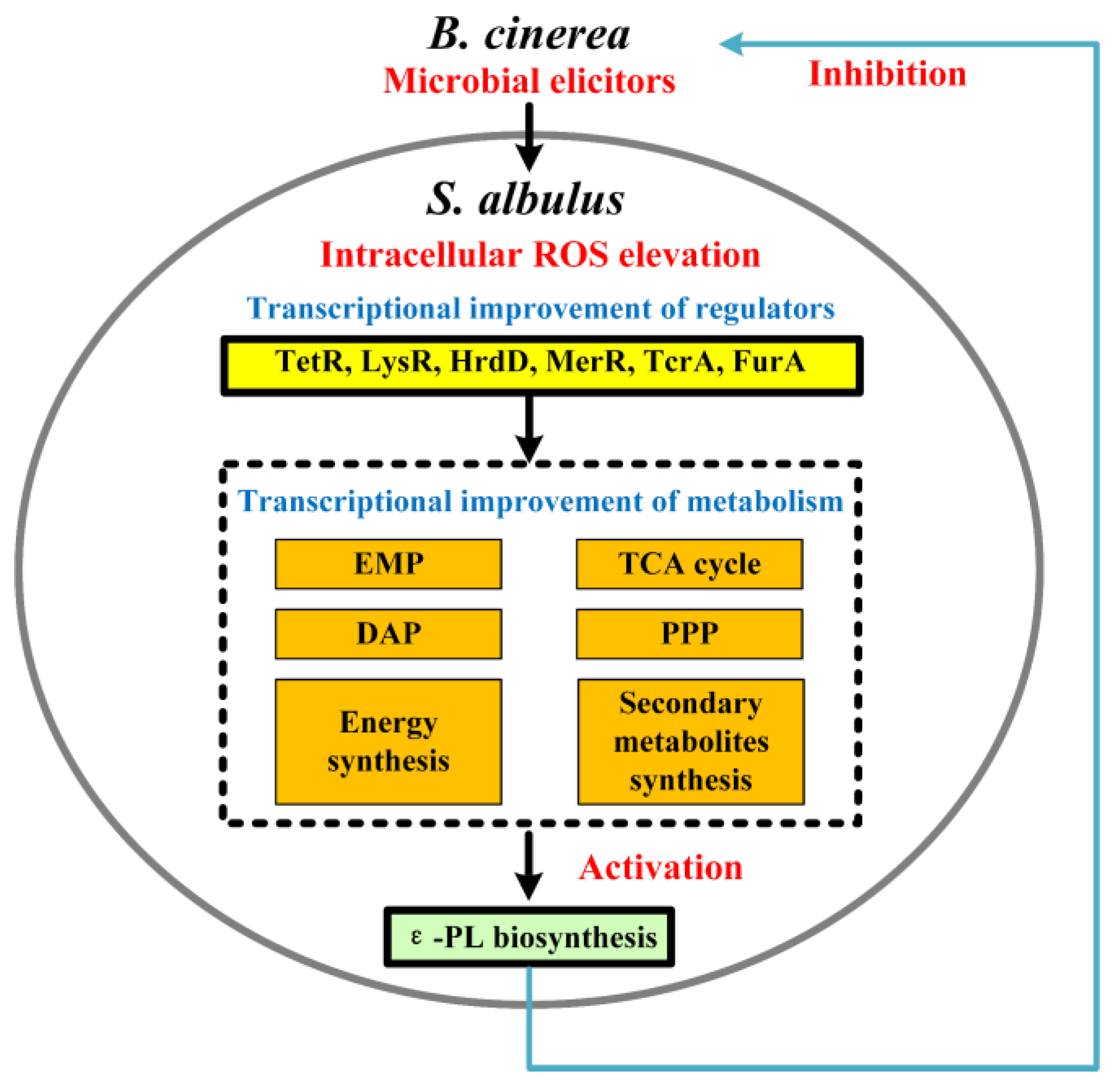
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Pollastro, S.; Faretra, F.; Di Canio, V.; De Guido, A. Characterization and genetic analysis of field isolates of Botryotinia fuckeliana (Botrytis cinerea) resistant to dichlofluanid. Eur. J. Plant Pathol. 1996, 102, 607–613. [Google Scholar] [CrossRef]
- Staats, M.; van Kan, J.A. Genome update of Botrytis cinerea strains B05.10 and T4. Eukaryot. Cell 2012, 11, 1413–1414. [Google Scholar] [CrossRef] [PubMed]
- Leroch, M.; Kleber, A.; Silva, E.; Coenen, T.; Koppenhöfer, D.; Shmaryahu, A.; Valenzuela, P.D.; Hahn, M. Transcriptome profiling of Botrytis cinerea conidial germination reveals upregulation of infection-related genes during the prepenetration stage. Eukaryot. Cell 2013, 12, 614–626. [Google Scholar] [CrossRef]
- Staples, R.C.; Mayer, A.M. Putative virulence factors of Botrytis cinerea acting as a wound pathogen. FEMS Microbiol. Lett. 1995, 134, 1–7. [Google Scholar] [CrossRef]
- Li, B.; Wang, W.; Zong, Y.; Qin, G.; Tian, S. Exploring pathogenic mechanisms of Botrytis cinerea secretome under different ambient pH based on comparative proteomic analysis. J. Proteome Res. 2012, 11, 4249–4260. [Google Scholar] [CrossRef] [PubMed]
- Wubben, J.P.; ten Have, A.; van Kan, J.A.; Visser, J. Regulation of endopolygalacturonase gene expression in Botrytis cinerea by galacturonic acid, ambient pH and carbon catabolite repression. Curr. Genet. 2000, 37, 152–157. [Google Scholar] [CrossRef] [PubMed]
- ten Have, A.; Breuil, W.O.; Wubben, J.P.; Visser, J.; van Kan, J.A. Botrytis cinerea endopolygalacturonase genes are differentially expressed in various plant tissues. Fungal Genet. Biol. 2001, 33, 97–105. [Google Scholar] [CrossRef]
- ten Have, A.; Espino, J.J.; Dekkers, E.; Van Sluyter, S.C.; Brito, N.; Kay, J.; González, C.; van Kan, J.A. The Botrytis cinerea aspartic proteinase family. Fungal Genet. Biol. 2010, 47, 53–65. [Google Scholar] [CrossRef]
- Wilson, C.L.; Wisniewski, M.E.; Biles, C.L.; McLaughlin, R.; Halutz, E.; Droby, S. Biological control of post-harvest diseases of fruits and vegetables: Alternatives to synthetic fungicides. Crop. Prot. 1991, 10, 172–177. [Google Scholar] [CrossRef]
- Shi, L.; Liu, B.; Wei, Q.; Ge, B.; Zhang, K. Genome-wide transcriptomic analysis of the response of Botrytis cinerea to wuyiencin. PLoS ONE 2020, 15, e0224643. [Google Scholar] [CrossRef] [PubMed]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.L. Fungi vs. Fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef]
- O’Brien, P.A. Biological control of plant diseases. Australas. Plant Pathol. 2017, 46, 293–304. [Google Scholar] [CrossRef]
- Stevens, C.; Khan, V.A.; Lu, J.Y.; Wilson, C.L.; Pusey, P.L.; Igwegbe, E.C.K.; Droby, S. Integration of ultraviolet (UV-C) light with yeast treatment for control of postharvest storage rots of fruit and vegetables. Biol. Control. 1997, 10, 98–103. [Google Scholar] [CrossRef]
- Singh, H. Management of plant pathogens with microorganisms. Proc. Indian Natl. Sci. Acad. 2014, 80, 443–454. [Google Scholar] [CrossRef]
- Shukla, S.C.; Singh, A.; Pandey, A.K.; Mishra, A. Review on production and medical applications of ε-polylysine. Biochem. Eng. J. 2012, 65, 70–81. [Google Scholar] [CrossRef]
- Hamano, Y.; Nicchu, I.; Hoshino, Y.; Kawai, T.; Nakamori, S.; Takagi, H. Development of gene delivery systems for the epsilon-poly-L-lysine producer, Streptomyces albulus. J. Biosci. Bioeng. 2005, 99, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yao, H.; Xu, Z. Production of ε-poly-lysine by Streptomyces albulus PD-1 via solid-state fermentation. Bioresour. Technol. 2017, 223, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Nagasawa, T. Epsilon-Poly-L-lysine: Microbial production, biodegradation and application potential. Appl. Microbiol. Biotechnol. 2003, 62, 21–26. [Google Scholar] [CrossRef]
- Chang, Y.; Mclandsborough, L.; Mcclements, D.J. Physicochemical properties and antimicrobial efficacy of electrostatic complexes based on cationic ε-Polylysine and anionic pectin. J. Agric. Food Chem. 2011, 59, 6776–6782. [Google Scholar] [CrossRef]
- Liu, K.; Zhou, X.; Fu, M. Inhibiting effects of epsilon-poly-lysine (ε-PL) on Pencillium digitatum and its involved mechanism. Postharvest Biol. Technol. 2017, 123, 94–101. [Google Scholar] [CrossRef]
- Wei, M.; Ge, Y.; Li, C. Antifungal activity of ε-poly-L-lysine on Trichothecium roseum in vitro and its mechanisms. Physiol. Mol. Plant Pathol. 2018, 103, 23–27. [Google Scholar] [CrossRef]
- Liu, H.; Chen, J.; Xia, Z. Effects of ε-poly-l-lysine on vegetative growth, pathogenicity and gene expression of Alternaria alternate infecting Nicotiana tabacum. Pestic. Biochem. Physiol. 2020, 163, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Liu, X.; Chen, Q. Epsilon-poly-L-lysine (ε-PL) exhibits antifungal activity in vivo and in vitro against Botrytis cinerea and mechanism involved. Postharvest Biol. Technol. 2020, 168, 111270. [Google Scholar] [CrossRef]
- Sun, G.; Yang, Q.; Zhang, A. Synergistic effect of the combined bio-fungicides ε-poly-l-lysine and chitooligosaccharide in controlling grey mould (Botrytis cinerea) in tomatoes. Int. J. Food Microbiol. 2018, 276, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Lu, Y.; Li, B.; Shi, L.; Zhang, K.; Ge, B. Effects of ε-Poly-L-Lysine Combined with Wuyiencin as a Bio-Fungicide against Botryris cinerea. Microorganisms 2022, 10, 971. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhang, C.; Yue, C.; Su, Z.; Tai, B.; Tang, H.; Zeng, H.; Xin, B.; Zhu, M. Transcriptome and metabolome analysis revealing the improved epsilon-poly-L-lysine production induced by a microbial call from Botrytis cinerea. Appl. Environ. Microbiol. 2022, 88, e00952-22. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Miao, W.; Wen, B.; Mao, Z.; Zhu, M.; Chen, X. Transcriptional study of the enhanced ε-poly-L-lysine productivity in culture using glucose and glycerol as a mixed carbon source. Bioproc. Biosyst. Eng. 2019, 42, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.S.; Ren, X.D.; Zeng, X.; Zhao, F.L.; Tang, L.; Zhang, H.J.; Zhang, J.H.; Mao, Z.G. Enhancement of ε-poly-L-lysine production coupled with precursor L-lysine feeding in glucose-glycerol co-fermentation by Streptomyces sp. M-Z18. Bioproc. Biosyst. Eng. 2013, 36, 1843–1849. [Google Scholar] [CrossRef]
- Rao, Y.M.; Sureshkumar, G.K. Improvement in bioreactor productivities using free radicals: HOCl-induced overproduction of xanthan gum from Xanthomonas campestris and its mechanism. Biotechnol. Bioeng. 2001, 72, 62–68. [Google Scholar] [CrossRef]
- Wei, Z.H.; Bai, L.; Deng, Z.; Zhong, J.J. Enhanced production of validamycin A by H2O2-induced reactive oxygen species in fermentation of Streptomyces hygroscopicus 5008. Bioresour. Technol. 2011, 102, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.H.; Bai, L.; Deng, Z.; Zhong, J.J. Temperature shift-induced reactive oxygen species enhanced validamycin A production in fermentation of Streptomyces hygroscopicus 5008. Bioproc. Biosyst. Eng. 2012, 35, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Beites, T.; Pires, S.D.S.; Santos, C.L.; Osorio, H.; Moradas-Ferreira, P.; Mendes, M.V. Crosstalk between ROS homeostasis and secondary metabolism in S. natalensis ATCC 27448: Modulation of pimaricin production by intracellular ROS. PLoS ONE 2011, 6, e27472. [Google Scholar] [CrossRef] [PubMed]
- Beites, T.; Rodríguez-García, A.; Moradas-Ferreira, P.; Aparicio, J.F.; Mendes, M.V. Genome-wide analysis of the regulation of pimaricin production in Streptomyces natalensis by reactive oxygen species. Appl. Microbiol. Biot. 2014, 98, 2231–2241. [Google Scholar] [CrossRef] [PubMed]
- Roukas, T. The role of oxidative stress on carotene production by Blakeslea trispora in submerged fermentation. Crit. Rev. Biotechnol. 2015, 36, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wei, L.; Zhang, Y.; Zhang, M.; Gu, S. Physicochemical and microbial responses of Streptomyces natalensis HW-2 to fungal elicitor. Appl. Microbiol. Biot. 2017, 101, 6705–6712. [Google Scholar] [CrossRef]
- Song, Z.Q.; Ma, Z.; Bechthold, A.; Yu, X.P. Effects of addition of elicitors on rimocidin biosynthesis in Streptomyces rimosus M527. Appl. Microbiol. Biot. 2020, 104, 4445–4455. [Google Scholar] [CrossRef]


| ε-PL Concentration (μg/mL) a | ||||||
|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | 800 | 1000 | |
| Mycelial diameter (cm) | 7.88 ± 0.72 | 6.45 ± 0.51 | 3.57 ± 0.33 | 1.89 ± 0.23 | 0.54 ± 0.08 | 0.17 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhang, Z.; Cheng, Y.; Ni, N.; Tong, S.; Da, W.; Liu, C.; Diao, Q.; Chen, Z.; Xin, B.; et al. Transcriptional Analysis Revealing the Improvement of ε-Poly-L-lysine Production from Intracellular ROS Elevation after Botrytis cinerea Induction. J. Fungi 2024, 10, 324. https://doi.org/10.3390/jof10050324
Zhang C, Zhang Z, Cheng Y, Ni N, Tong S, Da W, Liu C, Diao Q, Chen Z, Xin B, et al. Transcriptional Analysis Revealing the Improvement of ε-Poly-L-lysine Production from Intracellular ROS Elevation after Botrytis cinerea Induction. Journal of Fungi. 2024; 10(5):324. https://doi.org/10.3390/jof10050324
Chicago/Turabian StyleZhang, Chen, Zhanyang Zhang, Ya Cheng, Ni Ni, Siyu Tong, Wangbao Da, Chunyan Liu, Qiran Diao, Ziyan Chen, Bingyue Xin, and et al. 2024. "Transcriptional Analysis Revealing the Improvement of ε-Poly-L-lysine Production from Intracellular ROS Elevation after Botrytis cinerea Induction" Journal of Fungi 10, no. 5: 324. https://doi.org/10.3390/jof10050324






