Biological Activities of Secondary Metabolites from the Edible-Medicinal Macrofungi
Abstract
:1. Introduction
2. Biological Activities of Secondary Metabolites
2.1. Anti-Tumor Activity
2.2. Antioxidant Activity
2.3. Anti-Inflammatory Activity
2.4. Antimicrobial Activity
2.5. Antimalarial Activity
2.6. Neuro-Protective Activity
2.7. Hypoglycemic Activity
2.8. Hypolipidemic Activity
2.9. Other Bioactivities
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Himanshi, R.; Shalinee, P.; Satyawati, S. Mushroom nutraceuticals for improved nutrition and better human health: A review. PharmaNutrition 2017, 5, 35–46. [Google Scholar]
- Sergey, G.; Ramin, R.; Josef, D.; Aristidis, T. Poisoning associated with the use of mushrooms: A review of the global pattern and main characteristics. Food Chem. Toxicol. 2019, 128, 267–279. [Google Scholar]
- El-Maradny, Y.A.; El-Fakharany, E.M.; Abu-Serie, M.M.; Hashish, M.H.; Selim, H.S. Lectins purified from medicinal and edible mushrooms: Insights into their antiviral activity against pathogenic viruses. Int. J. Biol. Macromol. 2021, 179, 239–258. [Google Scholar] [CrossRef]
- Chen, Y.J.; Jiang, S.; Jin, Y.X.; Yin, Y.L.; Yu, G.J.; Lan, X.Q.; Cui, M.Y.; Liang, Y.; Wong, B.H.C.; Guo, L.; et al. Purification and characterization of an antitumor protein with deoxyribonuclease activity from edible mushroom Agrocybe aegerita. Mol. Nutr. Food Res. 2012, 56, 1729–1738. [Google Scholar] [CrossRef]
- Citores, L.; Ragucci, S.; Ferreras, J.M.; Di Maro, A.; Iglesias, R. Ageritin, a Ribotoxin from poplar mushroom (Agrocybe aegerita) with defensive and antiproliferative activities. ACS Chem. Biol. 2019, 14, 1319–1327. [Google Scholar] [CrossRef]
- Kalaras, M.D.; Richie, J.P.; Calcagnotto, A.; Beelman, R.B. Mushrooms: A rich source of the antioxidants ergothioneine and glutathione. Food Chem. 2017, 233, 429–433. [Google Scholar] [CrossRef]
- Tong, Z.J.; Chu, G.D.; Wan, C.M.; Wang, Q.Y.; Yang, J.L.; Meng, Z.L.; Du, L.N.; Yang, J.; Ma, H.X. Multiple metabolites derived from mushrooms and their beneficial effect on Alzheimer’s diseases. Nutrients 2023, 15, 2758. [Google Scholar] [CrossRef]
- Martel, J.; Ojcius, D.M.; Chang, C.J.; Lin, C.S.; Lu, C.C.; Ko, Y.F.; Tseng, S.F.; Lai, H.C.; Young, J.D. Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nat. Rev. Endocrinol. 2016, 13, 149–160. [Google Scholar] [CrossRef]
- Qi, J.S.; Duan, Y.; Li, Z.C.; Gao, J.M.; Qi, J.; Liu, C. The alkynyl-containing compounds from mushrooms and their biological activities. Nat. Prod. Bioprospect. 2023, 13, 50. [Google Scholar] [CrossRef]
- Wang, Y.X.; Wang, H.J.; Chai, K.Y.; Guo, S.Y.; Zhai, Y.Y.; Shi, R.; Zhang, F.Q.; Huang, J.Q.; Jin, Z.S.; Gao, Y.F.; et al. Systematic review and meta-analysis on the efficacy and safety of injectable lentinan combined with chemotherapy in the treatment of gastric cancer. Phytomedicine 2024, 123, 155242. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Wang, H.Z.; Zhang, J.C.; Guan, Y.; Zhang, Y.J. Single-injection subunit vaccine for rabies prevention using lentinan as adjuvant. Int. J. Biol. Macromol. 2024, 254, 128118. [Google Scholar] [CrossRef]
- Meenu, M.; Xu, B. Application of vibrational spectroscopy for classification, authentication and quality analysis of mushroom: A concise review. Food Chem. 2019, 289, 545–557. [Google Scholar] [CrossRef]
- Lee, S.R.; Roh, H.S.; Lee, S.; Park, H.B.; Jang, T.S.; Ko, Y.J.; Baek, K.H.; Kim, K.H. Bioactivity-guided isolation and chemical characterization of antiproliferative constituents from morel mushroom (Morchella esculenta) in human lung adenocarcinoma cells. J. Funct. Foods 2018, 40, 249–260. [Google Scholar] [CrossRef]
- Satria, D.; Amen, Y.; Niwa, Y.; Ashour, A.; Allam, A.E.; Shimizu, K. Lucidumol D, a new lanostane-type triterpene from fruiting bodies of reishi (Ganoderma lingzhi). Nat. Prod. Rep. 2018, 33, 189–195. [Google Scholar] [CrossRef]
- Chen, H.P.; Zhao, Z.Z.; Li, Z.H.; Huang, Y.; Zhang, S.B.; Tang, Y.; Yao, J.N.; Chen, L.; Isaka, M.; Feng, T.; et al. Anti-Proliferative and anti-inflammatory lanostane triterpenoids from the polish edible mushroom Macrolepiota procera. J. Agric. Food Chem. 2018, 66, 3146–3154. [Google Scholar] [CrossRef] [PubMed]
- Deveci, E.; Tel-Çayan, G.; Duru, M.E.; Öztürk, M. Isolation, characterization, and bioactivities of compounds from Fuscoporia torulosa mushroom. J. Food Biochem. 2019, 43, e13074. [Google Scholar] [CrossRef]
- Wu, P.F.; Ding, R.; Tan, R.; Liu, J.; Hu, E.M.; Li, C.Y.; Liang, G.Y.; Yi, P. Sesquiterpenes from cultures of the fungus Phellinus igniarius and their cytotoxicities. Fitoterapia 2020, 140, 104415. [Google Scholar] [CrossRef]
- Chen, B.S.; Zhang, J.J.; Han, J.J.; Zhao, R.L.; Bao, L.; Huang, Y.; Liu, H.W. Lanostane triterpenoids with glucose-uptake-stimulatory activity from peels of the cultivated edible mushroom Wolfiporia cocos. J. Agric. Food Chem. 2019, 67, 7348–7364. [Google Scholar] [CrossRef]
- Zhao, Z.Z.; Liang, X.B.; Feng, W.S.; Wu, Y.; Zhi, Y.L.; Xue, G.M.; Chen, H.P.; Liu, J.K. Unusual constituents from the medicinal mushroom Ganoderma lingzhi. RSC Adv. 2019, 9, 36931–36939. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.; Ryoo, R.; Kim, J.C.; Park, H.B.; Kang, K.S.; Kim, K.H. Calvatianone, a sterol possessing a 6/5/6/5-fused ring system with a contracted tetrahydrofuran B-ring, from the fruiting bodies of Calvatia nipponica. J. Nat. Prod. 2020, 83, 2737–2742. [Google Scholar] [CrossRef]
- Su, L.H.; Geng, C.A.; Li, T.Z.; Huang, X.Y.; Ma, Y.B.; Zhang, X.M.; Wu, G.; Yang, Z.L.; Chen, J.J. Spiroseoflosterol, a rearranged ergostane-steroid from the fruiting bodies of Butyriboletus roseoflavus. J. Nat. Prod. 2020, 83, 1706–1710. [Google Scholar] [CrossRef]
- Li, X.C.; Liu, F.; Su, H.G.; Peng, C.; Zhou, Q.M.; Liu, J.; Huang, Y.J.; Guo, L.; Xiong, L. Twelve undescribed derivatives of ganoderic acid isolated from Ganoderma luteomarginatum and their cytotoxicity against three human cancer cell lines. Phytochemistry 2021, 183, 112617. [Google Scholar] [CrossRef]
- Menaga, D.; Rahman, P.K.S.M.; Rajakumar, S.; Ayyasamy, P.M. Antioxidant and cytotoxic activities of a novel isomeric molecule (PF5) obtained from methanolic extract of Pleurotus Florida mushroom. J. Biosci. Bioeng. 2021, 6, 338–349. [Google Scholar] [CrossRef]
- Hassan, K.; Matio, K.B.; Stadler, M. Two new triterpenes from basidiomata of the medicinal and edible mushroom, Laetiporus sulphureus. Molecules 2021, 26, 7090. [Google Scholar] [CrossRef]
- Ullah, Z.; Öztürk, M.; Ertaş, A.; Wahab, A.; Ben, M.R.; Iqbal Choudhary, M. Insight into isolation and elucidation of cytotoxic ergostanoids from the mushroom Sarcosphaera crassa (Santi) Pouzar: An edible mushroom. Steroids 2022, 181, 108990. [Google Scholar] [CrossRef] [PubMed]
- Gilardoni, G.; Negri, F.; Vita, F.P.; Hussain, F.H.S.; Vidari, G. New tricholidic acid triterpenoids from the mushroom Tricholoma ustaloides collected in an Italian beech wood. Molecules 2023, 28, 3864. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Z.; Zhang, F.; Ji, B.Y.; Zhou, N.; Chen, H.; Sun, Y.J.; Feng, W.S.; Zheng, X.K. Pyrrole alkaloids from the fruiting bodies of edible mushroom Lentinula edodes. RSC Adv. 2023, 13, 18223–18228. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Z.; Ji, B.Y.; Wang, Z.Z.; Si, Y.Y.; Sun, Y.J.; Chen, H.; Feng, W.S.; Zheng, X.K.; Liu, J.K. Lanostane triterpenoids with anti-proliferative and anti-inflammatory activities from medicinal mushroom Ganoderma lingzhi. Phytochemistry 2023, 213, 113791. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, X.; Lian, C.; Ke, J.; Liu, J. Triterpenes and aromatic meroterpenoids with antioxidant activity and neuroprotective effects from Ganoderma lucidum. Molecules 2019, 24, 4353. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhou, X.; Meng, Q.; Wang, M.; Zhang, Y.; Fu, S. Secondary metabolites and antiradical activity of liquid fermentation of Morchella sp. isolated from southwest China. Molecules 2019, 24, 1706. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, C.; Meng, Q.; Cui, Y.; Wang, Y.; Chen, X.; Fu, S. Investigation of chemical compounds and DPPH radical scavenging activity of Oudemansiella raphanipes (Agaricomycetes) based on fermentation. Int. J. Med. Mushrooms 2020, 22, 299–304. [Google Scholar] [CrossRef]
- Zhang, J.J.; Chen, B.S.; Dai, H.Q.; Ren, J.W.; Zhou, L.W.; Wu, S.H.; Liu, H.W. Sesquiterpenes and polyphenols with glucose-uptake stimulatory and antioxidant activities from the medicinal mushroom Sanghuangporus sanghuang. Chin. J. Nat. Med. 2021, 19, 693–699. [Google Scholar] [CrossRef]
- Lv, J.H.; Yao, L.; Duan, C.; Li, Z.; Zhang, J.; Li, C.T.; Li, Y. New bioactive α-pyrone from wild mushroom Paxillus involutus. Nat. Prod. Rep. 2021, 36, 2707–2712. [Google Scholar] [CrossRef]
- Lv, J.H.; Yao, L.; Zhang, J.X.; Wang, L.A.; Zhang, J.; Wang, Y.P.; Xiao, S.Y.; Li, C.T.; Li, Y. Novel 2,5-Diarylcyclopentenone derivatives from the wild edible mushroom Paxillus involutus and their antioxidant activities. J. Agric. Food Chem. 2021, 69, 5040–5048. [Google Scholar] [CrossRef]
- Ge, X.X.; Wang, Y.; Sun, C.X.; Zhang, Z.P.; Song, L.; Tan, L.L.; Li, D.H.; Yang, S.; Yu, G.H. Secondary metabolites produced by coculture of Pleurotus ostreatus SY10 and Pleurotus eryngii SY302. Chem. Biodivers. 2022, 19, e202100832. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.F.; Tan, J.; Jiang, R.X.; Li, F.F.; Zheng, R.Q.; Yu, L.J.; Luo, L.Z.; Zheng, Y.B. DPPH radical scavenging activity of new phenolics from the fermentation broth of mushroom Morehella importuna. Molecules 2023, 28, 4760. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.T.; Li, Y.C.; He, Y.D.; Meng, Q.F.; Ju, J.H.; Fu, S.B. Chemical constituents from mycelia of Lepista sordida (Agaricomycetes) and their ABTS radical scavenging activity. Int. J. Med. Mushrooms 2023, 25, 31–39. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.; Lee, S.O.; Ryu, J.Y.; Choi, S.Z.; Kang, K.S.; Kim, K.H. Anti-inflammatory activity of the sclerotia of edible fungus, Poria Cocos wolf and their active ianostane triterpenoids. J. Funct. Foods 2017, 32, 27–36. [Google Scholar] [CrossRef]
- Wu, Y.L.; Han, F.; Luan, S.S.; Ai, R.; Zhang, P.; Li, H.; Chen, L.X. Triterpenoids from Ganoderma lucidum and their potential anti-inflammatory effects. J. Agric. Food Chem. 2019, 67, 5147–5158. [Google Scholar] [CrossRef]
- Zhang, J.J.; Chen, B.S.; Liang, J.; Han, J.J.; Zhou, L.W.; Zhao, R.L.; Liu, H.W.; Dai, H.Q. Lanostane triterpenoids with PTP1B inhibitory and glucose-uptake stimulatory activities from mushroom Fomitopsis pinicola collected in north America. J. Agric. Food Chem. 2020, 68, 10036–10049. [Google Scholar] [CrossRef]
- Yin, X.; Tuong, T.M.L.; Tian, J.M.; Pescitelli, G.; Gao, J.M. Ganorbifates A and B from Ganoderma orbiforme, determined by DFT calculations of NMR data and ECD spectra. Chem. Commun. 2020, 56, 10195–10198. [Google Scholar] [CrossRef] [PubMed]
- Kou, R.W.; Han, R.; Gao, Y.Q.; Li, D.; Yin, X.; Gao, J.M. Anti-neuroinflammatory polyoxygenated lanostanoids from Chaga mushroom Inonotus obliquus. Phytochemistry 2021, 184, 112647. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.B.; Tang, L.; Xie, Y.; Xie, L.Y. Secondary metabolites from Hericium erinaceus and their anti-inflammatory activities. Molecules 2022, 27, 2157. [Google Scholar] [CrossRef] [PubMed]
- Khalilov, Q.; Numonov, S.; Sukhrobov, P.; Bobakulov, K.; Sharopov, F.; Habasi, M.; Zhao, J.; Yuan, T.; Aisa, H.A. New triterpenoids from the fruiting bodies of Laetiporus sulphureus and their anti-inflammatory activity. ACS Omega 2022, 7, 27272–27277. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Wang, C.; Cui, B.; Zhang, J.; Zhou, S.; Pan, X.; Pan, F.; Dai, Y.; Feng, N. Lanostane triterpenoids from mycelia-associated Ganoderma sinense and their anti-inflammatory activity. Phytochemistry 2023, 215, 113870. [Google Scholar] [CrossRef]
- Isaka, M.; Chinthanom, P.; Sappan, M.; Supothina, S.; Vichai, V.; Danwisetkanjana, K.; Boonpratuang, T.; Hyde, K.D.; Choeyklin, R. Antitubercular activity of mycelium-associated Ganoderma lanostanoids. J. Nat. Prod. 2017, 80, 1361–1369. [Google Scholar] [CrossRef]
- Isaka, M.; Chinthanom, P.; Thummarukcharoen, T.; Boonpratuang, T.; Choowong, W. Highly modified lanostane triterpenes from fruiting bodies of the basidiomycete Tomophagus sp. J. Nat. Prod. 2019, 82, 1165–1176. [Google Scholar] [CrossRef]
- Sandargo, B.; Thongbai, B.; Stadler, M.; Surup, F. Cysteine-derived pleurotin congeners from the nematode-trapping basidiomycete Hohenbuehelia grisea. J. Nat. Prod. 2018, 81, 286–291. [Google Scholar] [CrossRef]
- Chepkirui, C.; Cheng, T.; Matasyoh, J.; Decock, C.; Stadler, M. An unprecedented spiro [furan-2,1′-indene]-3-one derivative and other nematicidal and antimicrobial metabolites from Sanghuangporus sp. (Hymenochaetaceae, Basidiomycota) collected in Kenya. Phytochem. Lett. 2018, 25, 141–146. [Google Scholar] [CrossRef]
- Cheng, T.; Chepkirui, C.; Decock, C.; Matasyoh, J.C.; Stadler, M. Sesquiterpenes from an eastern African medicinal mushroom belonging to the genus Sanghuangporus. J. Nat. Prod. 2019, 82, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.H.; Ge, X.X.; Wang, Y.; Mo, X.H.; Yu, H.; Tan, L.L.; Yang, S. Discovery of novel terpenoids from the basidiomycete Pleurotus ostreatus through genome mining and coculture optimization. J. Agric. Food Chem. 2023, 71, 11110–11123. [Google Scholar] [CrossRef]
- Wahba, A.E.; El, S.A.; El, A.A.; Soliman, E.M. New antimalarial lanostane triterpenes from a new isolate of Egyptian Ganoderma species. Med. Chem. Res. 2019, 28, 2246–2251. [Google Scholar] [CrossRef]
- Isaka, M.; Chinthanom, P.; Vichai, V.; Sommai, S.; Choeyklin, R. Ganoweberianones A and B, antimalarial lanostane dimers from cultivated fruiting bodies of the basidiomycete Ganoderma weberianum. J. Nat. Prod. 2020, 83, 3404–3412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Cao, C.Y.; Kubo, M.; Harada, K.; Yan, X.T.; Fukuyama, Y.; Gao, J.M. Chemical constituents from Hericium erinaceus promote neuronal survival and potentiate neurite outgrowth via the TrkA/Erk1/2 pathway. Int. J. Mol. Sci. 2017, 18, 1659. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Zhang, J.J.; Ren, J.W.; Wang, W.Z.; Xiong, W.P.; Zhang, Y.D.; Bao, L.; Liu, H.W. Triterpenes and meroterpenes with neuroprotective effects from Ganoderma leucocontextum. Chem. Biodivers. 2018, 15, e1700567. [Google Scholar] [CrossRef] [PubMed]
- Rupcic, Z.; Rascher, M.; Kanaki, S.; Köster, R.; Stadler, M.; Wittstein, K. Two new cyathane diterpenoids from mycelial cultures of the medicinal mushroom Hericium erinaceus and the rare species, Hericium flagellum. Int. J. Mol. Sci. 2018, 19, 740. [Google Scholar] [CrossRef] [PubMed]
- Kou, R.W.; Du, S.T.; Xia, B.; Zhang, Q.; Yin, X.; Gao, J.M. Phenolic and steroidal metabolites from the cultivated edible Inonotus hispidus mushroom and their bioactivities. J. Agric. Food Chem. 2021, 69, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Kou, R.W.; Xia, B.; Wang, Z.J.; Li, J.N.; Yang, J.R.; Gao, Y.Q.; Yin, X.; Gao, J.M. Triterpenoids and meroterpenoids from the edible Ganoderma resinaceum and their potential anti-inflammatory, antioxidant and anti-apoptosis activities. Bioorg. Chem. 2022, 121, 105689. [Google Scholar] [CrossRef] [PubMed]
- Kou, R.W.; Xia, B.; Han, R.; Li, Z.Q.; Yang, J.R.; Yin, X.; Gao, Y.Q.; Gao, J.M. Neuroprotective effects of a new triterpenoid from edible mushroom on oxidative stress and apoptosis through the BDNF/TrkB/ERK/CREB and Nrf2 signaling pathway in vitro and in vivo. Food Funct. 2022, 13, 12121–12134. [Google Scholar] [CrossRef] [PubMed]
- Sum, W.C.; Ebada, S.S.; Kirchenwitz, M.; Kellner, H.; Ibrahim, M.A.A.; Stradal, T.E.B.; Matasyoh, J.C.; Stadler, M. Hericioic acids A–G and hericiofuranoic acid; neurotrophic agents from cultures of the european mushroom Hericium flagellum. J. Agric. Food Chem. 2023, 71, 11094–11103. [Google Scholar] [CrossRef]
- Zhang, Y.; Xun, H.; Gao, Q.; Qi, F.; Sun, J.; Tang, F. Chemical constituents of the mushroom Dictyophora indusiata and their anti-inflammatory activities. Molecules 2023, 28, 2760. [Google Scholar] [CrossRef]
- Chen, B.S.; Tian, J.; Zhang, J.J.; Wang, K.; Liu, L.; Yang, B.; Bao, L.; Liu, H.W. Triterpenes and meroterpenes from Ganoderma lucidum with inhibitory activity against HMGs reductase, aldose reductase and α-glucosidase. Fitoterapia 2017, 120, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Z.H.; Yao, J.N.; Peng, Y.L.; Huang, R.; Feng, T.; Liu, J.K. Isoindolinone-containing meroterpenoids with α-glucosidase inhibitory activity from mushroom Hericium caput-medusae. Fitoterapia 2017, 122, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Ryu, S.H.; Turk, A.; Yeon, S.W.; Jo, Y.H.; Han, Y.K.; Hwang, B.Y.; Lee, K.Y.; Lee, M.K. Characterization of α-glucosidase inhibitory constituents of the fruiting body of lion’s mane mushroom (Hericium erinaceus). J. Ethnopharmacol. 2020, 262, 113197. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.H.; Yao, L.; Li, D.; Jia, C.W.; Zhang, J.X.; Wang, L.A.; Li, C.T.; Li, Y. Novel hypoglycemic compounds from wild mushroom Paxillus involutus. Bioorg. Chem. 2021, 112, 104984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Ma, K.; Han, J.J.; Wang, K.; Chen, H.Y.; Bao, L.; Liu, L.; Xiong, W.P.; Zhang, Y.D.; Huang, Y.; et al. Eight new triterpenoids with inhibitory activity against HMG-CoA reductase from the medical mushroom Ganoderma leucocontextum collected in Tibetan plateau. Fitoterapia 2018, 130, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Meng, Q.F.; Zhang, Y.; Hu, Y.; Xiao, S.J.; Zhang, Y.Q.; Ju, J.H.; Fu, S.B. Morelsins A–F, six sesquiterpenoids from the liquid culture of Morchella importuna. Tetrahedron 2020, 76, 31–32. [Google Scholar] [CrossRef]
- Meng, Q.F.; Hu, W.T.; Wang, H.; Wu, L.H.; Yang, C.L.; Zhou, X.; Tian, Y.; Fu, S. One new compound from fruiting body of cultivated caterpillar medicinal mushroom, Cordyceps militaris (ascomycetes), and its lipase inhibitory activity. Int. J. Med. Mushrooms 2021, 23, 9–16. [Google Scholar] [CrossRef]
- Pu, D.B.; Zheng, X.; Gao, J.B.; Zhang, X.J.; Qi, Y.; Li, X.S.; Wang, Y.M.; Li, X.N.; Li, X.L.; Wan, C.P.; et al. Highly oxygenated lanostane-type triterpenoids and their bioactivity from the fruiting body of Ganoderma gibbosum. Fitoterapia 2017, 119, 1–7. [Google Scholar] [CrossRef]
- Zhang, S.B.; Huang, Y.; He, S.J.; Chen, H.P.; Wu, B.; Li, S.Y.; Zhao, Z.Z.; Li, Z.H.; Wang, X.; Zuo, J.P.; et al. Heterocyclic compounds from the mushroom Albatrellus confluens and their inhibitions against lipopolysaccharides-induced B lymphocyte cell proliferation. J. Org. Chem. 2018, 83, 10158–10165. [Google Scholar] [CrossRef]
- Shi, Q.Q.; Huang, Y.J.; Su, H.G.; Gao, Y.; Lu, S.Y.; Peng, X.R.; Li, X.N.; Zhou, L.; Qiu, M.H. Structurally diverse lanostane triterpenoids from medicinal and edible mushroom Ganoderma resinaceum boud. Bioorg. Chem. 2020, 100, 103871. [Google Scholar] [CrossRef]
- Lee, S.R.; Lee, D.; Lee, H.J.; Noh, H.J.; Jung, K.; Kang, K.S.; Kim, K.H. Renoprotective chemical constituents from an edible mushroom, Pleurotus cornucopiae in cisplatin-induced nephrotoxicity. Bioorg. Chem. 2017, 71, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, J.; Chen, Q.B.; Liu, W.; Qi, Y.R.; Aisa, H.A.; Yuan, T. Applanaic acids A–C, three new highly oxygenated lanostane triterpenoids from the fruiting bodies of Ganoderma applanatum. Nat. Prod. Res. 2020, 35, 3918–3924. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Jia, J.H.; Wang, Q.; Wei, Y.L.; Yuan, H.S. Secondary metabolites from the cultures of medicinal mushroom Vanderbylia robiniophila and their tyrosinase inhibitory activities. J. Fungi 2023, 9, 702. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Nounou, M.I.; ElAmrawy, F.; Ahmed, N.; Abdelraouf, K.; Goda, S.; Syed-Sha-Qhattal, H. Breast cancer: Conventional diagnosis and treatment modalities and recent patents and technologies. Breast Cancer Res. 2015, 9 (Suppl. S2), 17–34. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Q.; Shao, H.L.; Gao, L.; Li, H.; Sheng, H.G.; Zhu, L.Q. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: A review. Drug Deliv. 2022, 29, 2130–2161. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Maestre, A.B.; Hernández, R.J.; Arnao, M.B. ABTS/TAC methodology: Main milestones and recent applications. Processes 2023, 11, 185. [Google Scholar] [CrossRef]
- Hou, C.Y.; Chen, L.L.; Yang, L.Z.; Ji, X.L. An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 2020, 153, 248–255. [Google Scholar] [CrossRef]
- Alessandri, A.L.; Sousa, L.P.; Lucas, C.D.; Rossi, A.G.; Pinho, V.; Teixeira, M.M. Resolution of inflammation: Mechanisms and opportunity for drug development. Chin. J. Nat. Med. 2013, 139, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, T.W.; Wallig, M.A.; Dobrucki, I.T.; Dobrucki, L.W.; Nelson, E.R.; Swanson, K.S.; Smith, A.M. Efficient targeting of adipose tissue macrophages in obesity with polysaccharide nanocarriers. ACS Nano 2016, 10, 6952–6962. [Google Scholar] [CrossRef] [PubMed]
- Suleyman, G.; Alangaden, G.J. Nosocomial fungal infections: Epidemiology, infection control, and prevention. Infect. Dis. Clin. N. Am. 2021, 35, 1027–1053. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Pang, X.; Zhang, H.; Ji, P. The cGAS-STING pathway in bacterial infection and bacterial immunity. Front. Immunol. 2021, 12, 814709. [Google Scholar] [CrossRef]
- McEwen, S.A.; Collignon, P.J. Antimicrobial resistance: A one health perspective. Microbiol. Spectr. 2018, 6, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Efferth, T. Cameroonian medicinal plants: Pharmacology and derived natural products. Front. Pharmacol. 2010, 1, 123. [Google Scholar] [CrossRef]
- Alaithan, H.; Kumar, N.; Islam, M.Z.; Liappis, A.P.; Nava, V.E. Novel therapeutics for malaria. Pharmaceutics 2023, 15, 1800. [Google Scholar] [CrossRef]
- Racca, F.; Vianello, A.; Mongini, T.; Ruggeri, P.; Versaci, A.; Vita, G.L.; Vita, G. Practical approach to respiratory emergencies in neurological diseases. Neurol. Sci. 2020, 41, 497–508. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Zhou, J.W. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef]
- Kshirsagar, R.P.; Kulkarni, A.A.; Chouthe, R.S.; Pathan, S.K.; Une, H.D.; Reddy, G.B.; Diwan, P.V.; Ansari, S.A.; Sangshetti, J.N. SGLT inhibitors as antidiabetic agents: A comprehensive review. RSC Adv. 2020, 10, 1733–1756. [Google Scholar] [CrossRef]
- Thakur, S.; Gupta, S.K.; Ali, V.; Singh, P.; Verma, M. Aldose Reductase: A cause and a potential target for the treatment of diabetic complications. Arch. Pharm. Res. 2021, 44, 655–667. [Google Scholar] [CrossRef]
- Abduldileep, S.; Narayanasamy, R.; Usharani, D.; Singh, A.; Rajasekharan, R. A bioactive polypeptide from sugarcane selectively inhibits intestinal sucrase. Int. J. Biol. Macromol. 2020, 156, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef]
- Sharma, B.; Xie, L.; Yang, F.; Wang, W.; Zhou, Q.; Xiang, M.; Zhou, S.; Lv, W.; Jia, Y.; Pokhrel, L.; et al. Recent advance on PTP1B inhibitors and their biomedical applications. Eur. J. Med. Chem. 2020, 199, 112376. [Google Scholar] [CrossRef]
- Ghani, U. Re-exploring promising α-glucosidase inhibitors for potential development into oral anti-diabetic drugs: Finding needle in the haystack. Eur. J. Med. Chem. 2015, 103, 133–162. [Google Scholar] [CrossRef] [PubMed]
- He, J.Y.; Hong, Q.; Chen, B.X.; Cui, S.Y.; Liu, R.; Cai, G.Y.; Guo, J.; Chen, X.M. Ginsenoside Rb1 alleviates diabetic kidney podocyte injury by inhibiting aldose reductase activity. Acta Pharmacol. Sin. 2022, 43, 342–353. [Google Scholar] [CrossRef]
- Kenner, K.A.; Anyanwu, E.; Olefsky, J.M.; Kusari, J. Protein-tyrosine phosphatase 1B is a negative regulator of insulin- and insulin-like growth factor-I-stimulated signaling. J. Biol. Chem. 1996, 271, 19810–19816. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, C.M.; Cohen, R.V.; Sumithran, P.; Clément, K.; Frühbeck, G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet. 2023, 401, 1116–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.S.; Li, L.X.; Liang, N.N.; Zhang, L.L.; Xu, X.D.; Chen, S.T.; Yin, H.Y. Acetaldehyde dehydrogenase 2 regulates HMG-CoA reductase stability and cholesterol synthesis in the liver. Redox Biol. 2021, 41, 101919. [Google Scholar] [CrossRef]
- Bialecka-Florjanczyk, E.; Fabiszewska, A.U.; Krzyczkowska, J.; Kurylowicz, A. Synthetic and natural lipase inhibitors. Mini. Rev. Med. Chem. 2018, 18, 672–683. [Google Scholar] [CrossRef]
- Pennerman, K.K.; Yin, G.; Bennett, J.W. Health effects of small volatile compounds from East Asian medicinal mushrooms. Mycobiology 2015, 43, 9–13. [Google Scholar] [CrossRef]
- Wang, X.M.; Zhang, J.; Wu, L.H.; Zhao, Y.L.; Li, T.; Li, J.Q.; Wang, Y.Z.; Liu, H.G. A mini-review of chemical composition and nutritional value of edible wild-grown mushroom from China. Food Chem. 2014, 151, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; de Mores, A.R.; Cohen, L.; Anwar, M.M.; Lazar, F.; Hicklen, R.; Lopez, G.; Yang, P.; Bruera, E. Medicinal mushroom supplements in cancer: A systematic review of clinical studies. Curr. Oncol. Rep. 2023, 25, 569–587. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart. Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Marek-Jozefowicz, L.; Nedoszytko, B.; Grochocka, M.; Żmijewski, M.A.; Czajkowski, R.; Cubała, W.J.; Slominski, A.T. Molecular mechanisms of neurogenic inflammation of the skin. Int. J. Mol. Sci. 2023, 24, 5001. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef] [PubMed]
- Bhambri, A.; Srivastava, M.; Mahale, V.G.; Mahale, S.; Karn, S.K. Mushrooms as potential sources of active metabolites and medicines. Front. Microbiol. 2022, 26, 837266. [Google Scholar] [CrossRef]
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible mushrooms: A comprehensive review on bioactive compounds with health benefits and processing aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef]
- Estella, T.F.; Mbong, G.A.; Lem, E.A.; Manju, E.B.; Tchadji, M.V.; Dobgima, J.F.; Charles, F. Medicinal mushroom of potential pharmaceutical toxic importance: Contribution in phytotherapy. In Current Topics in Functional Food; IntechOpen Limited: London, UK, 2022. [Google Scholar]
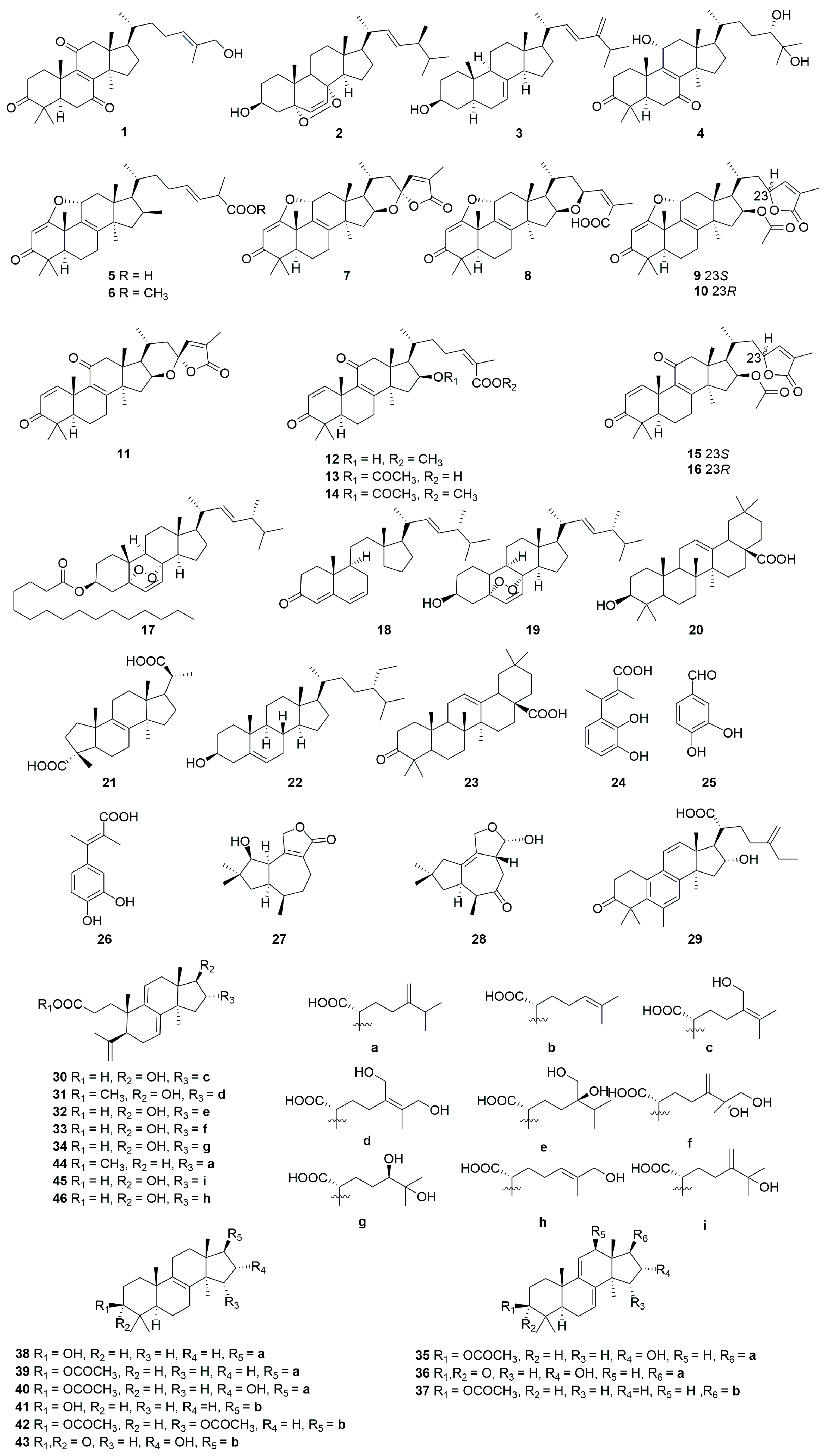

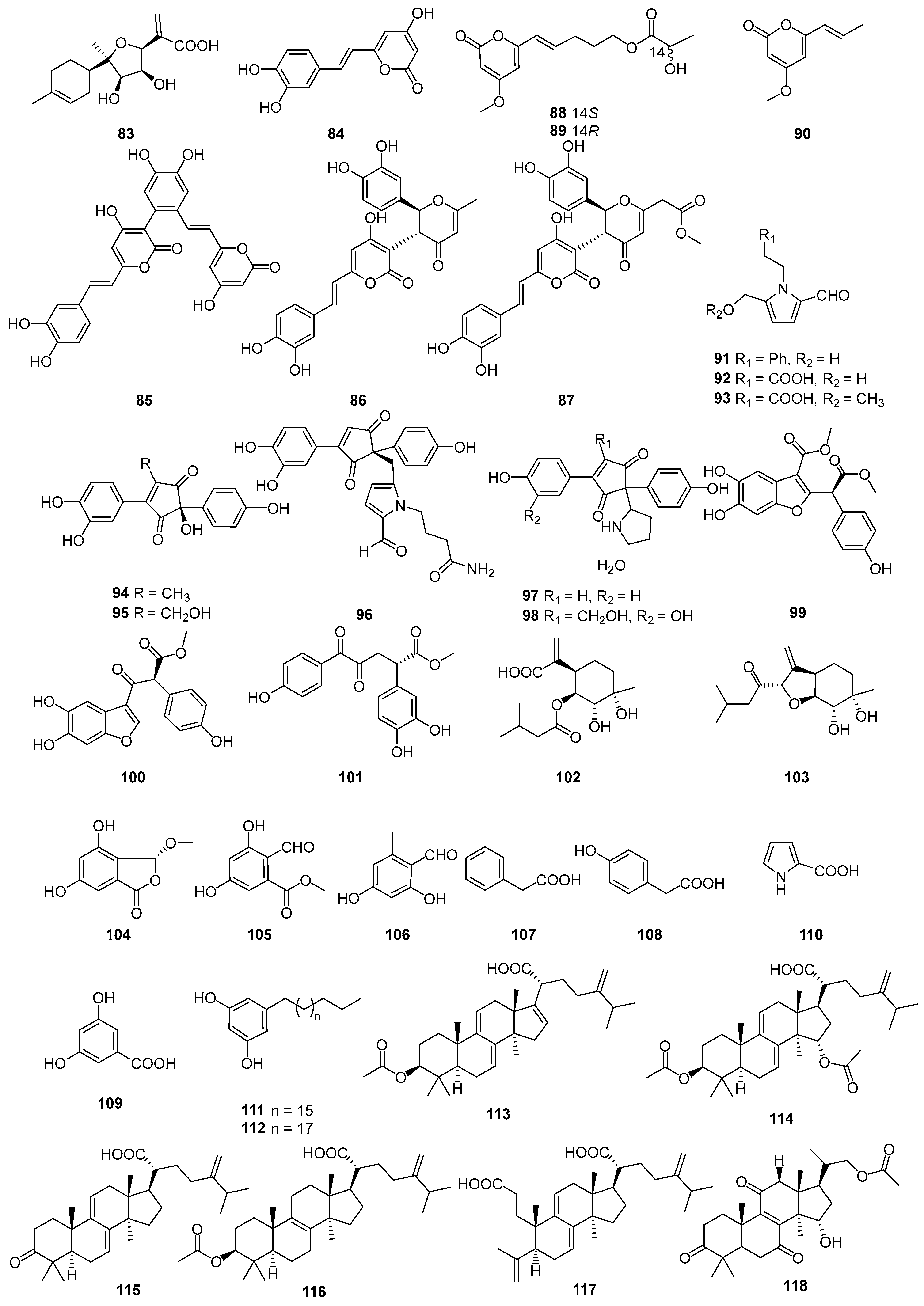

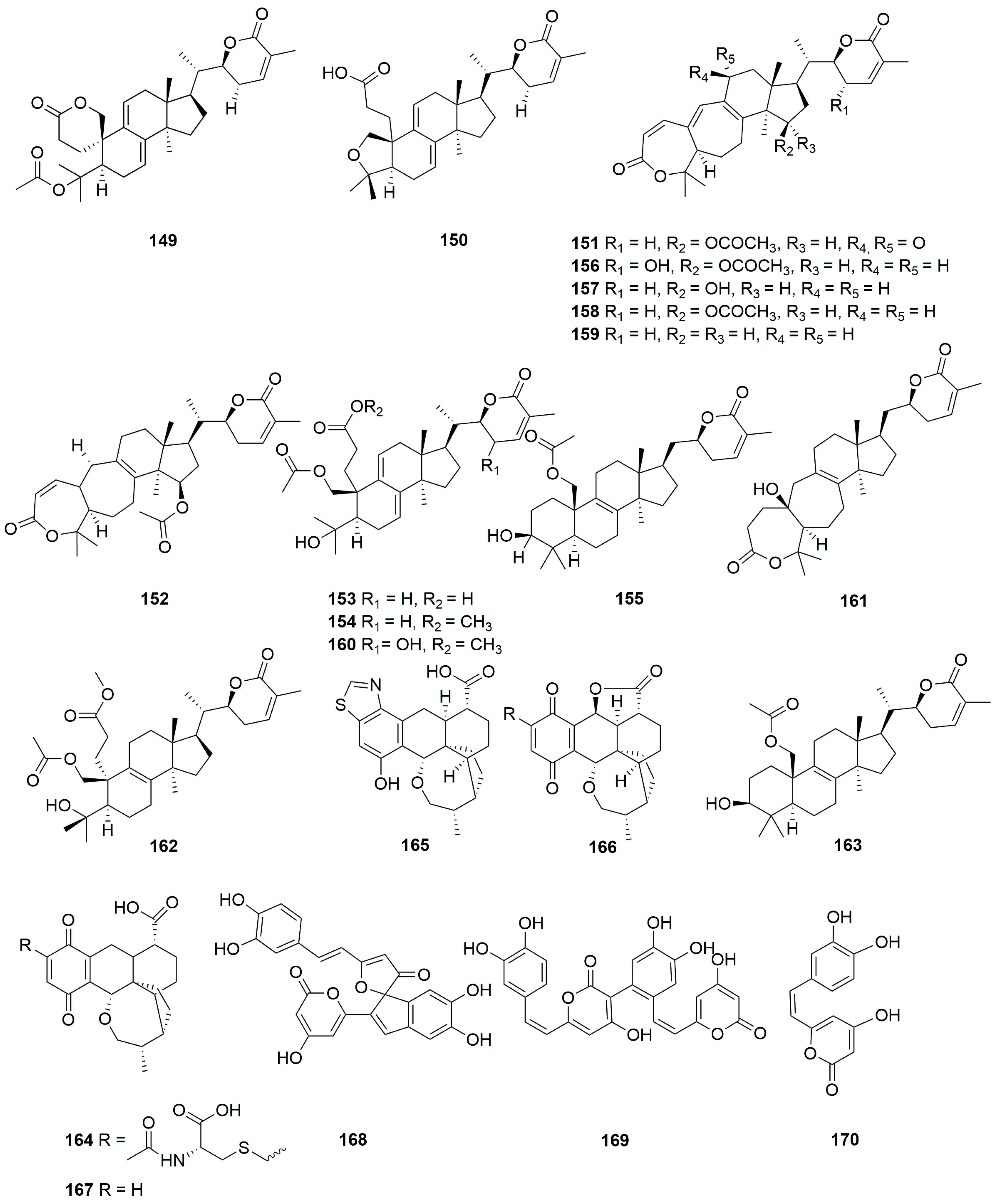
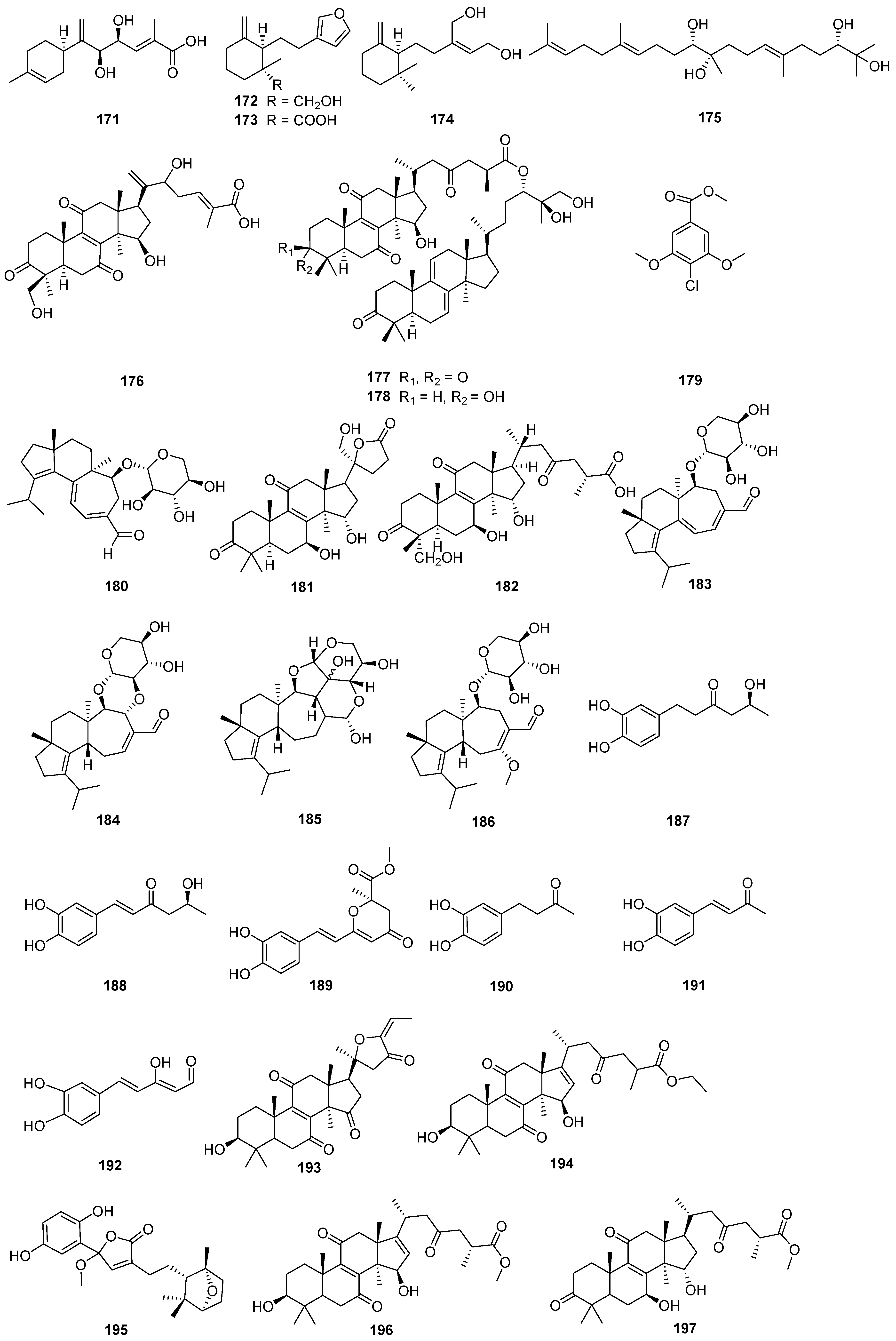


| Mushroom | Mushroom Family | Secondary Metabolites | Refs. |
|---|---|---|---|
| Morchella esculenta | Morchellaceae | 1–3 | [13] |
| Ganoderma lingzhi | Ganodermataceae | 4 | [14] |
| Macrolepiota procera | Agaricaceae | 5–16 | [15] |
| Fuscoporia torulosa | Hymenochaetaceae | 17–26 | [16] |
| Phellinus igniarius | Polyporaceae | 27–28 | [17] |
| Wolfiporia cocos | Polyporaceae | 29–46 | [18] |
| Ganoderma lingzhi | Ganodermataceae | 47 | [19] |
| Calvatia nipponica | Lycoperdaceae | 48–52 | [20] |
| Butyriboletus roseoflavus | Boletaceae | 53 | [21] |
| Ganoderma luteomarginatum | Ganodermataceae | 54–56 | [22] |
| Pleurotus florida | Pleurotaceae | 57 | [23] |
| Laetiporus sulphureus | Polyporaceae | 58–59 | [24] |
| Sarcosphaera crassa | Pezizaceae | 60–63 | [25] |
| Tricholoma ustaloides | Tricholomataceae | 64–65 | [26] |
| Lentinula edodes | Omphalotaceae | 66–68 | [27] |
| Ganoderma | Ganodermataceae | 69–72 | [28] |
| Ganoderma lucidum | Ganodermataceae | 73–75 | [29] |
| Morchella importuna | Morchellaceae | 76–78 | [30] |
| Oudemansiella raphanipes | Tricholomataceae | 79–81 | [31] |
| Sanghuangporus sanghuang | Hymenochaetaceae | 82–87 | [32] |
| Paxillus involutus | Paxillaceae | 88–93 | [33] |
| Paxillus involutus | Paxillaceae | 94–101 | [34] |
| Pleurotus ostreatus and Pleurotus eryngii | Pleurotaceae | 102–103 | [35] |
| Morehella importuna | Morchellaceae | 104–110 | [36] |
| Lepista sordida | Tricholomataceae | 111–112 | [37] |
| Poria cocos Wolf | Polyporaceae | 113–117 | [38] |
| Ganoderma lucidum | Ganodermataceae | 118 | [39] |
| Fomitopsis pinicola | Coriolaceae | 119–127 | [40] |
| Ganoderma orbiforme | Ganodermataceae | 128–129 | [41] |
| Inonotus obliquus | Hymenochaetaceae | 130–136 | [42] |
| Hericium erinaceus | Hericiaceae | 137–138 | [43] |
| Laetiporus sulphureus | Polyporaceae | 139–141 | [44] |
| Ganoderma sinense | Ganodermataceae | 142–143 | [45] |
| Ganoderma | Ganodermataceae | 144–148 | [46] |
| Tomophagus sp. | Ganodermataceae | 149–163 | [47] |
| Hohenbuehelia grisea | Pleurotaceae | 164–167 | [48] |
| Sanghuangporus | Hymenochaetaceae | 168–170 | [49] |
| Sanghuangporus | Hymenochaetaceae | 171–174 | [50] |
| Pleurotus ostreatus and Trametes robiniophila | Pleurotaceae and Polyporaceae | 175 | [51] |
| Ganoderma | Ganodermataceae | 176 | [52] |
| Ganoderma weberianum | Ganodermataceae | 177–178 | [53] |
| Hericium erinaceus | Hericiaceae | 179–180 | [54] |
| Ganoderma leucocontextum | Ganodermataceae | 181–182 | [55] |
| Hericium erinaceus and Hericium flagellum | Hericiaceae | 183–186 | [56] |
| Inonotus hispidus | Hymenochaetaceae | 187–192 | [57] |
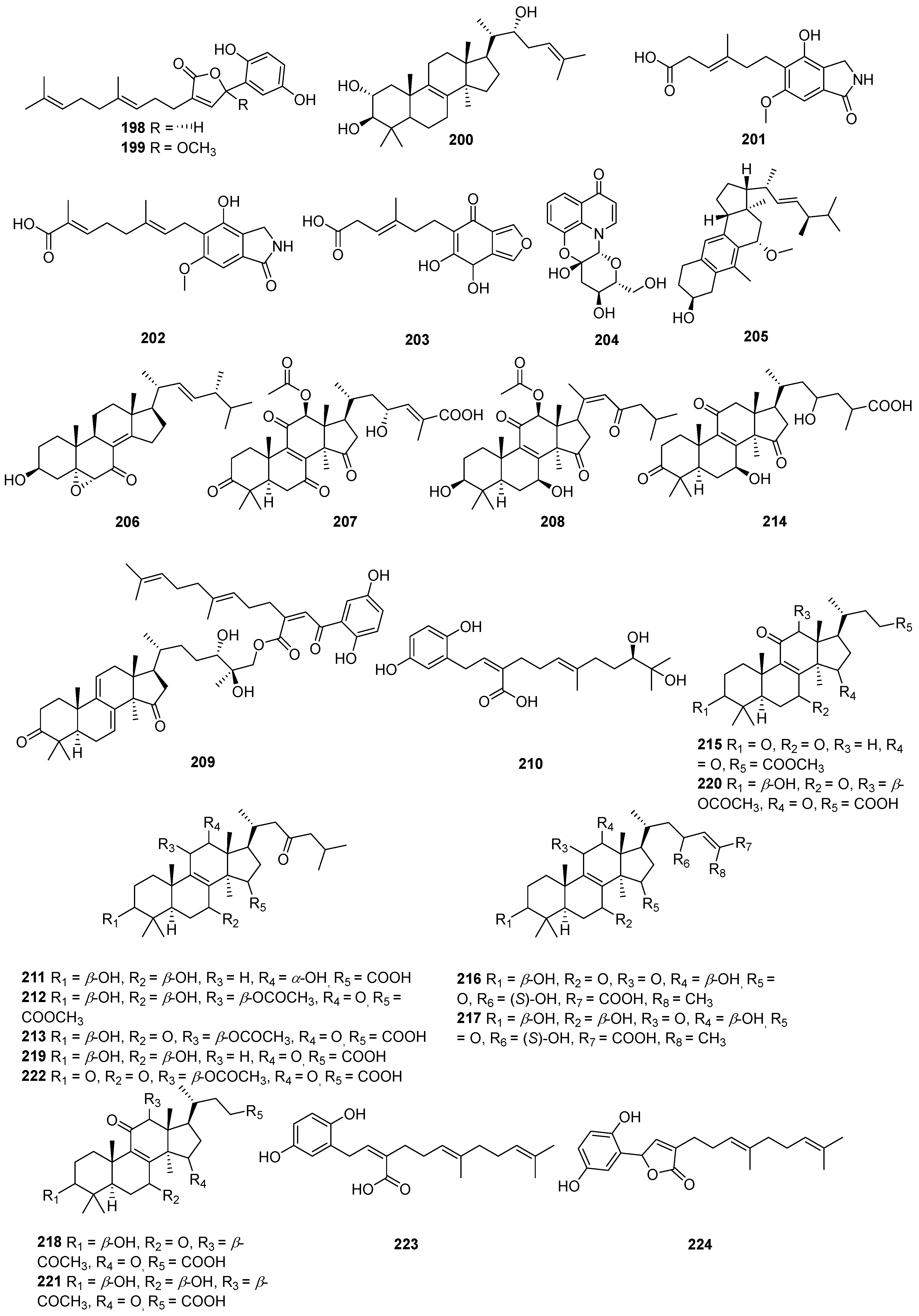
| Ganoderma resinaceum | Ganodermataceae | 193–199 | [58] |
| Inonotus obliquus | Hymenochaetaceae | 200 | [59] |
| Hericium flagellum | Hericiaceae | 201–203 | [60] |
| Dictyophora indusiata | Phallaceae | 204–206 | [61] |
| Ganoderma lucidum | Ganodermataceae | 207–230 | [62] |
| Hericium caput−medusae | Hericiaceae | 231–233 | [63] |
| Hericium erinaceus | Hericiaceae | 234–238 | [64] |
| Paxillus involutus | Paxillaceae | 239–242 | [65] |
| Ganoderma leucocontextum | Ganodermataceae | 243–245 | [66] |
| Morchella importuna | Morchellaceae | 246 | [67] |
| Cordyceps militaris | Cordycepitaceae | 247–251 | [68] |
| Ganoderma gibbosum | Ganodermataceae | 252 | [69] |
| Albatrellus confluens | Crypsinus | 253–254 | [70] |
| Ganoderma resinaceum | Ganodermataceae | 255–264 | [71] |
| Pleurotus cornucopiae | Pleurotaceae | 265–268 | [72] |
| Ganoderma applanatum | Ganodermataceae | 269 | [73] |
| Vanderbylia robiniophila | Polyporaceae | 270–274 | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Shi, Y.; Shi, D.; Tu, Y.; Liu, L. Biological Activities of Secondary Metabolites from the Edible-Medicinal Macrofungi. J. Fungi 2024, 10, 144. https://doi.org/10.3390/jof10020144
Sun X, Shi Y, Shi D, Tu Y, Liu L. Biological Activities of Secondary Metabolites from the Edible-Medicinal Macrofungi. Journal of Fungi. 2024; 10(2):144. https://doi.org/10.3390/jof10020144
Chicago/Turabian StyleSun, Xiaoqi, Ying Shi, Dongxiao Shi, Yu Tu, and Ling Liu. 2024. "Biological Activities of Secondary Metabolites from the Edible-Medicinal Macrofungi" Journal of Fungi 10, no. 2: 144. https://doi.org/10.3390/jof10020144





