Angiopoietin-Like 3 (ANGPTL3) and Atherosclerosis: Lipid and Non-Lipid Related Effects
Abstract
:1. Introduction
1.1. Genetics of ANGPTL3
1.2. Genome-Wide Association Studies (GWAS) and ANGPTL3
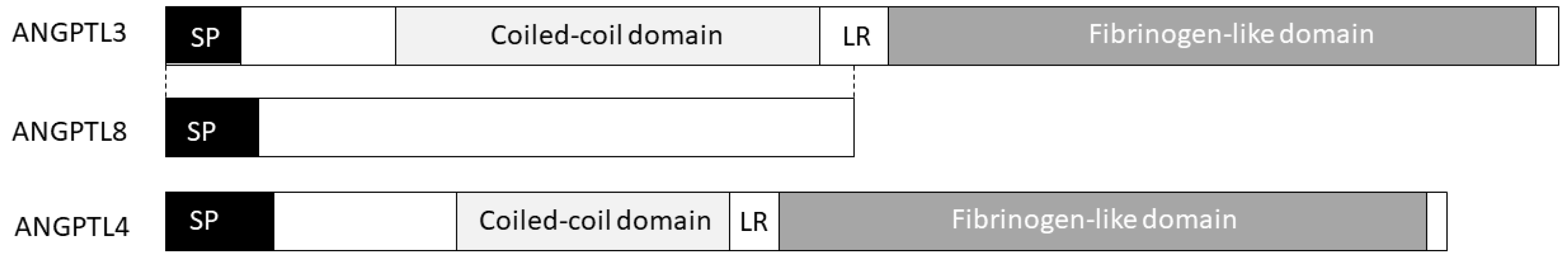
2. ANGPTL3 Structure
3. ANGPTL3 Post-Translational Modifications
4. ANGPTL3 Transcriptional Regulation
5. ANGPTL3 Coiled-Coil Fold: Its Role in Lipid Metabolism
6. The ANGPTL 3-4-8 Model
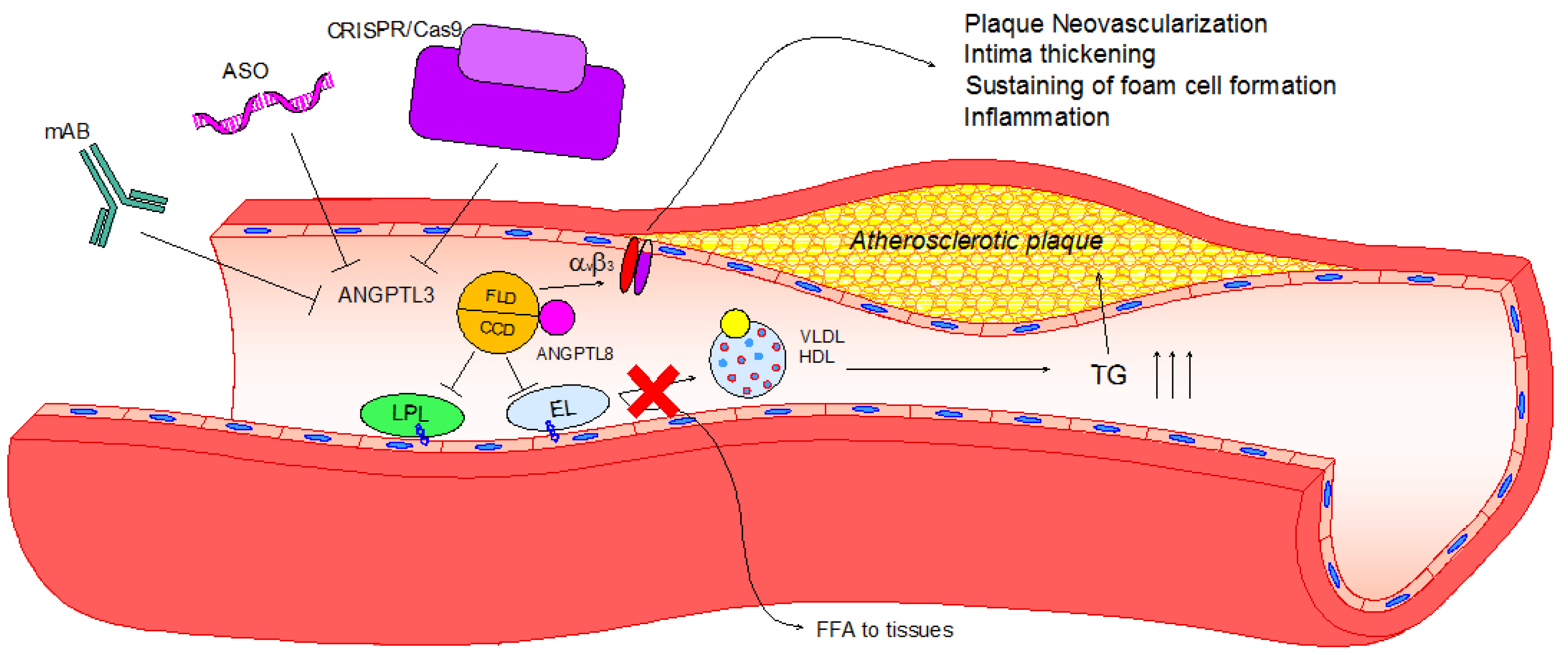
7. ANGPTL3 C-Terminal Domain: Fibrinogen-Like Domain Binds to Integrin αVβ3
8. Pharmacological Inhibition of ANGPTL3
9. Conclusions
Funding
Conflicts of Interest
References
- Conklin, D.; Gilbertson, D.; Taft, D.W.; Maurer, M.F.; Whitmore, T.E.; Smith, D.L.; Walker, K.M.; Chen, L.H.; Wattler, S.; Nehls, M.; et al. Identification of a mammalian angiopoietin-related protein expressed specifically in liver. Genomics 1999, 62, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Koishi, R.; Ando, Y.; Ono, M.; Shimamura, M.; Yasumo, H.; Fujiwara, T.; Horikoshi, H.; Furukawa, H. Angptl3 regulates lipid metabolism in mice. Nat. Genet. 2002, 30, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Shimizugawa, T.; Ono, M.; Shimamura, M.; Yoshida, K.; Ando, Y.; Koishi, R.; Ueda, K.; Inaba, T.; Minekura, H.; Kohama, T.; et al. ANGPTL3 decreases very low density lipoprotein triglyceride clearance by inhibition of lipoprotein lipase. J. Biol. Chem. 2002, 277, 33742–33748. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Shimizugawa, T.; Shimamura, M.; Yoshida, K.; Noji-Sakikawa, C.; Ando, Y.; Koishi, R.; Furukawa, H. Protein region important for regulation of lipid metabolism in angiopoietin-like 3 (ANGPTL3): ANGPTL3 is cleaved and activated in vivo. J. Biol. Chem. 2003, 278, 41804–41809. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, M.; Matsuda, M.; Yasumo, H.; Okazaki, M.; Fujimoto, K.; Kono, K.; Shimizugawa, T.; Ando, Y.; Koishi, R.; Kohama, T.; et al. Angiopoietin-like protein3 regulates plasma HDL cholesterol through suppression of endothelial lipase. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Nozawa, K.; Tomita, T. Inbred strains resulting from Japanese mice. Bull. Exp. Anim. 1957, 6, 107–112. [Google Scholar]
- Musunuru, K.; Pirruccello, J.P.; Do, R.; Peloso, G.M.; Guiducci, C.; Sougnez, C.; Garimella, K.V.; Fisher, S.; Abreu, J.; Barry, A.J.; et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N. Engl. J. Med. 2010, 363, 2220–2227. [Google Scholar] [CrossRef] [PubMed]
- Minicocci, I.; Montali, A.; Robciuc, M.R.; Quagliarini, F.; Censi, V.; Labbadia, G.; Gabiati, C.; Pigna, G.; Sepe, M.L.; Pannozzo, F.; et al. Mutations in the ANGPTL3 gene and familial combined hypolipidemia: A clinical and biochemical characterization. J. Clin. Endocrinol. Metab. 2012, 97, E1266–E1275. [Google Scholar] [CrossRef] [PubMed]
- Robciuc, M.R.; Maranghi, M.; Lahikainen, A.; Rader, D.; Bensadoun, A.; Oorni, K.; Metso, J.; Minicocci, I.; Ciociola, E.; Ceci, F.; et al. Angptl3 deficiency is associated with increased insulin sensitivity, lipoprotein lipase activity, and decreased serum free fatty acids. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Reiner, Z. Management of patients with familial hypercholesterolaemia. Nat. Rev. Cardiol. 2015, 12, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.P. Triglyceride-rich lipoproteins as a causal factor for cardiovascular disease. Vasc. Health Risk Manag. 2016, 12, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Musunuru, K.; Kathiresan, S. Cardiovascular endocrinology: Is ANGPTL3 the next PCSK9? Nat. Rev. Endocrinol. 2017, 13, 503–504. [Google Scholar] [CrossRef] [PubMed]
- Stitziel, N.O.; Khera, A.V.; Wang, X.; Bierhals, A.J.; Vourakis, A.C.; Sperry, A.E.; Natarajan, P.; Klarin, D.; Emdin, C.A.; Zekavat, S.M.; et al. ANGPTL3 Deficiency and Protection Against Coronary Artery Disease. J. Am. Coll. Cardiol. 2017, 69, 2054–2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatsuda, S.; Shoji, T.; Shinohara, K.; Kimoto, E.; Mori, K.; Fukumoto, S.; Koyama, H.; Emoto, M.; Nishizawa, Y. Association between plasma angiopoietin-like protein 3 and arterial wall thickness in healthy subjects. J. Vasc. Res. 2007, 44, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Shimizugawa, T.; Takeshita, S.; Ono, M.; Shimamura, M.; Koishi, R.; Furukawa, H. A decreased expression of angiopoietin-like 3 is protective against atherosclerosis in apoE-deficient mice. J. Lipid Res. 2003, 44, 1216–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korstanje, R.; Eriksson, P.; Samnegard, A.; Olsson, P.G.; Forsman-Semb, K.; Sen, S.; Churchill, G.A.; Rollins, J.; Harris, S.; Hamsten, A.; et al. Locating Ath8, a locus for murine atherosclerosis susceptibility and testing several of its candidate genes in mice and humans. Atherosclerosis 2004, 177, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.; Yin, W.; Kozlitina, J.; Pennacchio, L.A.; Boerwinkle, E.; Hobbs, H.H.; Cohen, J.C. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J. Clin. Investig. 2009, 119, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Pisciotta, L.; Favari, E.; Magnolo, L.; Simonelli, S.; Adorni, M.P.; Sallo, R.; Fancello, T.; Zavaroni, I.; Ardigo, D.; Bernini, F.; et al. Characterization of three kindreds with familial combined hypolipidemia caused by loss-of-function mutations of ANGPTL3. Circ. Cardiovasc. Genet. 2012, 5, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Noto, D.; Cefalu, A.B.; Valenti, V.; Fayer, F.; Pinotti, E.; Ditta, M.; Spina, R.; Vigna, G.; Yue, P.; Kathiresan, S.; et al. Prevalence of ANGPTL3 and APOB gene mutations in subjects with combined hypolipidemia. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Martin-Campos, J.M.; Roig, R.; Mayoral, C.; Martinez, S.; Marti, G.; Arroyo, J.A.; Julve, J.; Blanco-Vaca, F. Identification of a novel mutation in the ANGPTL3 gene in two families diagnosed of familial hypobetalipoproteinemia without APOB mutation. Clin. Chim. Acta 2012, 413, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Huijgen, R.; Sjouke, B.; Vis, K.; de Randamie, J.S.; Defesche, J.C.; Kastelein, J.J.; Hovingh, G.K.; Fouchier, S.W. Genetic variation in APOB, PCSK9, and ANGPTL3 in carriers of pathogenic autosomal dominant hypercholesterolemic mutations with unexpected low LDL-Cl Levels. Hum. Mutat. 2012, 33, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Teslovich, T.M.; Musunuru, K.; Smith, A.V.; Edmondson, A.C.; Stylianou, I.M.; Koseki, M.; Pirruccello, J.P.; Ripatti, S.; Chasman, D.I.; Willer, C.J.; et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 2010, 466, 707–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oldoni, F.; Palmen, J.; Giambartolomei, C.; Howard, P.; Drenos, F.; Plagnol, V.; Humphries, S.E.; Talmud, P.J.; Smith, A.J. Post-GWAS methodologies for localisation of functional non-coding variants: ANGPTL3. Atherosclerosis 2016, 246, 193–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kathiresan, S.; Melander, O.; Guiducci, C.; Surti, A.; Burtt, N.P.; Rieder, M.J.; Cooper, G.M.; Roos, C.; Voight, B.F.; Havulinna, A.S.; et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat. Genet. 2008, 40, 189–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aulchenko, Y.S.; Ripatti, S.; Lindqvist, I.; Boomsma, D.; Heid, I.M.; Pramstaller, P.P.; Penninx, B.W.; Janssens, A.C.; Wilson, J.F.; Spector, T.; et al. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat. Genet. 2009, 41, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kathiresan, S.; Willer, C.J.; Peloso, G.M.; Demissie, S.; Musunuru, K.; Schadt, E.E.; Kaplan, L.; Bennett, D.; Li, Y.; Tanaka, T.; et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat. Genet. 2009, 41, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Kamatani, Y.; Matsuda, K.; Okada, Y.; Kubo, M.; Hosono, N.; Daigo, Y.; Nakamura, Y.; Kamatani, N. Genome-wide association study of hematological and biochemical traits in a Japanese population. Nat. Genet. 2010, 42, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Chasman, D.I.; Pare, G.; Mora, S.; Hopewell, J.C.; Peloso, G.; Clarke, R.; Cupples, L.A.; Hamsten, A.; Kathiresan, S.; Malarstig, A.; et al. Forty-three loci associated with plasma lipoprotein size, concentration, and cholesterol content in genome-wide analysis. PLoS Genet. 2009, 5, e1000730. [Google Scholar] [CrossRef] [PubMed]
- Willer, C.J.; Schmidt, E.M.; Sengupta, S.; Peloso, G.M.; Gustafsson, S.; Kanoni, S.; Ganna, A.; Chen, J.; Buchkovich, M.L.; Mora, S.; et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 2013, 45, 1274–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spracklen, C.N.; Chen, P.; Kim, Y.J.; Wang, X.; Cai, H.; Li, S.; Long, J.; Wu, Y.; Wang, Y.X.; Takeuchi, F.; et al. Association analyses of East Asian individuals and trans-ancestry analyses with European individuals reveal new loci associated with cholesterol and triglyceride levels. Hum. Mol. Genet. 2017, 26, 1770–1784. [Google Scholar] [CrossRef] [PubMed]
- Camenisch, G.; Pisabarro, M.T.; Sherman, D.; Kowalski, J.; Nagel, M.; Hass, P.; Xie, M.H.; Gurney, A.; Bodary, S.; Liang, X.H.; et al. ANGPTL3 stimulates endothelial cell adhesion and migration via integrin alpha vbeta 3 and induces blood vessel formation in vivo. J. Biol. Chem. 2002, 277, 17281–17290. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, M.; Matsuda, M.; Kobayashi, S.; Ando, Y.; Ono, M.; Koishi, R.; Furukawa, H.; Makishima, M.; Shimomura, I. Angiopoietin-like protein 3, a hepatic secretory factor, activates lipolysis in adipocytes. Biochem. Biophys. Res. Commun. 2003, 301, 604–609. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Xu, H.; Fang, Z.; Yao, W.; Guo, W.; Rao, J.; Zha, X. Angiopoietin-like protein 3 modulates barrier properties of human glomerular endothelial cells through a possible signaling pathway involving phosphatidylinositol-3 kinase/protein kinase B and integrin alphaVbeta3. Acta Biochim. Biophys. Sin. 2008, 40, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Rao, J.; Zha, X.L.; Xu, H. Angiopoietin-like 3 induces podocyte F-actin rearrangement through integrin alpha(V)beta(3)/FAK/PI3K pathway-mediated Rac1 activation. BioMed Res. Int. 2013, 2013, 135608. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Lin, Y.; Liu, H.; Rao, J.; Zhai, Y.; Zha, X.; Fang, X.; Xu, H. A vital role for Angptl3 in the PAN-induced podocyte loss by affecting detachment and apoptosis in vitro. BMC Nephrol. 2015, 16, 38. [Google Scholar] [CrossRef] [PubMed]
- Schjoldager, K.T.; Vester-Christensen, M.B.; Bennett, E.P.; Levery, S.B.; Schwientek, T.; Yin, W.; Blixt, O.; Clausen, H. O-glycosylation modulates proprotein convertase activation of angiopoietin-like protein 3: Possible role of polypeptide GalNAc-transferase-2 in regulation of concentrations of plasma lipids. J. Biol. Chem. 2010, 285, 36293–36303. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Qian, W.J.; Gritsenko, M.A.; Camp, D.G., 2nd; Monroe, M.E.; Moore, R.J.; Smith, R.D. Human plasma N-glycoproteome analysis by immunoaffinity subtraction, hydrazide chemistry, and mass spectrometry. J. Proteome Res. 2005, 4, 2070–2080. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Jiang, X.; Sun, D.; Han, G.; Wang, F.; Ye, M.; Wang, L.; Zou, H. Glycoproteomics analysis of human liver tissue by combination of multiple enzyme digestion and hydrazide chemistry. J. Proteome Res. 2009, 8, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Rust, S.; Rosier, M.; Funke, H.; Real, J.; Amoura, Z.; Piette, J.C.; Deleuze, J.F.; Brewer, H.B.; Duverger, N.; Denefle, P.; et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 1999, 22, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Repa, J.J.; Mangelsdorf, D.J. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Ann. Rev. Cell Dev. Biol. 2000, 16, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.; Zhang, T.; Hernandez, M.; Gan, F.X.; Wright, S.D.; Waters, M.G.; Cai, T.Q. Regulation of the angiopoietin-like protein 3 gene by LXR. J. Lipid Res. 2003, 44, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Inaba, T.; Matsuda, M.; Shimamura, M.; Takei, N.; Terasaka, N.; Ando, Y.; Yasumo, H.; Koishi, R.; Makishima, M.; Shimomura, I. Angiopoietin-like protein 3 mediates hypertriglyceridemia induced by the liver X receptor. J. Biol. Chem. 2003, 278, 21344–21351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Repa, J.J.; Gauthier, K.; Mangelsdorf, D.J. Regulation of lipoprotein lipase by the oxysterol receptors, LXRalpha and LXRbeta. J. Biol. Chem. 2001, 276, 43018–43024. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hong, S.W.; Park, S.E.; Rhee, E.J.; Park, C.Y.; Oh, K.W.; Park, S.W.; Lee, W.Y. AMP-activated protein kinase suppresses the expression of LXR/SREBP-1 signaling-induced ANGPTL8 in HepG2 cells. Mol. Cell. Endocrinol. 2015, 414, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Fugier, C.; Tousaint, J.J.; Prieur, X.; Plateroti, M.; Samarut, J.; Delerive, P. The lipoprotein lipase inhibitor ANGPTL3 is negatively regulated by thyroid hormone. J. Biol. Chem. 2006, 281, 11553–11559. [Google Scholar] [CrossRef] [PubMed]
- Ptashne, M. How eukaryotic transcriptional activators work. Nature 1988, 335, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Inukai, K.; Nakashima, Y.; Watanabe, M.; Kurihara, S.; Awata, T.; Katagiri, H.; Oka, Y.; Katayama, S. ANGPTL3 is increased in both insulin-deficient and -resistant diabetic states. Biochem. Biophys. Res. Commun. 2004, 317, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, M.; Matsuda, M.; Ando, Y.; Koishi, R.; Yasumo, H.; Furukawa, H.; Shimomura, I. Leptin and insulin down-regulate angiopoietin-like protein 3, a plasma triglyceride-increasing factor. Biochem. Biophys. Res. Commun. 2004, 322, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Enerback, S.; Semb, H.; Bengtsson-Olivecrona, G.; Carlsson, P.; Hermansson, M.L.; Olivecrona, T.; Bjursell, G. Molecular cloning and sequence analysis of cDNA encoding lipoprotein lipase of guinea pig. Gene 1987, 58, 1–12. [Google Scholar] [CrossRef]
- Wang, C.S.; Hartsuck, J.; McConathy, W.J. Structure and functional properties of lipoprotein lipase. Biochim. Biophys. Acta 1992, 1123, 1–17. [Google Scholar] [CrossRef]
- Beigneux, A.P.; Davies, B.S.; Gin, P.; Weinstein, M.M.; Farber, E.; Qiao, X.; Peale, F.; Bunting, S.; Walzem, R.L.; Wong, J.S.; et al. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 2007, 5, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.E.; Severson, D.L. Regulation of the synthesis, processing and translocation of lipoprotein lipase. Biochem. J. 1992, 287 Pt 2, 337–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camps, L.; Reina, M.; Llobera, M.; Vilaro, S.; Olivecrona, T. Lipoprotein lipase: Cellular origin and functional distribution. Am. J. Physiol. 1990, 258, C673–C681. [Google Scholar] [CrossRef] [PubMed]
- LaRosa, J.C.; Levy, R.I.; Herbert, P.; Lux, S.E.; Fredrickson, D.S. A specific apoprotein activator for lipoprotein lipase. Biochem. Biophys. Res. Commun. 1970, 41, 57–62. [Google Scholar] [CrossRef]
- Miller, A.L.; Smith, L.C. Activation of lipoprotein lipase by apolipoprotein glutamic acid. Formation of a stable surface film. J. Biol. Chem. 1973, 248, 3359–3362. [Google Scholar] [PubMed]
- Mead, J.R.; Irvine, S.A.; Ramji, D.P. Lipoprotein lipase: Structure, function, regulation, and role in disease. J. Mol. Med. 2002, 80, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Merkel, M.; Eckel, R.H.; Goldberg, I.J. Lipoprotein lipase: Genetics, lipid uptake, and regulation. J. Lipid Res. 2002, 43, 1997–2006. [Google Scholar] [CrossRef] [PubMed]
- Mead, J.R.; Ramji, D.P. The pivotal role of lipoprotein lipase in atherosclerosis. Cardiovasc. Res. 2002, 55, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, K.; Koishi, R.; Shimizugawa, T.; Ando, Y. Angptl3-null mice show low plasma lipid concentrations by enhanced lipoprotein lipase activity. Exp. Anim. 2006, 55, 27–34. [Google Scholar] [CrossRef] [PubMed]
- McCoy, M.G.; Sun, G.S.; Marchadier, D.; Maugeais, C.; Glick, J.M.; Rader, D.J. Characterization of the lipolytic activity of endothelial lipase. J. Lipid Res. 2002, 43, 921–929. [Google Scholar] [PubMed]
- Lee, E.C.; Desai, U.; Gololobov, G.; Hong, S.; Feng, X.; Yu, X.C.; Gay, J.; Wilganowski, N.; Gao, C.; Du, L.L.; et al. Identification of a new functional domain in angiopoietin-like 3 (ANGPTL3) and angiopoietin-like 4 (ANGPTL4) involved in binding and inhibition of lipoprotein lipase (LPL). J. Biol. Chem. 2009, 284, 13735–13745. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Afroza, H.; Rader, D.J.; Jin, W. Angiopoietin-like protein 3 inhibits lipoprotein lipase activity through enhancing its cleavage by proprotein convertases. J. Biol. Chem. 2010, 285, 27561–27570. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.C.; Chickering, T.W.; Rosen, E.D.; Dussault, B.; Qin, Y.; Soukas, A.; Friedman, J.M.; Holmes, W.E.; Spiegelman, B.M. Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol. Cell. Biol. 2000, 20, 5343–5349. [Google Scholar] [CrossRef] [PubMed]
- Sukonina, V.; Lookene, A.; Olivecrona, T.; Olivecrona, G. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc. Natl. Acad. Sci. USA 2006, 103, 17450–17455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koster, A.; Chao, Y.B.; Mosior, M.; Ford, A.; Gonzalez-DeWhitt, P.A.; Hale, J.E.; Li, D.; Qiu, Y.; Fraser, C.C.; Yang, D.D.; et al. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: Regulation of triglyceride metabolism. Endocrinology 2005, 146, 4943–4950. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.; Pennacchio, L.A.; Fu, Y.; Boerwinkle, E.; Tybjaerg-Hansen, A.; Hobbs, H.H.; Cohen, J.C. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat. Genet. 2007, 39, 513–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, G.; Kim, J.Y.; Smas, C.M. Identification of RIFL, a novel adipocyte-enriched insulin target gene with a role in lipid metabolism. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E334–E351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R. Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochem. Biophys. Res. Commun. 2012, 424, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Quagliarini, F.; Wang, Y.; Kozlitina, J.; Grishin, N.V.; Hyde, R.; Boerwinkle, E.; Valenzuela, D.M.; Murphy, A.J.; Cohen, J.C.; Hobbs, H.H. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc. Natl. Acad. Sci. USA 2012, 109, 19751–19756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissglas-Volkov, D.; Aguilar-Salinas, C.A.; Nikkola, E.; Deere, K.A.; Cruz-Bautista, I.; Arellano-Campos, O.; Munoz-Hernandez, L.L.; Gomez-Munguia, L.; Ordonez-Sanchez, M.L.; Reddy, P.M.; et al. Genomic study in Mexicans identifies a new locus for triglycerides and refines European lipid loci. J. Med. Genet. 2013, 50, 298–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kersten, S.; Lichtenstein, L.; Steenbergen, E.; Mudde, K.; Hendriks, H.F.; Hesselink, M.K.; Schrauwen, P.; Muller, M. Caloric restriction and exercise increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Eklund, L.; Saharinen, P. Angiopoietin signaling in the vasculature. Exp. Cell Res. 2013, 319, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Hoshiga, M.; Alpers, C.E.; Smith, L.L.; Giachelli, C.M.; Schwartz, S.M. Alpha-v beta-3 integrin expression in normal and atherosclerotic artery. Circ. Res. 1995, 77, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Barger, A.C.; Beeuwkes, R., 3rd; Lainey, L.L.; Silverman, K.J. Hypothesis: Vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N. Engl. J. Med. 1984, 310, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Antonov, A.S.; Kolodgie, F.D.; Munn, D.H.; Gerrity, R.G. Regulation of macrophage foam cell formation by alphaVbeta3 integrin: Potential role in human atherosclerosis. Am. J. Pathol. 2004, 165, 247–258. [Google Scholar] [CrossRef]
- Scatena, M.; Almeida, M.; Chaisson, M.L.; Fausto, N.; Nicosia, R.F.; Giachelli, C.M. NF-kappaB mediates alphavbeta3 integrin-induced endothelial cell survival. J. Cell Biol. 1998, 141, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Dewey, F.E.; Gusarova, V.; Dunbar, R.L.; O’Dushlaine, C.; Schurmann, C.; Gottesman, O.; McCarthy, S.; Van Hout, C.V.; Bruse, S.; Dansky, H.M.; et al. Genetic and Pharmacologic Inactivation of ANGPTL3 and Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaudet, D.; Gipe, D.A.; Pordy, R.; Ahmad, Z.; Cuchel, M.; Shah, P.K.; Chyu, K.Y.; Sasiela, W.J.; Chan, K.C.; Brisson, D.; et al. ANGPTL3 Inhibition in Homozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2017, 377, 296–297. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.J.; Lee, R.G.; Brandt, T.A.; Tai, L.J.; Fu, W.; Peralta, R.; Yu, R.; Hurh, E.; Paz, E.; McEvoy, B.W.; et al. Cardiovascular and Metabolic Effects of ANGPTL3 Antisense Oligonucleotides. N. Engl. J. Med. 2017, 377, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Gusarova, V.; Alexa, C.A.; Wang, Y.; Rafique, A.; Kim, J.H.; Buckler, D.; Mintah, I.J.; Shihanian, L.M.; Cohen, J.C.; Hobbs, H.H.; et al. ANGPTL3 blockade with a human monoclonal antibody reduces plasma lipids in dyslipidemic mice and monkeys. J. Lipid Res. 2015, 56, 1308–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Vlijmen, B.J.; van den Maagdenberg, A.M.; Gijbels, M.J.; van der Boom, H.; HogenEsch, H.; Frants, R.R.; Hofker, M.H.; Havekes, L.M. Diet-induced hyperlipoproteinemia and atherosclerosis in apolipoprotein E3-Leiden transgenic mice. J. Clin. Investig. 1994, 93, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Bui, A.V.; Diesch, J.; Manasseh, R.; Hausding, C.; Rivera, J.; Haviv, I.; Agrotis, A.; Htun, N.M.; Jowett, J.; et al. A novel mouse model of atherosclerotic plaque instability for drug testing and mechanistic/therapeutic discoveries using gene and microRNA expression profiling. Circ. Res. 2013, 113, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Van der Donckt, C.; Van Herck, J.L.; Schrijvers, D.M.; Vanhoutte, G.; Verhoye, M.; Blockx, I.; Van Der Linden, A.; Bauters, D.; Lijnen, H.R.; Sluimer, J.C.; et al. Elastin fragmentation in atherosclerotic mice leads to intraplaque neovascularization, plaque rupture, myocardial infarction, stroke, and sudden death. Eur. Heart J. 2015, 36, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, A.C.; Evitt, N.H.; Lv, W.; Musunuru, K. Reduced Blood Lipid Levels With In Vivo CRISPR-Cas9 Base Editing of ANGPTL3. Circulation 2018, 137, 975–977. [Google Scholar] [CrossRef] [PubMed]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Mutation | Affected Domain | Phenotype | Reference |
|---|---|---|---|
| S17 * | Not CCD N-terminal region | Homozygous carriers: ↓ All lipids (no ANGPTL3 in the plasma) Heterozygous carriers: ↓ Total cholesterol ↓HDL-C (low ANGPTL3 in the plasma) | [7,8] |
| I19Lfs * | Not CCD N-terminal region | ↓ TG ↓ total cholesterol | [17,18] |
| D41N | Not CCD N-terminal region | ↓ TG | [17] |
| N42D | Not CCD N-terminal region | ↓ TG ↓ total cholesterol | [13] |
| G56V | Not CCD N-terminal region | ↓ LDL-C ↓ HDL-C | [19] |
| F60Lfs * | Not CCD N-terminal region | ↓ TG | [13] |
| K63T | Not CCD N-terminal region | ↓ TG (defective LPL inhibition) | [17] |
| F72L | Not CCD N-terminal region | ↓ TG | [17] |
| T83 * | Not CCD N-terminal region | ↓ TG ↓ total cholesterol | [13] |
| E91G | CCD | ↓ TG (defective LPL inhibition) | [17] |
| E98K | CCD | ↓ TG | [17] |
| N121Kfs | CCD | ↓ TG ↓ total cholesterol | [13,20] |
| S122fs | CCD | ↓ TG ↓ total cholesterol | [8,17] |
| L127F | CCD | ↓ TG ↓ LDL-C | [21] |
| E129 * | CCD | ↓ TG ↓ total cholesterol | [7] |
| K131T | CCD | ↓ TG | [17] |
| N147 * | CCD | ↓ TG ↓ total cholesterol | [13,17,18,19] |
| L164F | CCD | ↓ TG (defective LPL inhibition) | [17] |
| N173S | CCD | ↓ TG (defective LPL inhibition) | [17] |
| Y186 * | CCD | ↓ TG ↓ total cholesterol | [13] |
| Q192 * | CCD | ↓ TG ↓ total cholesterol | [13] |
| S215Lfs * | Linker Region | ↓ TG ↓ total cholesterol | [13] |
| N232fs | Linker Region | ↓ TG ↓ total cholesterol | [13] |
| M259T | FLD | Apparently nonpathogenic | [17] |
| R288Q | FLD | ↓ TG (lower ANGPTL3 secretion) | [13,17] |
| S292P | FLD | ↓ TG (lower ANGPTL3 secretion) | [13,17] |
| F295L | FLD | ↓ LDL-C ↓ HDL-C | [19] |
| F306Lfs * | FLD | ↓ TG ↓ total cholesterol | [13] |
| R332Q | FLD | ↓ LDL-C ↓ HDL-C | [19] |
| Y347 * | FLD | ↓ TG ↓ total cholesterol | [13] |
| E375K | FLD | ↓ TG (lower ANGPTL3 secretion) | [17] |
| T383S | FLD | ↓ TG ↓ total cholesterol | [13] |
| G400Vfs * | FLD | ↓ TG ↓ total cholesterol | [17,18] |
| W404 * | FLD | ↓ TG ↓ total cholesterol | [13] |
| Y417C | FLD | ↓ TG (lower ANGPTL3 secretion) | [17] |
| A422Qfs * | FLD | ↓ TG | [13] |
| R428M | FLD | ↓ TG | [17] |
| I444Yfs * | FLD | ↓ TG | [13] |
| T454Rfs * | FLD | ↓ TG | [13] |
| SNP ID | Normal Allele | Risk Allele | Phenotypic Trait | Ref. |
|---|---|---|---|---|
| rs12130333 | T | C | TG | [24] |
| rs10889353 | A | C | TG, TC, LDL-C | [25,26,27,28] |
| rs2131925 | T | G | TG, TC, LDL-C | [22,29,30] |
| rs10889352 | C | T | TG, LDL-C | [22,23] |
| rs6690733 | C | A | TG, LDL-C | [22,23] |
| rs11485618 | G | G | LDL-C | [30] |
| rs995000 | C | T | TG | [30] |
| rs11208004 | G | A | TC | [30] |
| PTM | Position(s) | Enzyme | Ref. |
|---|---|---|---|
| N-glycosilation | N115 | GlcNAc | [1,37] |
| O-glycosilation | T226 | GalNAc-T2 | [36] |
| Disulfide bond | C246 ↔ C274 | [1] | |
| N-glycosilation | N296 | GlcNAc | [37,38] |
| N-glycosilation | N357 | GlcNAc | [37] |
| Disulfide bond | C394 ↔ C408 | [1] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupo, M.G.; Ferri, N. Angiopoietin-Like 3 (ANGPTL3) and Atherosclerosis: Lipid and Non-Lipid Related Effects. J. Cardiovasc. Dev. Dis. 2018, 5, 39. https://doi.org/10.3390/jcdd5030039
Lupo MG, Ferri N. Angiopoietin-Like 3 (ANGPTL3) and Atherosclerosis: Lipid and Non-Lipid Related Effects. Journal of Cardiovascular Development and Disease. 2018; 5(3):39. https://doi.org/10.3390/jcdd5030039
Chicago/Turabian StyleLupo, Maria Giovanna, and Nicola Ferri. 2018. "Angiopoietin-Like 3 (ANGPTL3) and Atherosclerosis: Lipid and Non-Lipid Related Effects" Journal of Cardiovascular Development and Disease 5, no. 3: 39. https://doi.org/10.3390/jcdd5030039





