Never Too Late: A Case Report on Transcatheter Aortic Valve Implantation in a 97-Year-Old Patient
Abstract
:1. Introduction
2. Case Report
2.1. Preoperative Evaluation for TAVI
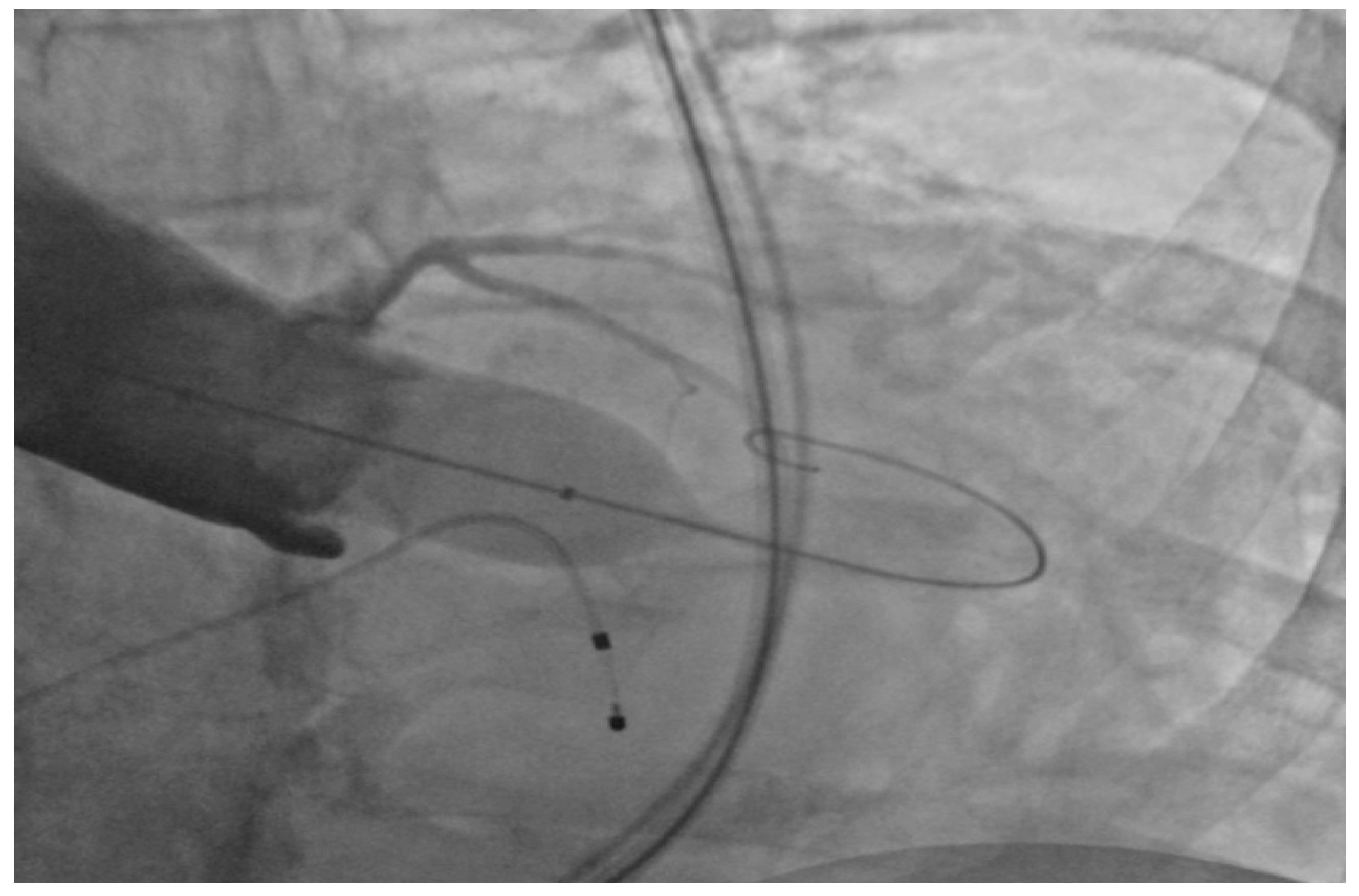
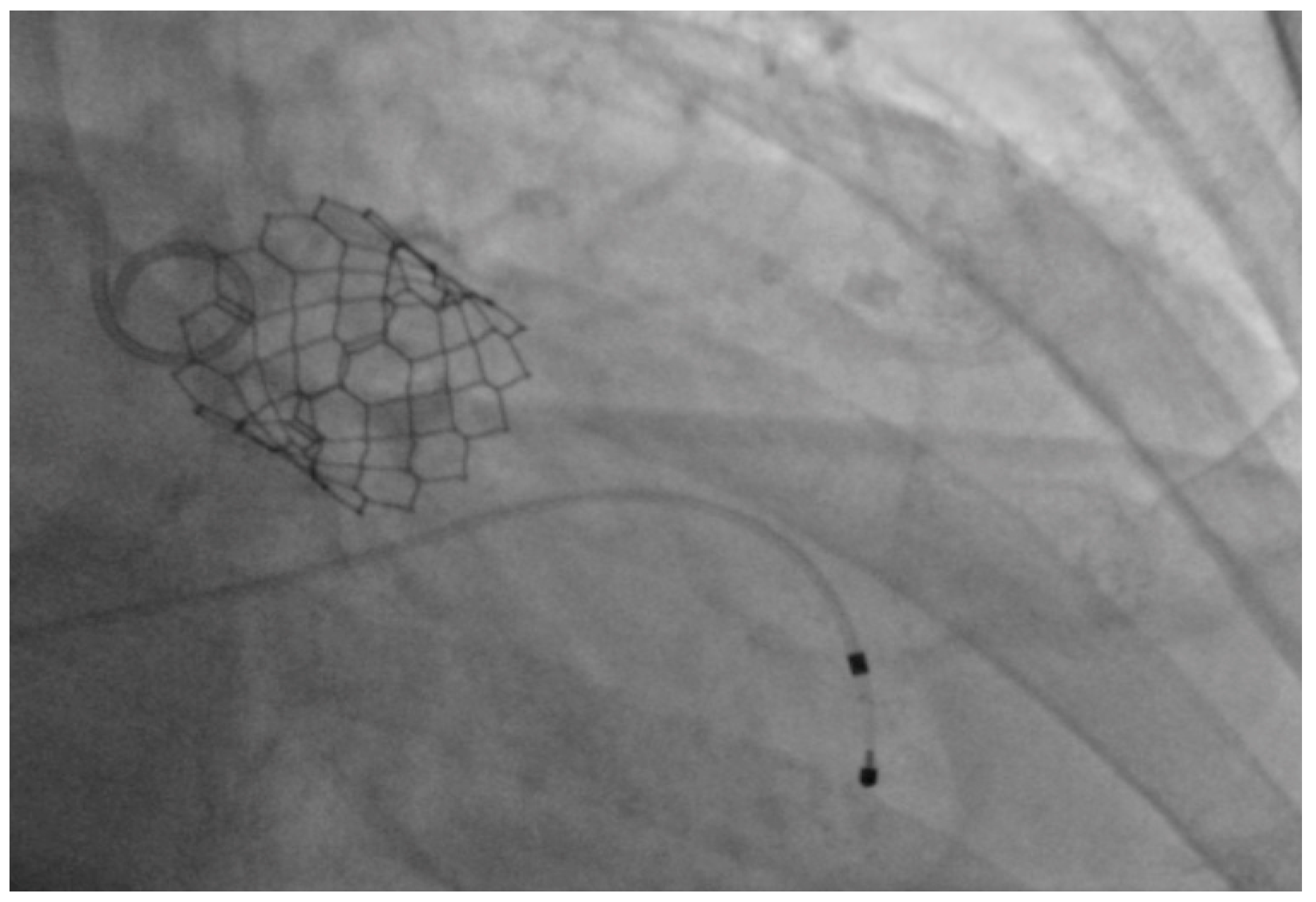
2.2. Performance of TAVI
2.3. Postoperative Course
2.4. Follow-up Visits
3. Literature Review
3.1. Complications after TAVI
3.2. Mortality after Hospital Discharge
3.3. TAVI in Centenarians
4. Discussion
5. Conclusions
Conflicts of Interest
References
- Andreoli, T.E.; Carpenter, C.C.J.; Griggs, R.C.; Benjamin, I.J. Acquired valvular heart disease. In Cecil’s Essentials of Medicine, 7th ed.; Saunders Elsevier: Philadelphia, PA, USA, 2007; Volume 8, pp. 86–87. [Google Scholar]
- Osnabrugge, R.L.J.; Mylotte, D.; Head, S.J.; Van Mieghem, N.M.; Nkomo, V.T.; LeReun, C.M.; Bogers, J.J.C.; Piazza, N.; Kappetein, A.P. Disease Prevalence and Number of Candidates for Transcatheter Aortic Valve Replacement: A Meta-analysis and Modeling Study. J. Am. Coll. Cardiol. 2013, 62, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Alsara, O.; Alsarah, A.; Laird-Fick, H. Advanced Age and the Clinical Outcomes of Transcatheter Aortic Valve Implantation. J. Geriatr. Cardiol. 2014, 11, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Daniels, D.V. Valvular diseases. In Concise Cardiology: An Evidence-Based Handbook; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; Volume 12, pp. 179–185. [Google Scholar]
- United States Census Bureau. Available online: https://www.census.gov/newsroom/releases/archives/aging_population/cb11-194.html (accessed on 27 June 2017).
- Cribier, A.G. The Odyssey of TAVR. Tex. Heart Inst. J. 2014, 41, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R; et al. Transcatheter Versus Surgical Aortic Valve Replacement in High Risk Patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017, 70, 252–289. [Google Scholar] [CrossRef] [PubMed]
- Online STS Adult Cardiac Surgery Risk Calculator. Available online: http://riskcalc.sts.org/stswebriskcalc/#/calculate (accessed on 28 June 2017).
- Barra, M.E.; Fanikos, J.; Connors, J.M.; Sylvester, K.W.; Piazza, G.; Goldhaber, S.Z. Evaluation of Dose-Reduced Direct Oral Anticoagulant Therapy. Am. J. Med. 2016, 129, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Jabs, A.; Kilic, T.; Schnelle, N.; Post, F.; Vosseler, M.; Vahl, C.-F.; Munzel, T.; Hink, U. Transcatheter Aortic Valve Implantation and Four-year Follow up in a 99-year-old Patient. J. Heart Valve Dis. 2013, 22, 261–264. [Google Scholar] [PubMed]
- Aslan, A.N.; Ayhan, H.; Ozdemir, E.; Bozkurt, E. A Centenarian Transcatheter Aortic Valve implantation case. J. Geriatr. Cardiol. 2016, 13, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Wendt, D.; Eggebrecht, H.; Kahlert, P.; Thielmann, M. Successful Transapical Aortic Valve Implantation Four Weeks before 97th Birthday. Interact. Cardiol. Thorac. Surg. 2009, 8, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Arsalan, M.; Szerlip, M.; Vemulapalli, S.; Holper, S.E.; Arnold, S.V.; Li, Z.; DiMaio, M.J.; Rumsfeld, J.S.; Brown, D.L.; Mack, M.J. Should Transcatheter Aortic Valve Replacement be Performed in Nonagenarians? Insights from the STS/ACC TVT Registry. J. Am. Coll. Cardiol. 2016, 67, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Kneitz, A.; Clifton, W.; Kar, B.; Delgado, R.M., III. Transcatheter Aortic Valve Replacement in a Nonagenarian. Tex. Heart Inst. J. 2013, 40, 196–197. [Google Scholar]
- Verouhis, D.; Yamasaki, K.; Ivert, T.; Ruck, A.; Settergren, M. Transcatheter Aortic Valve Implantation is Feasible and Safe in Nonagenarians. J. Am. Geriatr. Soc. 2014, 62, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Meguro, K.; Mouillet, G.; Bergoend, E.; Monin, J.-L.; Lim, P.; Dubois-Rande, J.-L.; Teiger, E. Comparison of Effectiveness and Safety of Transcatheter Aortic Valve Implantation in Patients Aged ≥90 Years Versus <90 Years. Am. J. Cardiol. 2012, 110, 1156–1163. [Google Scholar] [CrossRef]
- Abramowitz, Y.; Chakravarty, T.; Jilaihawi, H.; Kashif, M.; Zadikany, R.; Lee, C.; Matar, G.; Cheng, W.; Makkar, R.R. Comparison of Outcomes of Transcatheter Aortic Valve Implantation in Patients ≥90 years versus <90 years. Am. J. Cardiol. 2015, 116, 1110–1115. [Google Scholar] [CrossRef]
- Gurvitch, R.; Wood, D.A.; Tay, E.L.; Leipsic, J.; Ye, J.; Lichtenstein, S.V.; Thompson, C.R.; Carere, R.G.; Wijesinghe, N.; Nietlispach, F.; et al. Transcatheter Aortic Valve Implantation: Durability of Clinical and Hemodynamic Outcomes Beyond 3 years in a Large Patient Cohort. Circulation 2010, 122, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Escarcega, R.O.; Baker, N.C.; Lipinski, M.J.; Koifman, E.; Kiramijyan, S.; Magalhaes, M.A.; Gai, J.; Torguson, R.; Satler, L.F.; Pichard, A.D.; et al. Clinical Profiles and Correlates of Mortality in Nonagenarians with Severe Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement. Am. Heart J. 2016, 173, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Pascual, I.; Lopez-Otero, D.; Munoz-Garcia, A.J.; Alonso-Briales, J.H.; Avanzas, P.; Moris, C. Safety and Efficacy of Transcatheter Aortic Valve Implantation in Nonagenarian Patients. Rev. Esp. Cardiol. 2014, 67, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, G.L.; Chieffo, A.; Montorfano, M.; Maisano, F.; Latib, A.; Cioni, M.; Figini, F.; Carlino, M.; Gerli, C.; Franco, A.; et al. Feasibility of TAVI for Severe Aortic Stenosis in Nonagenarians. EuroIntervention 2013. Available online: https://www.pcronline.com/eurointervention/Abstracts2013_online_issue/abstracts-online-2013/265/feasibility-of-tavi-for-severe-aortic-stenosis-in-nonagenarians.html (accessed on 16 July 2017).
- Scholtz, S.; Dimitriadis, Z.; Vlachojannis, M.; Piper, C.; Horstkotte, D.; Wiemer, M.; Gummert, H.; Fujita, B. Transcatheter Aortic Valve Implantation in Nonagenarian: Procedural Outcome and Mid-term Results. Heart Lung Circ. 2017. [Google Scholar] [CrossRef] [PubMed]
- Penkalla, A.; Kempfert, J.; Unbehaun, A.; Buz, S.; Drews, T.; Pasic, M.; Falk, V. Transcatheter Aortic Valve Implantation in Nonagenarians. Innov. Tech. Tech. Cardiol. Vasc. Surg. 2016, 11, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Noble, S.; Frangos, S.; Ellenberger, C.; Frangos, C.; Cikirikcioglu, M.; Bendjelid, K.; Frei, A.; Myers, P.; Licker, M.; Roffi, M. Transcatheter Aortic Valve Implantation in Nonagenarians: Effective and Safe. Eur. J. Int. Med. 2013, 24, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Akin, I.; Kische, S.; Paranskaya, L.; Schneider, H.; Rehders, T.C.; Turan, G.R.; Divchev, D.; Kundt, G.; Bozdag-Turan, I.; Ortak, J. Morbidity and Mortality of Nonagenarians Undergoing CoreValve Implantation. BMC Cardiol. Dis. 2012, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Greason, K.L.; Pochettino, A.; Sandhu, G.S.; Mathew, V. Transcatheter Aortic Valve Insertion in the Nonagenarian Patient. J. Thorac. Cardiol. Surg. 2015, 150, 720–721. [Google Scholar] [CrossRef] [PubMed]
- Bleiziffer, S.; Krane, M.; Deutsch, M.A.; Elhmidi, Y.; Piazza, N.; Voss, B.; Lange, R. Which way in? The Necessity of Multiple Approaches to Transcatheter Valve Therapy. Curr. Cardiol. Rev. 2013, 9, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Zack, C.J.; Al-Qahtani, F.; Kawsara, A.; Al-Hijji, M.; Amin, A.H.; Alkhouli, M. Comparative Outcomes of Surgical and Transcatheter Aortic Valve Replacement for Aortic Stenosis in Nonagenarians. Am. J. Cardiol. 2017, 119, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.C.; Szerlip, M.; Herbert, M.A.; Akram, S.; Worley, C.; Kim, R.J.; Prince, B.A.; Harrington, K.B.; Mack, M.J.; Holper, E.M. Outcomes of Treatment of Nonagenarians with Severe Aortic Stenosis. Ann. Thorac. Surg. 2015, 100, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Murashita, T.; Greason, K.L.; Suri, R.M.; Nkomo, V.T.; Holmes, D.R.; Rihal, C.S.; Mathew, V. Aortic Valve Replacement for Severe Aortic Valve Stenosis in the Nonagenarian Patient. Ann. Thorac. Surg. 2014, 98, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.R.; Magnuson, E.A.; Wang, K.; Lei, Y.; Vilain, K.; Walczak, J.; Kodali, S.K.; Lasala, J.M.; O’Neill, W.W.; Davidson, C.J.; et al. Cost-effectiveness of Transcatheter Aortic Valve Replacement Compared With Standard Care Among Inoperable Patients with Severe Aortic Stenosis: Results from the Placement of Aortic Transcatheter Valves (PARTNER) Trial (Cohort B). Circulation 2012, 125, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.R.; Lei, Y.; Wang, K.; Chinnakondepalli, K.; Vilain, K.A.; Magnuson, E.A.; Galper, B.Z.; Meduri, C.U.; Arnold, S.V.; Baron, S.J.; et al. Cost-Effectiveness of Transcatheter Aortic Valve Replacement With a Self-Expanding Prosthesis Versus Surgical Aortic Valve Replacement. J. Am. Coll. Cardiol. 2016, 67, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Santarpino, G.; Pfeiffer, S.; Jess, J.; Dell'Aquila, A.; Vogt, F.; Wardenburg, C.; Schwab, J.; Sirch, J.; Pauschinger, M. Clinical Outcome and Cost Analysis of Sutureless Versus Transcatheter Aortic Valve Implantation With Propensity Score Matching Analysis. Am. J. Cardiol. 2015, 116, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zegrean, M. Never Too Late: A Case Report on Transcatheter Aortic Valve Implantation in a 97-Year-Old Patient. Geriatrics 2017, 2, 25. https://doi.org/10.3390/geriatrics2030025
Zegrean M. Never Too Late: A Case Report on Transcatheter Aortic Valve Implantation in a 97-Year-Old Patient. Geriatrics. 2017; 2(3):25. https://doi.org/10.3390/geriatrics2030025
Chicago/Turabian StyleZegrean, Mihaela. 2017. "Never Too Late: A Case Report on Transcatheter Aortic Valve Implantation in a 97-Year-Old Patient" Geriatrics 2, no. 3: 25. https://doi.org/10.3390/geriatrics2030025



