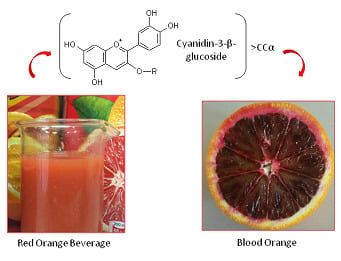
Blood Orange Anthocyanins in Fruit Beverages: How the Commercial Shelf Life Reflects the Quality Parameter
Abstract
:1. Introduction
 | |
| Name | R |
| Cyanidin-3-β-glucoside | Glucose |
| Cyanidin-3-(6”-malonyl)-β-glucoside | Malonyl-Glucose |

2. Materials and Methods
2.1. Chemicals
2.2. Chromatographic Analyses
2.3. Validation Parameters
2.3.1. Accordance
2.3.2. Specificity/Selectivity
2.3.3. Repeatability in Retention Times
2.3.4. Method limit of decision (CCα) and limit of detection (CCβ).
2.4. Samples
3. Results and Discussion
3.1. Method Validation
3.1.1. Accordance
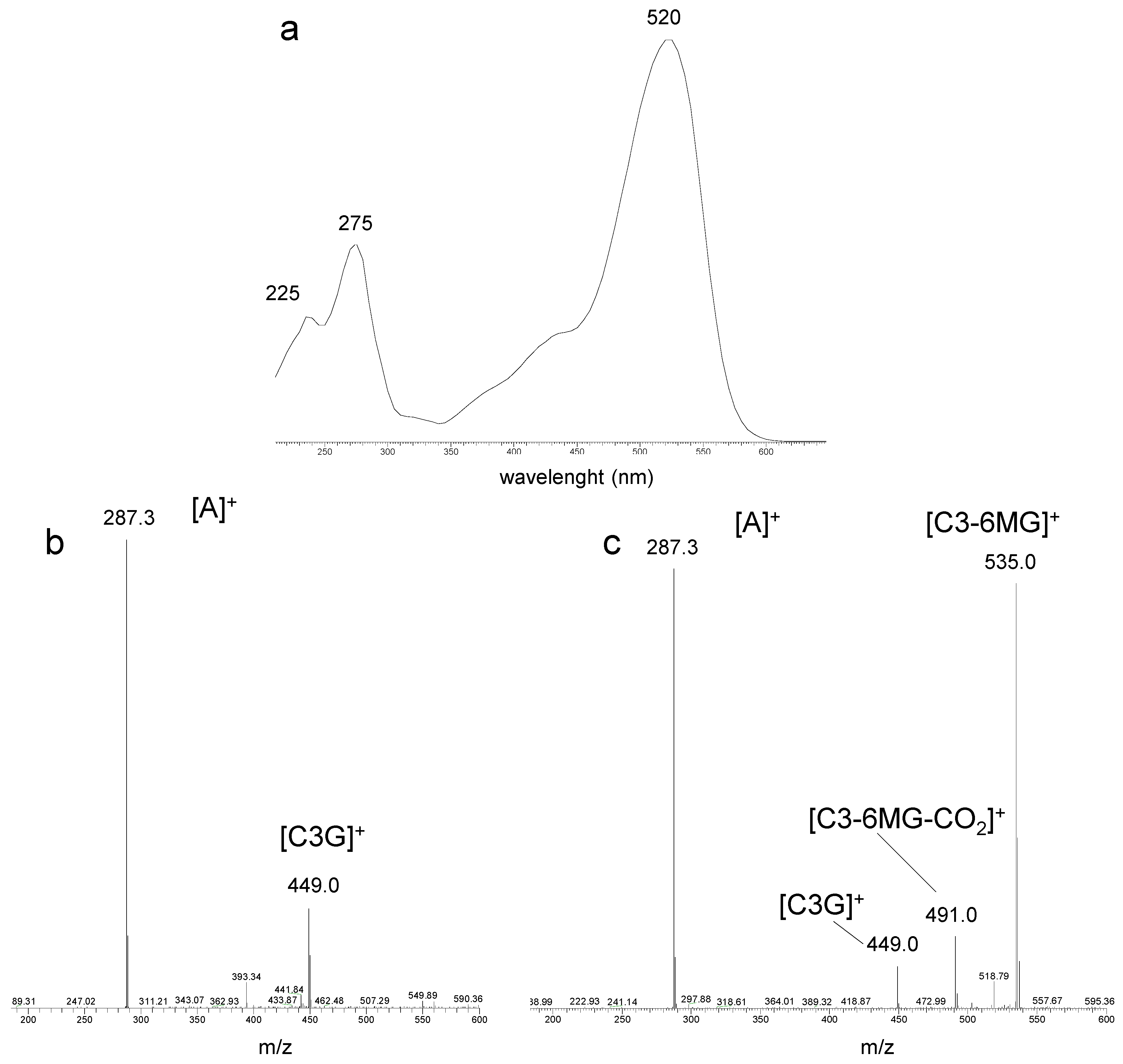
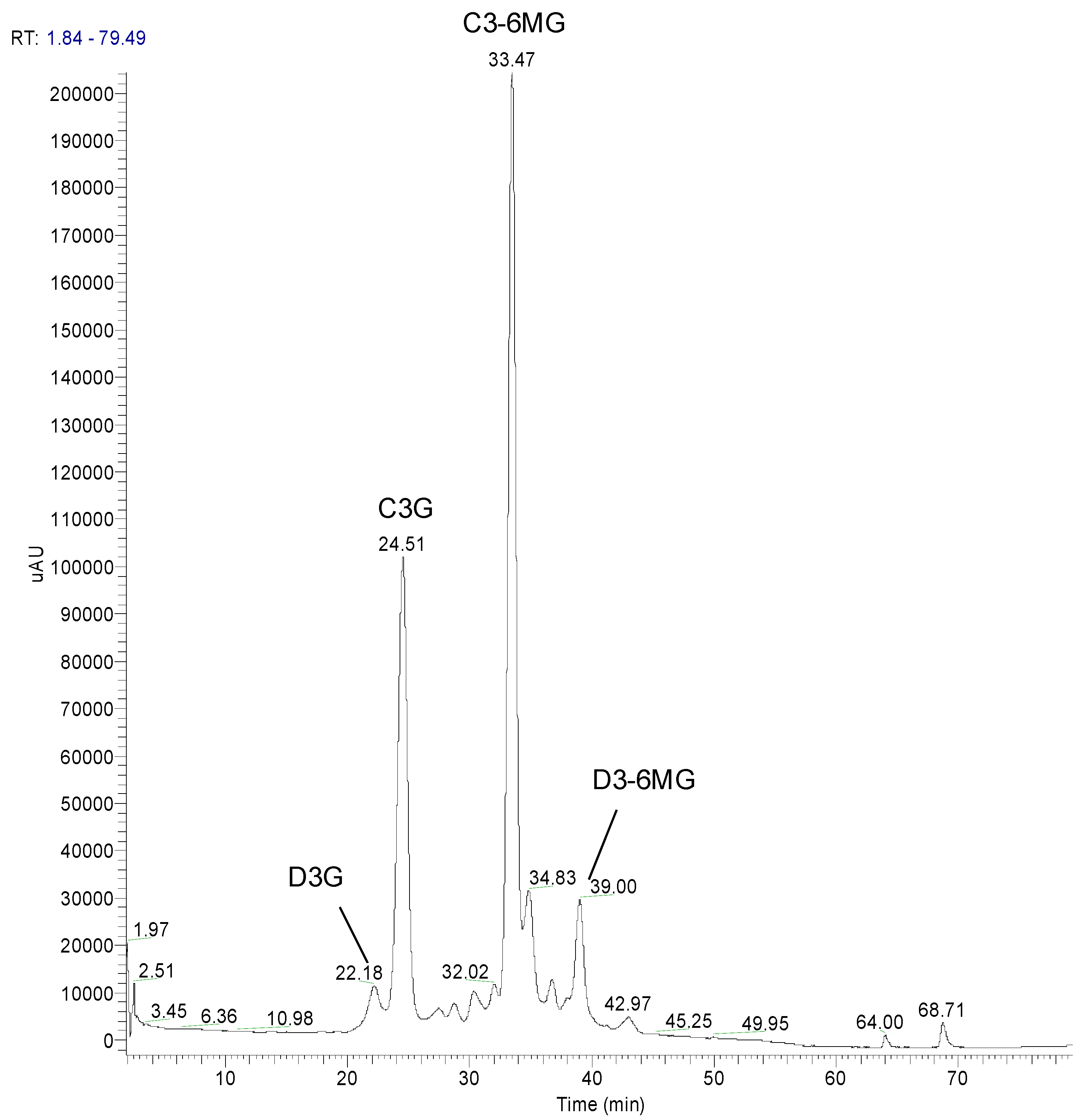
3.1.2. Specificity/Selectivity

3.1.3. Repeatability in Retention Times
3.1.4. Method limit of decision (CCα) and limit of detection (CCβ).
- CCα: 0.36 mg/L;
- CCβ: 0.41 mg/L.
- the presence of blood orange juice in the analyzed sample is confirmed if the amount of cyanidin 3-glucoside >CCα;
- the absence of blood orange juice in the analyzed sample is established if the amount of cyanidin 3-glucoside <CCα.
3.2. Assessment of C3G Occurrence on Commercial Red Orange Fruit Beverages
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Scordino, M.; Sabatino, L. Characterization of polyphenolic profile of Citrus fruit by HPLC/PDA/ESI/MS-MS. In Polyphenols in Plants: Isolation, Purification and Extract Preparation; Watson, R.R., Ed.; Academic Press: London, UK, 2014; pp. 187–199. [Google Scholar]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Horowitz, R.M. The Citrus flavonoids. In The Orange: Its Biochemistry and Physiology; Sinclair, W.B., Ed.; University of California Press: Berkeley, CA, USA, 1961; pp. 334–372. [Google Scholar]
- Maccarone, E.; Rapisarda, P.; Fanella, F.; Arena, E.; Mondello, L. Cyanidin 3-(6”-malonyl)-β-glucoside. One of the major anthocyanins in blood orange juice. Ital. J. Food Sci. 1998, 10, 367–372. [Google Scholar]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid composition of Citrus juice. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef] [PubMed]
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural bioactive compounds of Citrus limon for food and health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Scordino, M.; Sabatino, L.; Belligno, A.; Gagliano, G. Flavonoids and furanocoumarins distribution of unripe Chinotto (Citrus × myrtifolia Rafinesque) fruit: Beverage processing homogenate and juice characterization. Eur. Food Res. Technol. 2011, 233, 759–767. [Google Scholar] [CrossRef]
- Scordino, M.; Sabatino, L.; Traulo, P.; Gargano, M.; Pantó, V.; Gagliano, G. HPLC/PDA/ESI-MS/MS detection of polymethoxylated flavonoids highly degraded citrus juice: A quality control case study. Eur. Food Res. Technol. 2011, 232, 275–280. [Google Scholar] [CrossRef]
- Hwang, S.L.; Shih, P.H.; Yen, G.C. Neuroprotective effects of Citrus flavonoids. J. Agric. Food Chem. 2012, 60, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Dugo, P.; Mondello, L.; Morabito, D.; Dugo, G. Characterization of the anthocyanins fraction of sicilian blood orange juice by micro-HPLC-ESI/MS. J. Agric. Food Chem. 2003, 51, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Dannehl, D.; Josuttis, M. Cultivar and production effects on bioactive polyphenols. In Polyphenols in Plants: Isolation, Purification and Extract Preparation; Watson, R.R., Ed.; Academic Press: London, UK, 2014; pp. 3–13. [Google Scholar]
- Gómez-Caravaca, A.M.; Verardo, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Caboni, M.F. Phenolic compounds and saponins in plants grown under different irrigation regimes. In Polyphenols in Plants: Isolation, Purification and Extract Preparation; Watson, R.R., Ed.; Academic Press: London, UK, 2014; pp. 37–52. [Google Scholar]
- Butelli, E.; Licciardello, C.; Zhang, Y.; Liu, J.; Mackay, S.; Bailey, P.; Reforgiato-Recupero, G.; Martin, C. Retrotransposons control fruit-specific, cold-dependent accumulation of anthocyanins in blood oranges. Plant Cell 2012, 24, 1242–1255. [Google Scholar] [CrossRef] [PubMed]
- Council Directive 2000/13/EC. Labelling, Presentation and Advertising of Foodstuffs. Available online: http://europa.eu/legislation_summaries/consumers/product_labelling_and_packaging/l21090_en.htm (accessed on 19 May 2015).
- Rapisarda, P.; Fanella, F.; Maccarone, E. Reliability of analytical methods for determining anthocyanins in blood orange juices. J. Agric. Food Chem. 2000, 48, 2249–2252. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, X.; Zeng, L.; Liu, H.; Xie, M. Determination of cyanidin-3-glucoside (red kernel food colour) in beverages by high performance liquid chromatography and a study of its degradation by quadruple time-of-flight mass spectrometry. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 1645–1656. [Google Scholar] [PubMed]
- Fabre, N.; Rustan, I.; Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Abad-García, B.; Berrueta, L.A.; Garmón-Lobato, S.; Gallo, B.; Vicente, F. A general analytical strategy for the characterization of phenolic compounds in fruit juices by high performance liquid chromatography with diode array detection coupled to electrospray ionization and triple quadrupole mass spectrometry. J. Chromatogr. A 2009, 1216, 5398–5415. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Silva, M.; Becerra, J.; Schmeda-Hirschmann, G. Direct characterization of phenolic antioxidants in infusions from four Mapuche medicinal plants by liquid chromatography with diode array detection (HPLC-DAD) and electrospray ionization tandem mass spectrometry (HPLC-ESI–MS). Food Chem. 2012, 131, 318–327. [Google Scholar] [CrossRef]
- Magnusson, B.; Örnemark, U. Eurachem Guide: The Fitness for Purpose of Analytical Methods: A Laboratory Guide to Method Validation and Related Topics, Second Edition. Available online: http://www.eurachem.org/images/stories/Guides/pdf/MV_guide_2nd_ed_EN.pdf (accessed on 19 May 2015).
- Di Mauro, A.; Passerini, A.; Rapisarda, P.; Maccarone, E. Distribution of hydroxycinnamic acids as a criterion to evaluate variety and geographical origin of Italian orange juices. Ital. J. Food Sci. 2002, 14, 301–315. [Google Scholar]
- Maccarone, E.; Campisi, S.; Fallico, B.; Rapisarda, P.; Sgarlata, R. Flavor components of Italian orange juices. J. Agric. Food Chem. 1998, 46, 2293–2298. [Google Scholar] [CrossRef]
- Arena, E.; Campisi, S.; Fallico, B.; Maccarone, E. Fatty acids of Italian blood orange juices. J. Agric. Food Chem. 1998, 46, 4138–4143. [Google Scholar] [CrossRef]
- Licciardello, F.; Muratore, G.; Avola, C.; Tomaselli, F.; Maccarone, E. Geographical origin assessment of orange juices by comparison of free aminoacids distribution. Acta Hortic. 2011, 892, 389–394. [Google Scholar]
- Commission Decision of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results. European Commission Decision 2002/657/EC. Available online: http://cemu10.fmv.ulg.ac.be/OSTC/2002657EC.pdf (accessed on 19 May 2015).
- Remini, H.; Mertz, C.; Belbahi, A.; Achir, N.; Dornier, M.; Madani, K. Degradation kinetic modelling of ascorbic acid and colour intensity in pasteurised blood orange juice during storage. Food Chem. 2015, 173, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Torres, B.; Tiwari, B.K.; Patras, A.; Cullen, P.J.; Brunton, N.; Odonnell, C.P. Stability of anthocyanins and ascorbic acid of high pressure processed blood orange juice during storage. Innov. Food Sci. Emerg. Technol. 2011, 12, 93–97. [Google Scholar] [CrossRef]
- Kırca, A.; Cemeroğlu, B. Degradation kinetics of anthocyanins in blood orange juice and concentrate. Food Chem. 2003, 81, 583–587. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scordino, M.; Sabatino, L.; Lazzaro, F.; Borzì, M.A.; Gargano, M.; Traulo, P.; Gagliano, G. Blood Orange Anthocyanins in Fruit Beverages: How the Commercial Shelf Life Reflects the Quality Parameter. Beverages 2015, 1, 82-94. https://doi.org/10.3390/beverages1020082
Scordino M, Sabatino L, Lazzaro F, Borzì MA, Gargano M, Traulo P, Gagliano G. Blood Orange Anthocyanins in Fruit Beverages: How the Commercial Shelf Life Reflects the Quality Parameter. Beverages. 2015; 1(2):82-94. https://doi.org/10.3390/beverages1020082
Chicago/Turabian StyleScordino, Monica, Leonardo Sabatino, Francesco Lazzaro, Marco Antonio Borzì, Maria Gargano, Pasqualino Traulo, and Giacomo Gagliano. 2015. "Blood Orange Anthocyanins in Fruit Beverages: How the Commercial Shelf Life Reflects the Quality Parameter" Beverages 1, no. 2: 82-94. https://doi.org/10.3390/beverages1020082