Improvement of Enzymatic Saccharification and Ethanol Production from Rice Straw Using Recycled Ionic Liquid: The Effect of Anti-Solvent Mixture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass Preparation
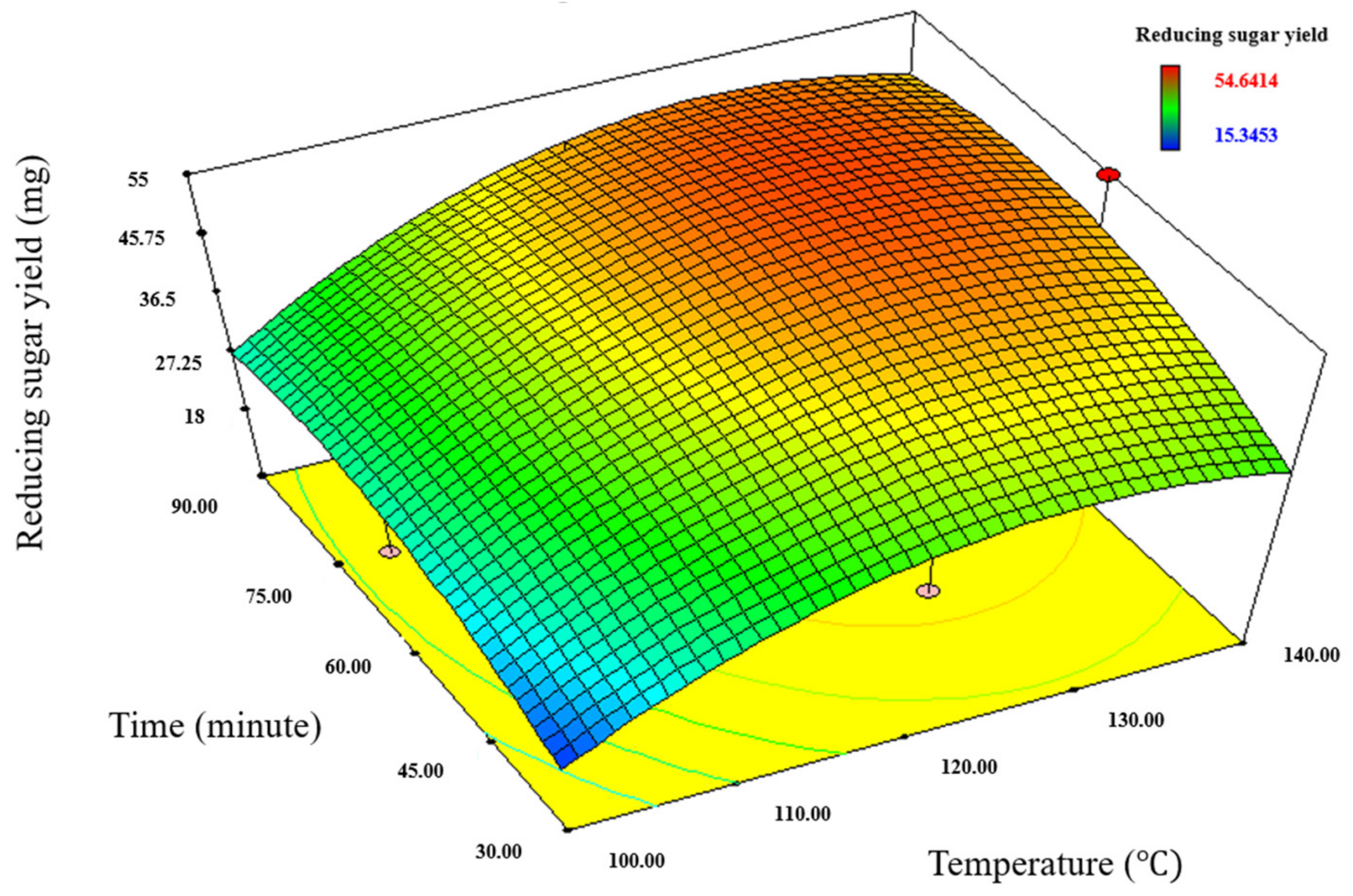
2.2. Optimization of Rice Straw Pretreatment for Reducing Sugar Production
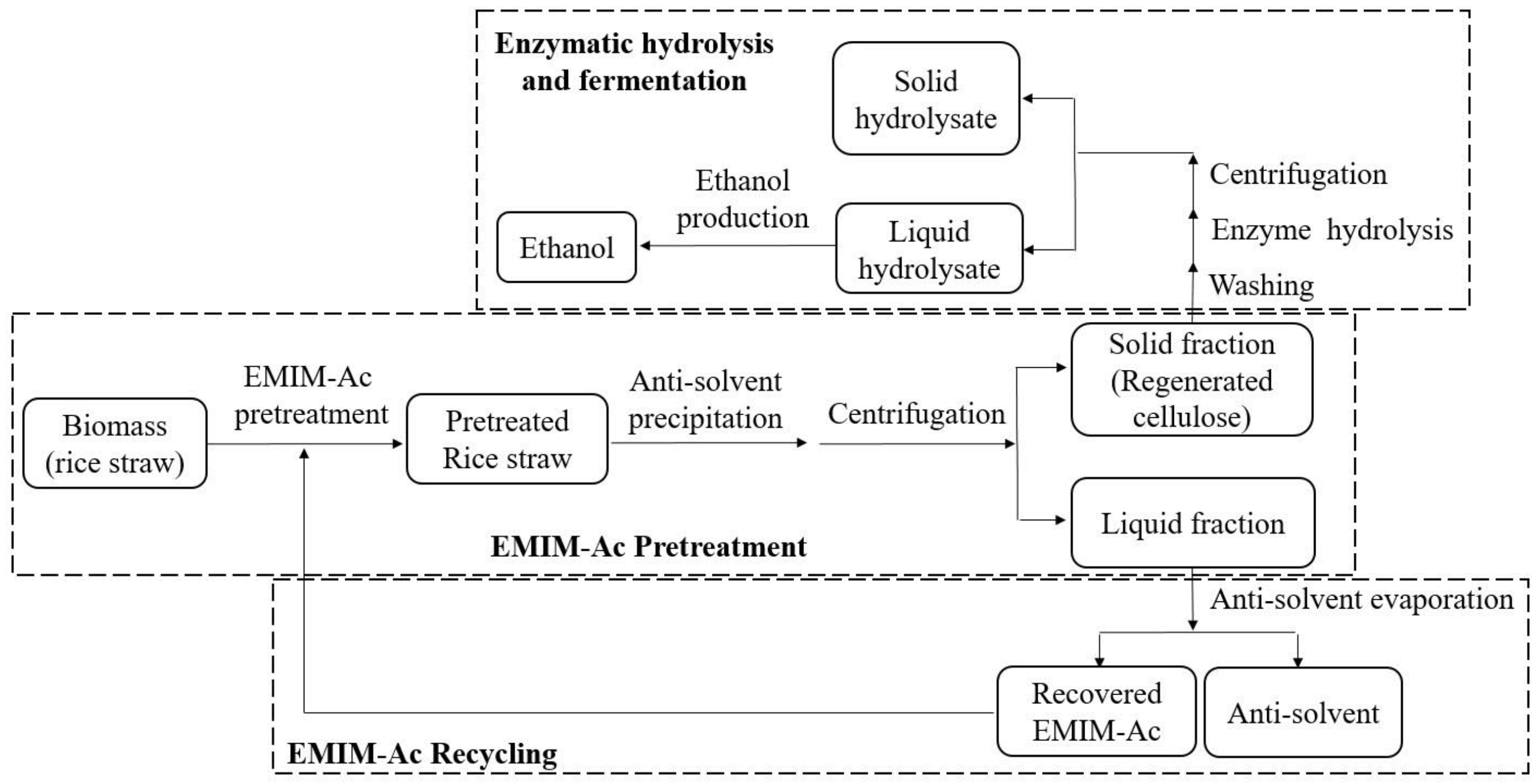
2.3. EMIM-Ac Pretreatment Procedure
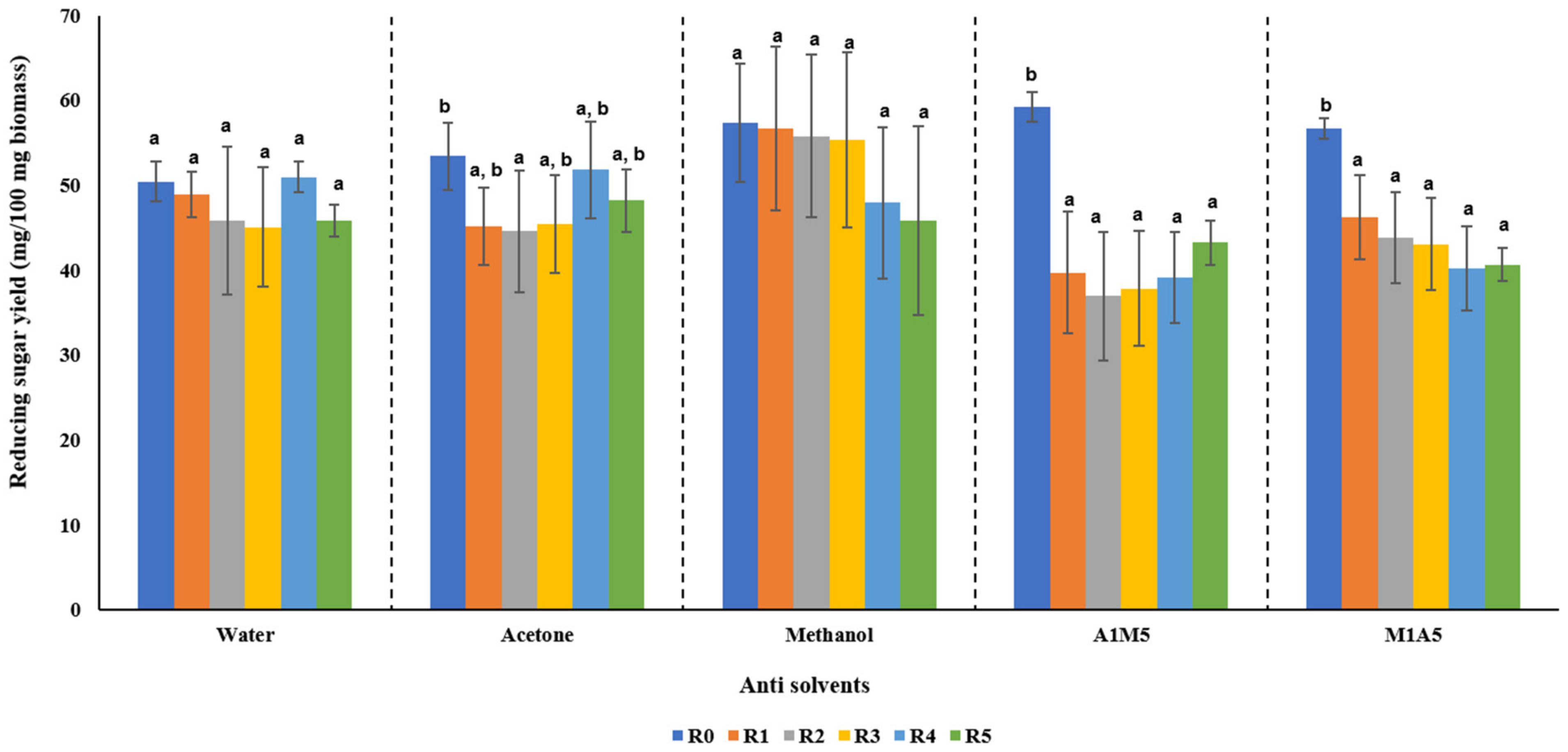
2.4. Recyclability of EMIM-Ac with Different Anti-Solvents
2.5. Enzymatic Hydrolysis and Measurement of a Sugar Yield
2.6. Fermentation and Analysis of Ethanol Yield
2.7. Analysis of Chemical Changes in Biomass
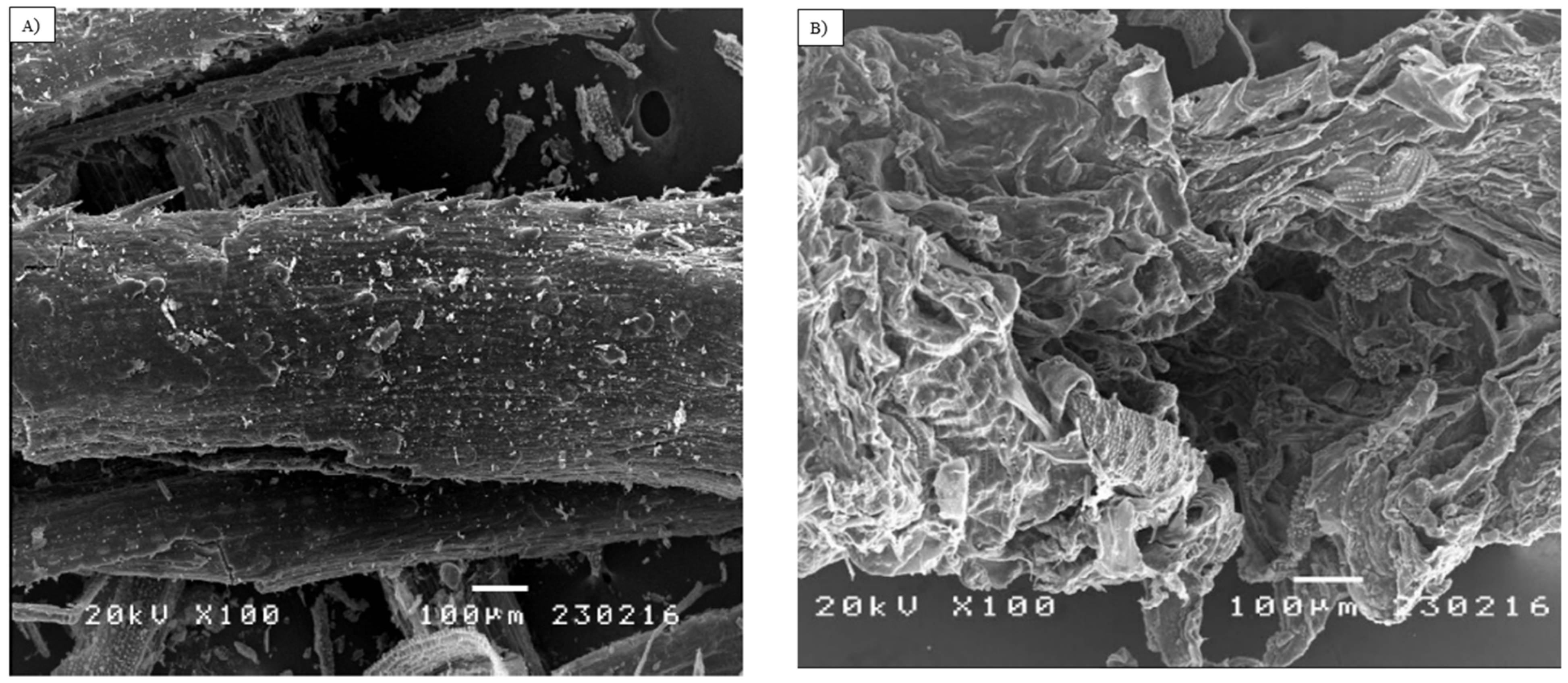
2.8. Analysis of Morphological Changes
3. Results and Discussion
3.1. EMIMAc Pretreatment and Its Optimization
3.2. Analysis of Morphological Changes
3.3. Effect of Different Anti Solvents and Recycled EMIM-Ac on Reducing Sugar Yields
3.4. Analysis of Chemical Changes in Biomass
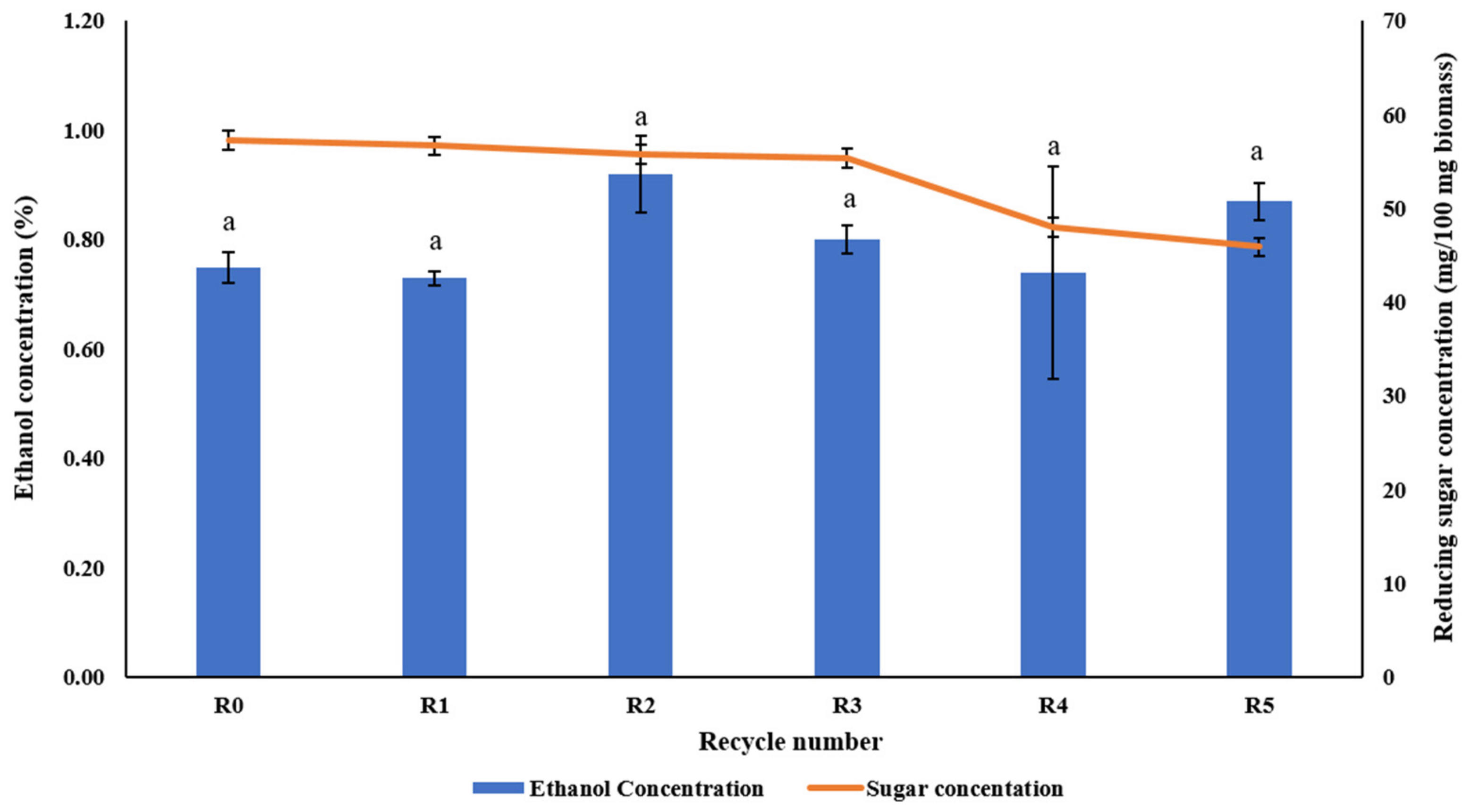
3.5. Effect of Recycled EMIM-AC on Sugar Yield and Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haq, I.U.; Qaisar, K.; Nawaz, A.; Akram, F.; Mukhtar, H.; Zohu, X.; Xu, Y.; Mumtaz, M.W.; Rashid, U.; Ghani, W.A.W.A.K.; et al. Advances in Valorization of Lignocellulosic Biomass towards Energy Generation. Catalysts 2021, 11, 309. [Google Scholar] [CrossRef]
- Sriariyanun, M.; Heitz, J.H.; Yasurin, P.; Asavasanti, S.; Tantayotai, P. Intaconic Acid: A Promising and Sustainable Platform Chemical? Appl. Sci. Eng. Prog. 2019, 12, 75–82. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Karishma, S.; Vo, D.-V.N. Recent advances and sustainable development of biofuels production from lignocellulosic biomass. Bioresour. Technol. 2022, 344, 126203. [Google Scholar] [CrossRef] [PubMed]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Tian, S.-Q.; Zhao, R.-Y.; Chen, Z.-C. Review of the pretreatment and bioconversion of lignocellulosic biomass from wheat straw materials. Renew. Sustain. Energy Rev. 2018, 91, 483–489. [Google Scholar] [CrossRef]
- Qin, L.; Li, W.-C.; Liu, L.; Zhu, J.-Q.; Li, X.; Li, B.-Z.; Yuan, Y.-J. Inhibition of lignin-derived phenolic compounds to cellulase. Biotechnol. Biofuels 2016, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Aftab, M.N.; Iqbal, I.; Riaz, F.; Karadag, A.; Tabatabaei, M. Different Pretreatment Methods of Lignocellulosic Biomass for Use in Biofuel Production. In Biomass for Bioenergy-Recent Trends and Future Challenges; IntechOpen: London, UK, 2019. [Google Scholar]
- Panakkal, E.J.; Cheenkachorn, K.; Gundupalli, M.P.; Kitiborwornkul, N.; Sriariyanun, M. Impact of sulfuric acid pretreatment of durian peel on the production of fermentable sugar and ethanol. J. Indian Chem. Soc. 2021, 98, 100264. [Google Scholar] [CrossRef]
- Norrrahim, M.N.F.; Ilyas, R.A.; Nurazzi, N.M.; Rani, M.S.A.; Atikah, M.S.N.; Shazleen, S.S. Chemical Pretreatment of Lignocellulosic Biomass for the Production of Bioproducts: An Overview. Appl. Sci. Eng. Prog. 2021, 14, 588–605. [Google Scholar] [CrossRef]
- Areepak, C.; Jiradechakorn, T.; Chuetor, S.; Phalakornkule, C.; Sriariyanun, M.; Raita, M.; Champreda, V.; Laosiripojana, N. Improvement of lignocellulosic pretreatment efficiency by combined chemo-Mechanical pretreatment for energy consumption reduction and biofuel production. Renew. Energy 2021, 182, 1094–1102. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, D.L.; Rodríguez-Jasso, R.M.; Zanuso, E.; Lara-Flores, A.A.; Aguilar, C.N.; Sanchez, A.; Ruiz, H.A. Operational Strategies for Enzymatic Hydrolysis in a Biorefinery. In Biorefining of Biomass to Biofuels: Opportunities and Perception; Kumar, S., Sani, R.K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 223–248. [Google Scholar]
- Zhao, X.; Liu, D. Multi-products co-production improves the economic feasibility of cellulosic ethanol: A case of Formiline pretreatment-based biorefining. Appl. Energy 2019, 250, 229–244. [Google Scholar] [CrossRef]
- Tayyab, M.; Noman, A.; Islam, W.; Waheed, S.; Arafat, Y.; Ali, F.; Zaynab, M.; Lin, S.; Zhang, H.; Khan, D. Bioethanol production from lignocellulosic biomass by environment-friendly pretreatment methods: A review. Appl. Ecol. Environ. Res. 2018, 16, 225–249. [Google Scholar] [CrossRef]
- Abushammala, H.; Mao, J. A Review on the Partial and Complete Dissolution and Fractionation of Wood and Lignocelluloses Using Imidazolium Ionic Liquids. Polymers 2020, 12, 195. [Google Scholar] [CrossRef] [Green Version]
- Casas, A.; Palomar, J.; Alonso, M.V.; Oliet, M.; Omar, S.; Rodriguez, F. Comparison of lignin and cellulose solubilities in ionic liquids by COSMO-RS analysis and experimental validation. Ind. Crop. Prod. 2012, 37, 155–163. [Google Scholar] [CrossRef]
- Nguyen, T.-A.D.; Kim, K.-R.; Han, S.J.; Cho, H.Y.; Kim, J.W.; Park, S.M.; Park, J.C.; Sim, S.J. Pretreatment of rice straw with ammonia and ionic liquid for lignocellulose conversion to fermentable sugars. Bioresour. Technol. 2010, 101, 7432–7438. [Google Scholar] [CrossRef]
- Reddy, P. A critical review of ionic liquids for the pretreatment of lignocellulosic biomass. S. Afr. J. Sci. 2015, 111, 1–9. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Hu, L.; Xia, J.; Wu, Z.; Xu, N.; Dai, B.; Wu, B. A novel ionic liquid-tolerant Fusarium oxysporum BN secreting ionic liquid-stable cellulase: Consolidated bioprocessing of pretreated lignocellulose containing residual ionic liquid. Bioresour. Technol. 2015, 181, 18–25. [Google Scholar] [CrossRef]
- Trivedi, N.; Gupta, V.; Reddy, C.; Jha, B. Detection of ionic liquid stable cellulase produced by the marine bacterium Pseudoalteromonas sp. isolated from brown alga Sargassum polycystum C. Agardh. Bioresour. Technol. 2013, 132, 313–319. [Google Scholar] [CrossRef]
- Auxenfans, T.; Buchoux, S.; Larcher, D.; Husson, G.; Husson, E.; Sarazin, C. Enzymatic saccharification and structural properties of industrial wood sawdust: Recycled ionic liquids pretreatments. Energy Convers. Manag. 2014, 88, 1094–1103. [Google Scholar] [CrossRef]
- Lopes, A.S.D.C.; Joao, K.G.; Morais, A.R.C.; Lukasik, E.B.; Lukasik, R.B. Ionic liquids as a tool for lignocellulosic biomass fractionation. Sustain. Chem. Processes 2013, 1, 1–31. [Google Scholar]
- Auxenfans, T.; Buchoux, S.; Djellab, K.; Avondo, C.; Husson, E.; Sarazin, C. Mild pretreatment and enzymatic saccharification of cellulose with recycled ionic liquids towards one-batch process. Carbohydr. Polym. 2012, 90, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Weerachanchai, P.; Lee, J.-M. Recyclability of an ionic liquid for biomass pretreatment. Bioresour. Technol. 2014, 169, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Hamidah, U.; Arakawa, T.; H’Ng, Y.Y.; Nakagawa-Izumi, A.; Kishino, M. Recycled ionic liquid 1-ethyl-3-methylimidazolium acetate pretreatment for enhancing enzymatic saccharification of softwood without cellulose regeneration. J. Wood Sci. 2017, 64, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccha-rides in Relation to Animal Nutrition. Diary Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Box, G.E.P.; Wilson, K.B. On the Experimental Attainment of Optimum Conditions on JSTOR. J.R. Stat. Soc. 1951, 13, 1–45. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Amnuaycheewa, P.; Hengaroonprasan, R.; Rattanaporn, K.; Kirdponpattara, S.; Cheenkachorn, K.; Sriariyanun, M. Enhancing enzymatic hydrolysis and biogas production from rice straw by pretreatment with organic acids. Ind. Crop. Prod. 2016, 87, 247–254. [Google Scholar] [CrossRef]
- Rattanaporn, K.; Tantayotai, P.; Phusantisampan, T.; Pornwongthong, P.; Sriariyanun, M. Organic acid pretreatment of oil palm trunk: Effect on enzymatic saccharification and ethanol production. Bioprocess Biosyst. Eng. 2018, 41, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Panakkal, E.J.; Sriariyanun, M.; Ratanapoompinyo, J.; Yasurin, P.; Cheenkachorn, K.; Rodiahwati, W.; Tantayotai, P. Influence of Sulfuric Acid Pretreatment and Inhibitor of Sugarcane Bagasse on the Production of Fermentable Sugar and Ethanol. Appl. Sci. Eng. Prog. 2021, 15. [Google Scholar] [CrossRef]
- Gundupalli, M.P.; Cheng, Y.-S.; Chuetor, S.; Bhattacharyya, D.; Sriariyanun, M. Effect of dewaxing on saccharification and ethanol production from different lignocellulosic biomass. Bioresour. Technol. 2021, 339, 125596. [Google Scholar] [CrossRef] [PubMed]
- Gundupalli, M.P.; Chuetor, S.; Cheenkachorn, K.; Rattanaporn, K.; Show, P.-L.; Cheng, Y.-S.; Sriariyanun, M. Interferences of Waxes on Enzymatic Saccharification and Ethanol Production from Lignocellulose Biomass. Bioengineering 2021, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.S.; Lee, S.-H.; Endo, T.; Bon, E.P. Major improvement in the rate and yield of enzymatic saccharification of sugarcane bagasse via pretreatment with the ionic liquid 1-ethyl-3-methylimidazolium acetate ([Emim] [Ac]). Bioresour. Technol. 2011, 102, 10505–10509. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Ionic liquid-mediated selective extraction of lignin from wood leading to enhanced enzymatic cellulose hydrolysis. Biotechnol. Bioeng. 2008, 102, 1368–1376. [Google Scholar] [CrossRef]
- Li, C.; Knierim, B.; Manisseri, C.; Arora, R.; Scheller, H.; Auer, M.; Vogel, K.P.; Simmons, B.; Singh, S. Comparison of dilute acid and ionic liquid pretreatment of switchgrass: Biomass recalcitrance, delignification and enzymatic saccharification. Bioresour. Technol. 2010, 101, 4900–4906. [Google Scholar] [CrossRef] [Green Version]
- Tantayotai, P.; Gundupalli, M.P.; Panakkal, E.J.; Sriariyanun, M.; Rattanaporn, K.; Bhattacharyya, D. Differential Influence of Imidazolium Ionic Liquid on Cellulase Kinetics in Saccharification of Cellulose and Lignocellulosic Biomass Substrate. Appl. Sci. Eng. Prog. 2021, 15, 5510. [Google Scholar] [CrossRef]
- Rao, J.; Kumar, B. 3D Blade root shape optimization. In Proceedings of the 10th International Conference on Vibrations in Rotating Machinery, London, UK, 11–13 September 2012. [Google Scholar]
- Poy, H.; Lladosa, E.; Gabaldón, C.; Loras, S. Optimization of rice straw pretreatment with 1-ethyl-3-methylimidazolium acetate by the response surface method. Biomass-Convers. Biorefinery 2021, 1–16. [Google Scholar] [CrossRef]
- Raeisi, S.M.; Tabatabaei, M.; Ayati, B.; Ghafari, A.; Mood, S.H. A Novel Combined Pretreatment Method for Rice Straw Using Optimized EMIM[Ac] and Mild NaOH. Waste Biomass-Valorization 2015, 7, 97–107. [Google Scholar] [CrossRef]
- Nakasu, P.Y.S.; Pin, T.C.; Hallett, J.P.; Rabelo, S.C.; Costa, A.C. In-depth process parameter investigation into a protic ionic liquid pretreatment for 2G ethanol production. Renew. Energy 2021, 172, 816–828. [Google Scholar] [CrossRef]
- Wei, H.L.; Wang, Y.T.; Hong, Y.Y.; Zhu, M.J. Pretreatment of rice straw with recycled ionic liquids by phase-separation process for low-cost biorefinery. Biotechnol. Appl. Biochem. 2020, 68, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Haarmeyer, C.; Balan, V.; Whitehead, T.A.; Dale, B.E.; Chundawat, S.P. Lignin triggers irreversible cellulase loss during pretreated lignocellulosic biomass saccharification. Biotechnol. Biofuels 2014, 7, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahar, P. Synergistic Effects of Pretreatment Process on Enzymatic Digestion of Rice Straw for Efficient Ethanol Fermentation. In Environmental Biotechnology-New Approaches and Prospective Applications; BoD–Books on Demand: Norderstedt, Germany, 2013. [Google Scholar]
- Dash, M.; Mohanty, K. Effect of different ionic liquids and anti-solvents on dissolution and regeneration of Miscanthus towards bioethanol. Biomass-Bioenergy 2019, 124, 33–42. [Google Scholar] [CrossRef]
- Dai, Y.; Si, M.; Chen, Y.; Zhang, N.; Zhou, M.; Liao, Q.; Shi, D.; Liu, Y.-N. Combination of biological pretreatment with NaOH/Urea pretreatment at cold temperature to enhance enzymatic hydrolysis of rice straw. Bioresour. Technol. 2015, 198, 725–731. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, H.; Han, H.; Qiu, Z.; Achal, V. Stimulatory effect of in-situ detoxification on bioethanol production by rice straw. Energy 2017, 135, 32–39. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. IR Study on Cellulose with the Varied Moisture Contents: Insight into the Supramolecular Structure. Materials 2020, 13, 4573. [Google Scholar] [CrossRef]
- Poornejad, N.; Karimi, K.; Behzad, T. Ionic Liquid Pretreatment of Rice Straw to Enhance Saccharification and Bioethanol Production. J. Biomass-Biofuel 2014, 1, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Kubovský, I.; Kačíková, D.; Kačík, F. Structural Changes of Oak Wood Main Components Caused by Thermal Modification. Polymers 2020, 12, 485. [Google Scholar] [CrossRef] [Green Version]
- Han, S.Y.; Park, C.W.; Park, J.B.; Ha, S.J.; Kim, N.H.; Lee, S.H. Ethanol Fermentation of the Enzymatic Hydrolysates from the Products Pretreated using [EMIM]Ac and Its Co-Solvents with DMF. J. For. Environ. Sci. 2020, 36, 62–66. [Google Scholar] [CrossRef]
- Elmacı, S.B.; Ozcelik, F. Ionic liquid pretreatment of yellow pine followed by enzymatic hydrolysis and fermentation. Biotechnol. Prog. 2018, 34, 1242–1250. [Google Scholar] [CrossRef]
- Lara-Serrano, M.; Angulo, F.S.; Negro, M.J.; Morales-Delarosa, S.; Campos-Martin, J.M.; Fierro, J.L.G. Second-Generation Bioethanol Production Combining Simultaneous Fermentation and Saccharification of IL-Pretreated Barley Straw. ACS Sustain. Chem. Eng. 2018, 6, 7086–7095. [Google Scholar] [CrossRef]
- Rezania, S.; Alizadeh, H.; Park, J.; Md Din, M.F.; Darajeh, N.; Shafiei Ebrahimi, S.; Saha, B.B.; Kamyab, H. Effect of various pretreatment methods on sugar and ethanol production from cellulosic water hyacinth. BioResources 2019, 14, 592–606. [Google Scholar]

| Pretreatment Factor | Level of Factor | ||
|---|---|---|---|
| Low | Med | High | |
| Abbreviation | −1 | 0 | 1 |
| Loading ratio (wt%) (X1) | 5 | 10 | 15 |
| Temperature (°C) (X2) | 100 | 120 | 140 |
| Time (min) (X3) | 30 | 60 | 90 |
| Run | Pretreatment Condition | Reducing Sugar (Y) (mg) | ||
|---|---|---|---|---|
| Loading Ratio (X1) (%) | Temperature (X2) (°C) | Time (X3) (min) | ||
| 1 | 15 | 120 | 30 | 22.36 |
| 2 | 5 | 120 | 90 | 44.19 |
| 3 | 5 | 140 | 60 | 54.64 |
| 4 | 10 | 120 | 60 | 48.31 |
| 5 | 15 | 120 | 90 | 45.14 |
| 6 | 10 | 120 | 60 | 43.52 |
| 7 | 10 | 120 | 60 | 45.70 |
| 8 | 15 | 100 | 60 | 16.99 |
| 9 | 10 | 120 | 60 | 46.57 |
| 10 | 5 | 120 | 30 | 32.48 |
| 11 | 10 | 100 | 30 | 15.35 |
| 12 | 15 | 140 | 60 | 29.95 |
| 13 | 5 | 100 | 60 | 23.09 |
| 14 | 10 | 120 | 60 | 44.10 |
| 15 | 10 | 140 | 90 | 31.90 |
| 16 | 10 | 140 | 30 | 40.70 |
| 17 | 10 | 100 | 90 | 27.98 |
| EMIM-Ac Pretreatment | Mathematical models | Sugar content (mg) = - 429.80831 − (0.99874 × Conc.) + (7.03793 × Temp.) + (0.97107 × Time) − (0.027403 × Temp2) − (0.00676158 × Time2) |
| Optimal pretreatment condition | 5% loading ratio, 128.4 °C temperature, 71.83 min time | |
| Predicted sugar yield | 51.96 mg |
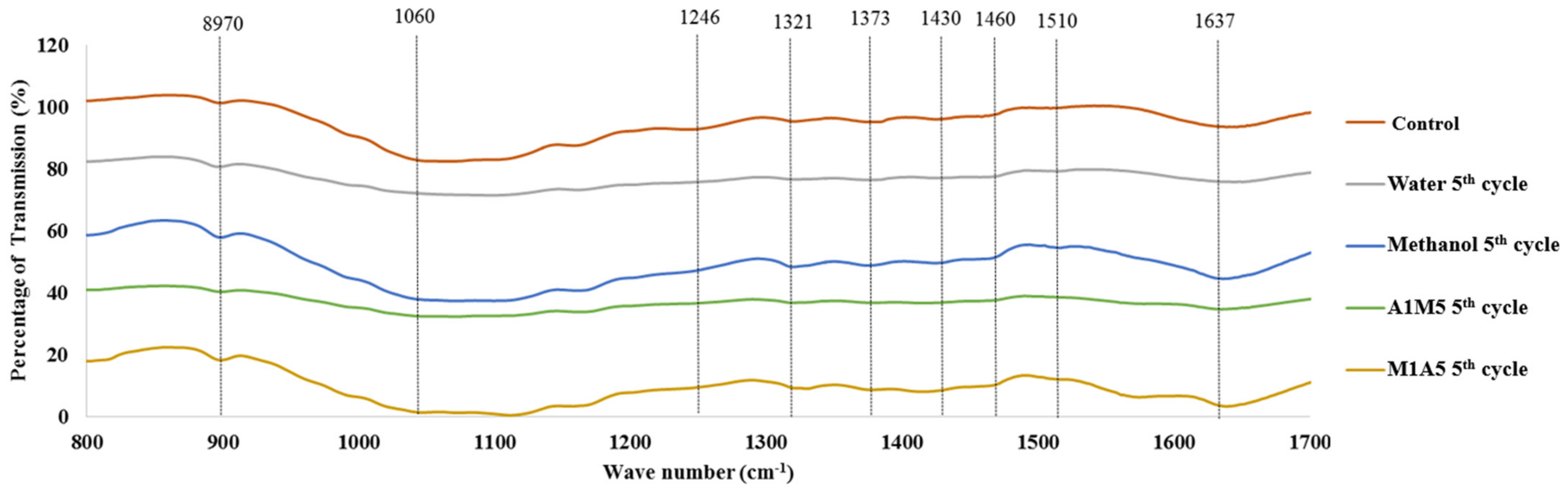
| Peak, cm–1 | Functional Group Assignment | References |
|---|---|---|
| 897 | β-glycosidic linkage; vibration of amorphous cellulose | [48,50] |
| 1060 | Bond Stretching in C–O of homo and heteropolysaccharide | [38] |
| 1246 | C–O stretching of phenolics in lignin | [46] |
| 1321 | Stretching vibration of C=O in syringyl, guaiacyl group | [33] |
| 1373 | Deformation of C–H in homo and heteropolysaccharide | [38] |
| 1430 | C–H2 bending of cellulose | [51] |
| 1460 | Deformations in C–H bonds of lignin | [52] |
| 1510 | Vibration in aromatic skeleton of lignin | [48] |
| 1637 | Phenolics in lignin | [46] |
| Biomass | Pretreatment Conditions | Anti-Solvent | Sugar Concentration | Ethanol Concentration | References |
|---|---|---|---|---|---|
| Wood powder | 15% solid loading, 120 °C, 2 h | Dimethyl formamide | Glucose: 31 g/100 g biomass Xylose: 314.4 g/100 g biomass | 3 g/L | [53] |
| Yellow pine wood | 5% solid loading, 140 °C, 45 min | - | 26.89 g/100 g biomass | 2.6 g/L | [54] |
| Barely straw | 5.26% solid loading, 105 °C, 7.5 h | Water | 36.3 g glucose/100 g biomass 13.2 Xylose/100 g biomass | 18.5 g/L | [55] |
| Water hyacinth | 5.89% solid loading, 120 °C, 180 min | Water | 4.5 g/100 g biomass | 1.3 g/L | [56] |
| Rice straw | 15% solid loading, 120 °C, 5 h | Water | 44.3 g glucose/100 g biomass | 1.92 g/L | [51] |
| Rice straw | R0, 5% solid loading, 128.4 °C, 71.83 min | Methanol | 57.3 mg/100 mg biomass | 5.9 g/L | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuetor, S.; Panakkal, E.J.; Ruensodsai, T.; Cheenkachorn, K.; Kirdponpattara, S.; Cheng, Y.-S.; Sriariyanun, M. Improvement of Enzymatic Saccharification and Ethanol Production from Rice Straw Using Recycled Ionic Liquid: The Effect of Anti-Solvent Mixture. Bioengineering 2022, 9, 115. https://doi.org/10.3390/bioengineering9030115
Chuetor S, Panakkal EJ, Ruensodsai T, Cheenkachorn K, Kirdponpattara S, Cheng Y-S, Sriariyanun M. Improvement of Enzymatic Saccharification and Ethanol Production from Rice Straw Using Recycled Ionic Liquid: The Effect of Anti-Solvent Mixture. Bioengineering. 2022; 9(3):115. https://doi.org/10.3390/bioengineering9030115
Chicago/Turabian StyleChuetor, Santi, Elizabeth Jayex Panakkal, Thanagorn Ruensodsai, Kraipat Cheenkachorn, Suchata Kirdponpattara, Yu-Shen Cheng, and Malinee Sriariyanun. 2022. "Improvement of Enzymatic Saccharification and Ethanol Production from Rice Straw Using Recycled Ionic Liquid: The Effect of Anti-Solvent Mixture" Bioengineering 9, no. 3: 115. https://doi.org/10.3390/bioengineering9030115








