Development of High Cell Density Cultivation Strategies for Improved Medium Chain Length Polyhydroxyalkanoate Productivity Using Pseudomonas putida LS46
Abstract
:1. Introduction
2. Materials and Methods
2.1. Micro-Organism, Medium, and Substrates
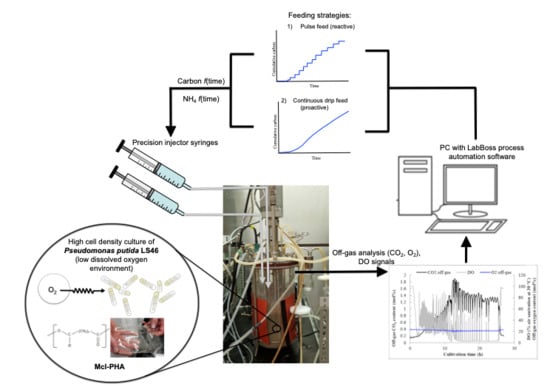
2.2. Reactor Setup and Operation
2.3. Feeding Strategies
2.4. Measurement of CO2 and Mass Balancing
2.5. Yield Coefficients
2.6. Sample Treatment
2.7. Measurement of Residual Carbon, NH4-N PO43−-P, and Trace Medium Components
3. Results
3.1. Pulse Feed Strategy
3.2. Residual Concentrations of Octanoic Acid, NH4+-N, and PO43−-P
3.3. Residual Trace Elements
3.4. Modeling of Feeding Rates—Continuous Drip Feed Strategy
3.5. PHA Composition
3.6. Carbon Flux and Yield Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CDM | cell dry mass (g L−1) |
| C(t) | cumulative octanoic acid uptake as a function of time (g L−1) |
| N(t) | cumulative (NH4)2SO4 uptake as a function of time (g L−1) |
| Qv | overall volumetric productivity of PHA (g L−1 h−1) |
| Qs | specific productivity or PHA synthesis rate (g g Xr−1 h−1) |
| XPHA | PHA biomass (g) |
| Xr | non-PHA (residual) cell mass (g) |
| Xt | total biomass (g) |
| YPHA/S | yield coefficient of PHA per unit carbon substrate consumed (g g−1 or C-mol C-mol−1) |
| YXr/N | yield coefficient of non-PHA cell mass per unit ammonium consumed (g g−1 or mol mol−1) |
| YXr/S | yield coefficient of non-PHA cell mass per unit carbon substrate consumed (g g−1 or C-mol C-mol−1) |
| YX/S | yield coefficient of total biomass per unit carbon substrate consumed (g g−1 or mol mol−1) |
| μavg/Xr | average specific growth rate over a defined period (h−1, calculated using increases in Xr) |
| %PHA | intracellular PHA content (% of CDM) |
| [ ] | concentration braces (g L−1 or mg L−1) |
References
- Dauvergne, P. The power of environmental norms: Marine plastic pollution and the politics of microbeads. Environ. Polit. 2018, 27, 579–597. [Google Scholar] [CrossRef]
- Schnurr, R.E.J.; Alboiu, V.; Chaudhary, M.; Corbett, R.A.; Quanz, M.E.; Sankar, K.; Srain, H.S.; Thavarajah, V.; Xanthos, D.; Walker, T.R. Reducing marine pollution from single-use plastics (SUPs): A review. Mar. Pollut. Bull. 2018, 137, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.R.; Xanthos, D. A call for Canada to move toward zero plastic waste by reducing and recycling single-use plastics. Resour. Conserv. Recycl. 2018, 133, 99–100. [Google Scholar] [CrossRef]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The chemomechanical properties of microbial polyhydroxyalkanoates. Prog. Polym. Sci. 2013, 38, 536–583. [Google Scholar] [CrossRef]
- Noda, I.; Lindsey, S.B.; Caraway, D. NodaxTM Class PHA Copolymers: Their Properties and Applications. In Plastics from Bacteria; Chen, G.G.-Q., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 14, pp. 237–255. [Google Scholar]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, J.; Chen, G.-Q. Polyhydroxyalkanoates, challenges and opportunities. Curr. Opin. Biotechnol. 2014, 30, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q. New challenges and opportunities for industrial biotechnology. Microb. Cell Factories 2012, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Możejko-Ciesielska, J.; Kiewisz, R. Bacterial polyhydroxyalkanoates: Still fabulous? Microbiol. Res. 2016, 192, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Roy, I. Strategies for large-scale production of polyhydroxyalkanoates. Chem. Biochem. Eng. Q. 2015, 29, 157–172. [Google Scholar] [CrossRef]
- Koller, M.; Maršálek, L.; de Sousa Dias, M.M.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef]
- Koller, M.; Braunegg, G. Advanced approaches to produce polyhydroxyalkanoate (PHA) biopolyesters in a sustainable and economic fashion. EuroBiotech J. 2018, 2, 89–103. [Google Scholar] [CrossRef] [Green Version]
- Heijnen, J.J.; Terwisscha van Scheltinga, A.H.; Straathof, A.J. Fundamental bottlenecks in the application of continuous bioprocesses. J. Biotechnol. 1992, 22, 3–20. [Google Scholar] [CrossRef]
- Ienczak, J.L.; Schmidell, W.; de Aragão, G.M.F. High-cell-density culture strategies for polyhydroxyalkanoate production: A review. J. Ind. Microbiol. Biotechnol. 2013, 40, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Cerrone, F.; Duane, G.; Casey, E.; Davis, R.; Belton, I.; Kenny, S.T.; Guzik, M.W.; Woods, T.; Babu, R.P.; O’Connor, K. Fed-batch strategies using butyrate for high cell density cultivation of Pseudomonas putida and its use as a biocatalyst. Appl. Microbiol. Biotechnol. 2014, 98, 9217–9228. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Sun, Z.; Ramsay, J.A.; Ramsay, B.A. Fed-batch production of MCL-PHA with elevated 3-hydroxynonanoate content. AMB Express 2013, 3, 50. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Wong, H.H.; Choi, J.; Lee, S.H.; Lee, S.C.; Han, C.S. Production of medium-chain-length polyhydroxyalkanoates by high-cell-density cultivation of Pseudomonas putida under phosphorus limitation. Biotechnol. Bioeng. 2000, 68, 466–470. [Google Scholar] [CrossRef]
- Maclean, H.; Sun, Z.; Ramsay, J.A.; Ramsay A., B. Decaying exponential feeding of nonanoic acid for the production of medium-chain-length poly(3-hydroxyalkanoates) by Pseudomonas putida KT2440. Can. J. Chem. 2008, 86, 564–569. [Google Scholar] [CrossRef]
- Ryu, H.W.; Hahn, S.K.; Chang, Y.K.; Chang, H.N. Production of poly (3-hydroxybutyrate) by high cell density fed-batch culture of Alcaligenes eutrophus with phospate limitation. Biotechnol. Bioeng. 1997, 55, 28–32. [Google Scholar] [CrossRef]
- Shang, L.; Jiang, M.; Chang, H.N. Poly (3-hydroxybutyrate) synthesis in fed-batch culture of Ralstonia eutropha with phosphate limitation under different glucose concentrations. Biotechnol. Lett. 2003, 25, 1415–1419. [Google Scholar] [CrossRef]
- Wang, F.; Lee, S.Y. Poly (3-hydroxybutyrate) production with high productivity and high polymer content by a fed-batch culture of Alcaligenes latus under nitrogen limitation. Appl. Environ. Microbiol. 1997, 63, 3703–3706. [Google Scholar]
- Blunt, W.; Levin, D.; Cicek, N. Bioreactor Operating Strategies for Improved Polyhydroxyalkanoate (PHA) Productivity. Polymers 2018, 10, 1197. [Google Scholar] [CrossRef] [PubMed]
- Bylund, F.; Collet, E.; Enfors, S.-O.; Larsson, G. Substrate gradient formation in the large-scale bioreactor lowers cell yield and increases by-product formation. Bioprocess Eng. 1998, 18, 171. [Google Scholar] [CrossRef]
- Lara, A.R.; Galindo, E.; Ramírez, O.T.; Palomares, L.A. Living with heterogeneities in bioreactors. Mol. Biotechnol. 2006, 34, 355–381. [Google Scholar] [CrossRef]
- Koller, M.; Sandholzer, D.; Salerno, A.; Braunegg, G.; Narodoslawsky, M. Biopolymer from industrial residues: Life cycle assessment of poly (hydroxyalkanoates) from whey. Resour. Conserv. Recycl. 2013, 73, 64–71. [Google Scholar] [CrossRef]
- Blunt, W.; Dartiailh, C.; Sparling, R.; Gapes, D.; Levin, D.B.; Cicek, N. Carbon flux to growth or polyhydroxyalkanoate synthesis under microaerophilic conditions is affected by fatty acid chain-length in Pseudomonas putida LS46. Appl. Microbiol. Biotechnol. 2018, 102, 6437–6449. [Google Scholar] [CrossRef] [PubMed]
- Blunt, W.; Dartiailh, C.; Sparling, R.; Gapes, D.; Levin, D.B.; Cicek, N. Microaerophilic environments improve the productivity of medium chain length polyhydroxyalkanoate biosynthesis from fatty acids in Pseudomonas putida LS46. Process Biochem. 2017, 59, 18–25. [Google Scholar] [CrossRef]
- Riesenberg, D.; Guthke, R. High-cell-density cultivation of microorganisms. Appl. Microbiol. Biotechnol. 1999, 51, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Ramsay, J.A.; Guay, M.; Ramsay, B.A. Automated feeding strategies for high-cell-density fed-batch cultivation of Pseudomonas putida KT2440. Appl. Microbiol. Biotechnol. 2006, 71, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Kellerhals, M.B.; Kessler, B.; Witholt, B. Closed-loop control of bacterial high-cell-density fed-batch cultures: Production of mcl-PHAs by Pseudomonas putida KT2442 under single-substrate and cofeeding conditions. Biotechnol. Bioeng. 1999, 65, 306–315. [Google Scholar] [CrossRef]
- Kim, B.S. Production of medium chain length polyhydroxyalkanoates by fed-batch culture of Pseudomonas oleovorans. Biotechnol. Lett. 2002, 24, 125–130. [Google Scholar] [CrossRef]
- Kim, G.J.; Lee, I.Y.; Yoon, S.C.; Shin, Y.C.; Park, Y.H. Enhanced yield and a high production of medium-chain-length poly (3-hydroxyalkanoates) in a two-step fed-batch cultivation of Pseudomonas putida by combined use of glucose and octanoate. Enzyme Microb. Technol. 1997, 20, 500–505. [Google Scholar] [CrossRef]
- Gao, J.; Ramsay, J.A.; Ramsay, B.A. Fed-batch production of poly-3-hydroxydecanoate from decanoic acid. J. Biotechnol. 2016, 218, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Ramsay, J.A.; Guay, M.; Ramsay, B.A. Carbon-limited fed-batch production of medium-chain-length polyhydroxyalkanoates from nonanoic acid by Pseudomonas putida KT2440. Appl. Microbiol. Biotechnol. 2007, 74, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Fu, J.; Cicek, N.; Sparling, R.; Levin, D.B. Kinetics of medium-chain-length polyhydroxyalkanoate production by a novel isolate of Pseudomonas putida LS46. Can. J. Microbiol. 2012, 58, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, B.A.; Lomaliza, K.; Chavarie, C.; Dubé, B.; Ramsay, J.A. Production of poly-(beta-hydroxybutyric-co-beta-hydroxyvaleric) acids. Appl. Environ. Microbiol. 1990, 56, 2093–2098. [Google Scholar] [PubMed]
- Blunt, W.; Hossain, M.E.; Gapes, D.J.; Sparling, R.; Levin, D.B.; Cicek, N. Real-time monitoring of microbial fermentation end-products in biofuel production with Titrimetric Off-Gas Analysis (TOGA). Biol. Eng. Trans. 2014, 6, 203–219. [Google Scholar]
- Preusting, H.; Hazenberg, W.; Witholt, B. Continuous production of poly (3-hydroxyalkanoates) by Pseudomonas oleovorans in a high-cell-density, two-liquid-phase chemostat. Enzyme Microb. Technol. 1993, 15, 311–316. [Google Scholar] [CrossRef]
- Brandl, H.; Gross, R.A.; Lenz, R.W.; Fuller, R.C. Pseudomonas oleovorans as a source of poly (β-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl. Environ. Microbiol. 1988, 54, 1977–1982. [Google Scholar]
- Fu, J.; Sharma, U.; Sparling, R.; Cicek, N.; Levin, D.B. Evaluation of medium-chain-length polyhydroxyalkanoate production by Pseudomonas putida LS46 using biodiesel by-product streams. Can. J. Microbiol. 2014, 60, 461–468. [Google Scholar] [CrossRef]
- Sun, Z.; Ramsay, J.A.; Guay, M.; Ramsay, B.A. Enhanced yield of medium-chain-length polyhydroxyalkanoates from nonanoic acid by co-feeding glucose in carbon-limited, fed-batch culture. J. Biotechnol. 2009, 143, 262–267. [Google Scholar] [CrossRef]
- Andin, N.; Longieras, A.; Veronese, T.; Marcato, F.; Molina-Jouve, C.; Uribelarrea, J.-L. Improving carbon and energy distribution by coupling growth and medium chain length polyhydroxyalkanoate production from fatty acids by Pseudomonas putida KT2440. Biotechnol. Bioprocess Eng. 2017, 22, 308–318. [Google Scholar] [CrossRef]
- Grousseau, E.; Blanchet, E.; Déléris, S.; Albuquerque, M.G.E.; Paul, E.; Uribelarrea, J.-L. Impact of sustaining a controlled residual growth on polyhydroxybutyrate yield and production kinetics in Cupriavidus necator. Bioresour. Technol. 2013, 148, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Możejko, J.; Ciesielski, S. Pulsed feeding strategy is more favorable to medium-chain-length polyhydroxyalkanoates production from waste rapeseed oil. Biotechnol. Prog. 2014, 30, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Peña, C.; López, S.; García, A.; Espín, G.; Romo-Uribe, A.; Segura, D. Biosynthesis of poly-β-hydroxybutyrate (PHB) with a high molecular mass by a mutant strain of Azotobacter vinelandii (OPN). Ann. Microbiol. 2014, 64, 39–47. [Google Scholar] [CrossRef]
- Myshkina, V.L.; Nikolaeva, D.A.; Makhina, T.K.; Bonartsev, A.P.; Bonartseva, G.A. Effect of growth conditions on the molecular weight of poly-3-hydroxybutyrate produced by Azotobacter chroococcum 7B. Appl. Biochem. Microbiol. 2008, 44, 482–486. [Google Scholar] [CrossRef]
- Shang, L.; Jiang, M.; Ryu, C.H.; Chang, H.N.; Cho, S.H.; Lee, J.W. Inhibitory effect of carbon dioxide on the fed-batch culture of Ralstonia eutropha: Evaluation by CO2 pulse injection and autogenous CO2 methods. Biotechnol. Bioeng. 2003, 83, 312–320. [Google Scholar] [CrossRef] [PubMed]






| Medium Component | Yield Coefficient |
|---|---|
| Octanoic Acid (g g−1) | 0.62 a |
| NH4+ (g g−1) | 6.1 a |
| PO43− (g g−1) | 13.5 |
| Ca2+ (g mg−1) | 2.6 |
| Cu2+ (g mg−1) | 15.5 |
| Fe3+ (g mg−1) | 2.2 |
| Mg2+ (g mg−1) | 0.5 |
| Process Performance Indicator | Previous Batch Results a | Pulse Feed Strategy (at 27 h Unless Otherwise Stated) | Continuous, Drip Feed Strategy (at 27 h Unless Otherwise Stated) |
|---|---|---|---|
| [Xt] (g L−1) | 2.37 ± 0.1 | 28.9 ± 4.0 | 32.4 ± 0.9 |
| %PHA (g g−1) | 44.4 ± 1.3 | 60.6 ± 8.2 | 52.9 ± 2.5 |
| [Xr] (g L−1) | 2.37 ± 0.5 | 11.2 ± 1.5 | 17.4 ± 2.1 b |
| [XPHA] (g L−1) | 1.01 ± 0.12 | 17.7 ± 4.8 | 15.4 ± 1.2 |
| μavg/Xr, growth phase (h−1) | 0.29 ± 0.03 | 0.35 ± 0.11 (0–14 h) | 0.31 ± 0.03 (0–14 h) |
| μavg/Xr, storage phase (h−1) | 0.11 ± 0.01 | 0.03 ± 0.01 (14–27 h) | 0.03 ± 0.01 (14–27 h) |
| Qv, final (g L−1 h−1) | 0.08 ± 0.00 | 0.61 ± 0.12 | 0.60 ± 0.04 |
| Qv, max (g L−1 h−1) | 0.08 ± 0.01 | 0.66 ± 0.14 (23–27 h) | 0.60 ± 0.04 (27 h) |
| Qs, max (g PHA g Xr−1 h−1) | 0.18 ± 0.03 | 0.18 ± 0.03 (15–19 h) | 0.10 ± 0.03 (23–25 h) b |
| Qs, avg (g PHA g Xr−1 h−1) | 0.11 ± 0.00 | 0.09 ± 0.01 (14–27 h) | 0.06 ± 0.01 (14–27 h) b |
| YPHA/S, overall (C-mol C-mol−1) | 0.35 ± 0.04 | 0.33 ± 0.05 | 0.26 ± 0.04 |
| YPHA/S, storage phase (C-mol C-mol−1) | 0.57 ± 0.05 | 0.52 ± 0.13 (14–27 h) | 0.31 ± 0.06 (14–27 h) b |
| Carbon Recovery | 1.04 ± 0.00 | 0.90 ± 0.15 | 0.89 ± 0.04 |
| Feeding Strategy | C6 | C8 | C10 | C12 |
|---|---|---|---|---|
| (Mol %) | ||||
| Pulse | 5.8 ± 0.6 | 92.9 ± 0.6 | 1.1 ± 0.2 | 0.6 ± 0.2 |
| Continuous drip | 7.4 ± 0.2 | 89.7± 0.3 | 2.2 ± 0.1 | 0.6 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blunt, W.; Dartiailh, C.; Sparling, R.; Gapes, D.J.; Levin, D.B.; Cicek, N. Development of High Cell Density Cultivation Strategies for Improved Medium Chain Length Polyhydroxyalkanoate Productivity Using Pseudomonas putida LS46. Bioengineering 2019, 6, 89. https://doi.org/10.3390/bioengineering6040089
Blunt W, Dartiailh C, Sparling R, Gapes DJ, Levin DB, Cicek N. Development of High Cell Density Cultivation Strategies for Improved Medium Chain Length Polyhydroxyalkanoate Productivity Using Pseudomonas putida LS46. Bioengineering. 2019; 6(4):89. https://doi.org/10.3390/bioengineering6040089
Chicago/Turabian StyleBlunt, Warren, Christopher Dartiailh, Richard Sparling, Daniel J. Gapes, David B. Levin, and Nazim Cicek. 2019. "Development of High Cell Density Cultivation Strategies for Improved Medium Chain Length Polyhydroxyalkanoate Productivity Using Pseudomonas putida LS46" Bioengineering 6, no. 4: 89. https://doi.org/10.3390/bioengineering6040089