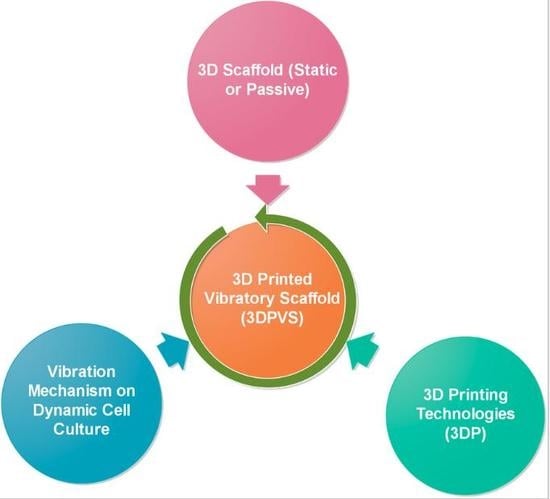
Trinity of Three-Dimensional (3D) Scaffold, Vibration, and 3D Printing on Cell Culture Application: A Systematic Review and Indicating Future Direction
Abstract
:1. Introduction
2. 3D Scaffold Utilized for 3D Cell Culture
2.1. Definition and Categorization of 3D Scaffold
2.2. GMB Characterization of 3D Scaffold and Properties
2.2.1. Geometrical Characters
2.2.2. Mechanics Properties
2.2.3. Biochemical Controls
2.2.4. Material Composition
3. Fabrication Methods and 3DP for 3D Scaffold
3.1. Conventional Means for Scaffold Fabrication
3.2. Concept and Scope of 3DP
3.3. Novel 3DP Methods for 3D Scaffold
3.3.1. 3DP Laser-Based Systems
3.3.2. 3DP Nozzle-Based Systems
3.3.3. 3DP Droplet-Based Systems
4. Vibration Mechanisms Applied for Cell Cultivation
4.1. Vibration and Dynamicity
4.2. Vibration Systems Utilized for Cell Culture
4.2.1. Bioreactor-Based Vibration System
4.2.2. Loudspeaker-Based Vibration System
4.2.3. Vibration System from Mechanical Stimulators
4.2.4. Vibration System from Ultrasonic Generators
4.2.5. Vibration System from 3D Micro-Vibration Stages
4.2.6. Vibration System from Mechanical Micro-Vibrators
5. Discussion
5.1. Current Limitations and Gaps
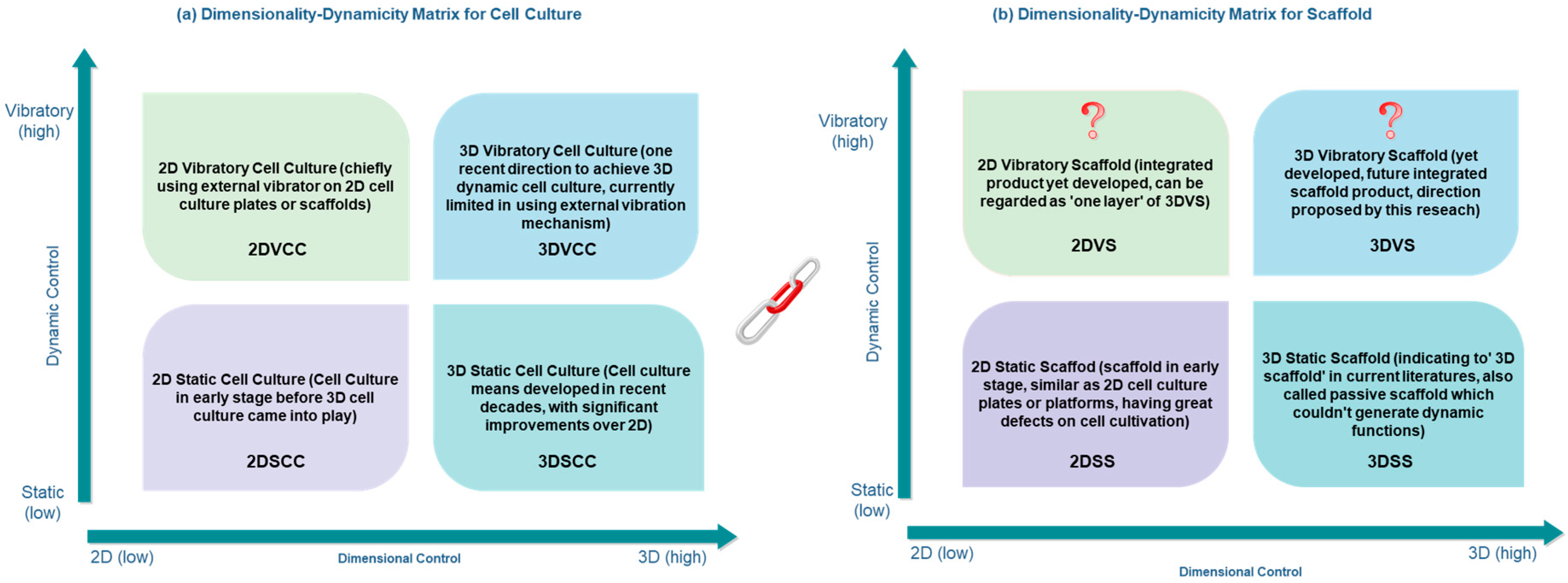
5.2. Future Trends and 3D Vibratory Scaffold
5.2.1. Trends Regards Cell Culture Dimensionality and Dynamicity
5.2.2. 3D Vibratory Scaffold in Future
5.2.3. 3DP as Bridging Technology for Future Scaffold
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Cheng, F.; Grenman, H.; Spoljaric, S.; Seppala, J.; Eriksson, J.E.; Willfor, S.; Xu, C. Development of nanocellulose scaffolds with tunable structures to support 3D cell culture. Carbohydr. Polym. 2016, 148, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Greiner, A.M.; Richter, B.; Bastmeyer, M. Micro-engineered 3D scaffolds for cell culture studies. Macromol. Biosci. 2012, 12, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.; Hernández, R.M.; Pedraz, J.L.; Orive, G. Novel advances in the design of three-dimensional bio-scaffolds to control cell fate: Translation from 2D to 3D. Trends Biotechnol. 2012, 30, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug. Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Shamir, E.R.; Ewald, A.J. Three-dimensional organotypic culture: Experimental models of mammalian biology and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 647–664. [Google Scholar] [CrossRef] [PubMed]
- Sawkins, M.J.; Shakesheff, K.M.; Bonassar, L.J.; Kirkham, G.R. 3D cell and scaffold patterning strategies in tissue engineering. Recent Pat. Biomed. Eng. 2013, 6, 3–21. [Google Scholar] [CrossRef]
- Sun, J.; Tan, R. Technology assessment: Triz technology system evolution theory. In Managing Technological Innovation: Tools and Methods; Daim, T.U., Ed.; World Scientific: Singapore, 2017. [Google Scholar]
- Cavallucci, D.; Rousselot, F. Evolution hypothesis as a means for linking system parameters and laws of engineering system evolution. Procedia Eng. 2011, 9, 484–499. [Google Scholar] [CrossRef]
- Bukhman, I. Triz Technology for Innovation; Cubic Creativity Company: Taipei, Taiwan, 2012; pp. 1–362. [Google Scholar]
- Wu, J.; Chen, Q.; Liu, W.; He, Z.; Lin, J.-M. Recent advances in microfluidic 3D cellular scaffolds for drug assays. TrAC Trends Anal. Chem. 2017, 87, 19–31. [Google Scholar] [CrossRef]
- Correia, D.M.; Ribeiro, C.; Sencadas, V.; Vikingsson, L.; Oliver Gasch, M.; Gómez Ribelles, J.L.; Botelho, G.; Lanceros-Méndez, S. Strategies for the development of three dimensional scaffolds from piezoelectric poly(vinylidene fluoride). Mater. Des. 2016, 92, 674–681. [Google Scholar] [CrossRef] [Green Version]
- Benders, K.E.; van Weeren, P.R.; Badylak, S.F.; Saris, D.B.; Dhert, W.J.; Malda, J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol. 2013, 31, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Shim, K.; Kim, S.H.; Lee, D.; Kim, B.; Kim, T.H.; Jung, Y.; Choi, N.; Sung, J.H. Fabrication of micrometer-scale porous gelatin scaffolds for 3D cell culture. J. Ind. Eng. Chem. 2017, 50, 183–189. [Google Scholar] [CrossRef]
- Ronca, D.; Langella, F.; Chierchia, M.; D’Amora, U.; Russo, T.; Domingos, M.; Gloria, A.; Bartolo, P.; Ambrosio, L. Bone tissue engineering: 3D pcl-based nanocomposite scaffolds with tailored properties. Procedia CIRP 2016, 49, 51–54. [Google Scholar] [CrossRef]
- Chung, S.; King, M.W. Design concepts and strategies for tissue engineering scaffolds. Biotechnol. Appl. Biochem. 2011, 58, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Richter, B.; Pauloehrl, T.; Kaschke, J.; Fichtner, D.; Fischer, J.; Greiner, A.M.; Wedlich, D.; Wegener, M.; Delaittre, G.; Barner-Kowollik, C.; et al. Three-dimensional microscaffolds exhibiting spatially resolved surface chemistry. Adv. Mater. 2013, 25, 6117–6122. [Google Scholar] [CrossRef] [PubMed]
- Kantaros, A.; Chatzidai, N.; Karalekas, D. 3D printing-assisted design of scaffold structures. Int. J. Adv. Manuf. Technol. 2016, 82, 559–571. [Google Scholar] [CrossRef]
- An, J.; Teoh, J.E.M.; Suntornnond, R.; Chua, C.K. Design and 3D printing of scaffolds and tissues. Engineering 2015, 1, 261–268. [Google Scholar] [CrossRef]
- Aliramaji, S.; Zamanian, A.; Mozafari, M. Super-paramagnetic responsive silk fibroin/chitosan/magnetite scaffolds with tunable pore structures for bone tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Eydivand, M.; Solati-Hashjin, M.; Fathi, A.; Padashi, M.; Abu Osman, N.A. Optimal design of a 3D-printed scaffold using intelligent evolutionary algorithms. Appl. Soft Comput. 2016, 39, 36–47. [Google Scholar] [CrossRef]
- Vaezi, M.; Seitz, H.; Yang, S. A review on 3D micro-additive manufacturing technologies. Int. J. Adv. Manuf. Technol. 2012, 67, 1721–1754. [Google Scholar] [CrossRef]
- Tan, Z.; Liu, T.; Zhong, J.; Yang, Y.; Tan, W. Control of cell growth on 3D-printed cell culture platforms for tissue engineering. J. Biomed. Mater. Res. Part A 2017, 105, 3281–3292. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Y.; Li, S.; Liu, C.; Hu, Q. Novel compound-forming technology using bioprinting and electrospinning for patterning a 3D scaffold construct with multiscale channels. Micromachines 2016, 7, 238. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, D.; Yoon, T.R.; Kim, H.K.; Jo, H.H.; Park, J.S.; Lee, J.H.; Kim, W.D.; Kwon, I.K.; Park, S.A. Surface modification of 3D-printed porous scaffolds via mussel-inspired polydopamine and effective immobilization of rhbmp-2 to promote osteogenic differentiation for bone tissue engineering. Acta Biomater. 2016, 40, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.V.; Grigoryev, A.M.; Krotova, L.I.; Skaletsky, N.N.; Popov, V.K.; Sevastianov, V.I. 3D printing of PLGA scaffolds for tissue engineering. J. Biomed. Mater. Res. Part A 2017, 105, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, S.J.; Jo, H.H.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. Fabrication and characterization of 3D-printed bone-like β-tricalcium phosphate/polycaprolactone scaffolds for dental tissue engineering. J. Ind. Eng. Chem. 2017, 46, 175–181. [Google Scholar] [CrossRef]
- Lima, M.J.; Correlo, V.M.; Reis, R.L. Micro/nano replication and 3D assembling techniques for scaffold fabrication. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Chen, W.; Ma, J.; Zhu, L.; Morsi, Y.; Ei-Hamshary, H.; Al-Deyab, S.S.; Mo, X. Superelastic, superabsorbent and 3D nanofiber-assembled scaffold for tissue engineering. Colloids Sur. B Biointerfaces 2016, 142, 165–172. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.M.; Holmes, B.; Faucett, S.; Zhang, L.G. Three-dimensional printing of nanomaterial scaffolds for complex tissue regeneration. Tissue Eng. Part B Rev. 2015, 21, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, A.A.; Saha, K. Manufacturing cell therapies using engineered biomaterials. Trends Biotech. 2017, 35, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.A.; Won, J.E.; Knowles, J.C.; Kim, H.W. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2013, 65, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Li, D.; Tian, X. Development trends in additive manufacturing and 3D printing. Engineering 2015, 1, 085–089. [Google Scholar] [CrossRef]
- Cox, S.C.; Thornby, J.A.; Gibbons, G.J.; Williams, M.A.; Mallick, K.K. 3D printing of porous hydroxyapatite scaffolds intended for use in bone tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 47, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.G.; Kaplan, D.L. Neural engineering: From advanced biomaterials to 3D fabrication techniques; Springer: Cham, Switzerland, 2016; pp. 1–306. [Google Scholar]
- Colaco, M.; Igel, D.A.; Atala, A. The potential of 3d printing in urological research and patient care. Nat. Rev. Urol. 2018, 15, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neufurth, M.; Wang, X.; Wang, S.; Steffen, R.; Ackermann, M.; Haep, N.D.; Schroder, H.C.; Muller, W.E.G. 3D printing of hybrid biomaterials for bone tissue engineering: Calcium-polyphosphate microparticles encapsulated by polycaprolactone. Acta Biomater. 2017, 64, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Vasireddi, R.; Basu, B. Conceptual design of three-dimensional scaffolds of powder-based materials for bone tissue engineering applications. Rapid Prototyp. J. 2015, 21, 716–724. [Google Scholar] [CrossRef]
- Do, A.V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D printing of scaffolds for tissue regeneration applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef] [PubMed]
- Serra, T. Development of 3D-Printed Biodegradable Composite Scaffolds for Tissue Engineering Applications. Ph.D. Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 29 April 2014. [Google Scholar]
- Park, H.K.; Shin, M.; Kim, B.; Park, J.W.; Lee, H. A visible light-curable yet visible wavelength-transparent resin for stereolithography 3D printing. NPG Asia Mater. 2018, 10, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Gu, B.K.; Choi, D.J.; Park, S.J.; Kim, M.S.; Kang, C.M.; Kim, C.H. 3-dimensional bioprinting for tissue engineering applications. Biomater. Res. 2016, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Kuiper, J. Functional 3D Tissue Engineering Scaffolds: Materials, Technologies, and Applications; Woodhead Publishing: Duxford, UK, 2017; pp. 1–482. [Google Scholar]
- Kumaresan, T.; Gandhinathan, R.; Ramu, M.; Ananthasubramanian, M. Conceptual design and fabrication of porous structured scaffold for tissue engineering applications. Biomed. Res. 2015, 4, 42–48. [Google Scholar]
- Taherkhani, S.; Moztarzadeh, F. Fabrication of a poly (ɛ-caprolactone)/starch nanocomposite scaffold with a solvent-casting/salt-leaching technique for bone tissue engineering applications. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Durgalakshmi, D.; Balakumar, S. Phase separation induced shell thickness variations in electrospun hollow bioglass 45s5 fiber mats for drug delivery applications. Phys. Chem. Chem. Phys. 2015, 17, 15316–15323. [Google Scholar] [CrossRef] [PubMed]
- Khoshroo, K.; Jafarzadeh Kashi, T.S.; Moztarzadeh, F.; Tahriri, M.; Jazayeri, H.E.; Tayebi, L. Development of 3D PCL microsphere/TiO2 nanotube composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, F.; Yan, Z.; Ma, Q.; Li, X.; Huang, Y.; Rogers, J.A. Printing, folding and assembly methods for forming 3D mesostructures in advanced materials. Nat. Rev. Mater. 2017, 2. [Google Scholar] [CrossRef]
- Singh, D.; Singh, D.; Han, S. 3D printing of scaffold for cells delivery: Advances in skin tissue engineering. Polymers 2016, 8, 19. [Google Scholar] [CrossRef]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Pumera, M. 3D-printing technologies for electrochemical applications. Chem. Soc. Rev. 2016, 45, 2740–2755. [Google Scholar] [CrossRef] [PubMed]
- Mazzoli, A. Selective laser sintering in biomedical engineering. Med. Biol. Eng. Comput. 2013, 51, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Selimis, A.; Mironov, V.; Farsari, M. Direct laser writing: Principles and materials for scaffold 3D printing. Microelectron. Eng. 2015, 132, 83–89. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, Q.; Yao, Y.; Liu, L.; Liu, Y.; Leng, J. Direct-write fabrication of 4D active shape-changing structures based on a shape memory polymer and its nanocomposite. ACS Appl. Mater. Interfaces 2017, 9, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Khoo, Z.X.; Teoh, J.E.M.; Liu, Y.; Chua, C.K.; Yang, S.; An, J.; Leong, K.F.; Yeong, W.Y. 3D printing of smart materials: A review on recent progresses in 4D printing. Virtual Phys. Prototyp. 2015, 10, 103–122. [Google Scholar] [CrossRef]
- Hendrikson, W.J.; Rouwkema, J.; Clementi, F.; van Blitterswijk, C.A.; Fare, S.; Moroni, L. Towards 4d printed scaffolds for tissue engineering: Exploiting 3D shape memory polymers to deliver time-controlled stimulus on cultured cells. Biofabrication 2017, 9, 031001. [Google Scholar] [CrossRef] [PubMed]
- Farran, A.J.E.; Teller, S.S.; Jia, F.; Clifton, R.J.; Duncan, R.L.; Jia, X. Design and characterization of a dynamic vibrational culture system. J. Tissue Eng. Regen. Med. 2013, 7, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, C.S.; Leonard, M.B.; Kobe, E.A.; Slinger, M.A.; Borges, K.A.; Billig, E.; Rubin, C.T.; Wehrli, F.W. The efficacy of low-intensity vibration to improve bone health in patients with end-stage renal disease is highly dependent on compliance and muscle response. Acad. Radiol. 2017, 24, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Vining, K.H.; Mooney, D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell. Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Goto, D. Effect of low-intensity whole-body vibration on bone defect repair and associated vascularization in mice. Med. Biol. Eng. Comput. 2017, 55, 2257–2266. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Zerdoum, A.B.; Duncan, R.L.; Jia, X. Dynamic vibration cooperates with connective tissue growth factor to modulate stem cell behaviors. Tissue Eng. Part A 2014, 20, 1922–1934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, J.; Zhang, L.; Zhou, Y.; Hou, W.; Quan, H.; Li, X.; Chen, Y.; Yu, H. Effects of mechanical vibration on proliferation and osteogenic differentiation of human periodontal ligament stem cells. Arch. Oral. Biol. 2012, 57, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hayward, R.C. Mimicking dynamic in vivo environments with stimuli-responsive materials for cell culture. Trends Biotechnol. 2012, 30, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.P.; Chinnathambi, S.; Titze, I.R.; Sander, E.A. Vibratory stimulation enhances thyroid epithelial cell function. Biochem. Biophys. Rep. 2016, 8, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Pongkitwitoon, S.; Weinheimer-Haus, E.M.; Koh, T.J.; Judex, S. Low-intensity vibrations accelerate proliferation and alter macrophage phenotype in vitro. J. Biomech. 2016, 49, 793–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajama, H.; Hashimoto, S. Effect of ultrasonic vibration on culture of myoblast. In Proceedings of the 18th World Multi-Conference on Systemics, Cybernetics and Informatics, Orlando, FL, USA, 6 February 2014; pp. 144–149. [Google Scholar]
- Hou, Y.; Hao, D.; Shen, J.; Li, P.; Zhang, T.; Wang, H. Effect of strengthened road vibration on performance degradation of pem fuel cell stack. Int. J. Hydrog. Energy 2016, 41, 5123–5134. [Google Scholar] [CrossRef]
- Paces, W.R.; Holmes, H.R.; Vlaisavljevich, E.; Snyder, K.L.; Tan, E.L.; Rajachar, R.M.; Ong, K.G. Application of sub-micrometer vibrations to mitigate bacterial adhesion. J. Funct. Biomater. 2014, 5, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Yeatts, A.B.; Choquette, D.T.; Fisher, J.P. Bioreactors to influence stem cell fate: Augmentation of mesenchymal stem cell signaling pathways via dynamic culture systems. Biochim. Biophys. Acta 2013, 1830, 2470–2480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Law, A.W.-K.; Jiang, Y.; Harijanto, A.K.; Fane, A.G. Fouling control of submerged hollow fibre membrane bioreactor with transverse vibration. J. Membr. Sci. 2016, 505, 216–224. [Google Scholar] [CrossRef]
- Diban, N.; Sánchez-González, S.; Lázaro-Díez, M.; Ramos-Vivas, J.; Urtiaga, A. Facile fabrication of poly(ε-caprolactone)/graphene oxide membranes for bioreactors in tissue engineering. J. Membr. Sci. 2017, 540, 219–228. [Google Scholar] [CrossRef]
- Kola, A.; Ye, Y.; Le-Clech, P.; Chen, V. Transverse vibration as novel membrane fouling mitigation strategy in anaerobic membrane bioreactor applications. J. Membr. Sci. 2014, 455, 320–329. [Google Scholar] [CrossRef]
- Wolchok, J.C.; Brokopp, C.; Underwood, C.J.; Tresco, P.A. The effect of bioreactor induced vibrational stimulation on extracellular matrix production from human derived fibroblasts. Biomaterials 2009, 30, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Titze, I.R.; Broadhead, K.; Tresco, P.; Gray, S. Strain distribution in an elastic substrate vibrated in a bioreactor for vocal fold tissue engineering. J. Biomech. 2005, 38, 2406–2414. [Google Scholar] [CrossRef] [PubMed]
- Puig, F.; Rico, F.; Almendros, I. Vibration enhances interleukin-8 release in a cell model of snoring-induced airway inflammatio. Sleep 2005, 28, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, W.; Tan, L.; Mu, D.; Zhu, D.; Wang, J.; Zhao, B. Low-magnitude mechanical vibration regulates expression of osteogenic proteins in ovariectomized rats. Biochem. Biophys. Res. Commun. 2015, 465, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Shikata, T.; Shiraishi, T.; Morishita, S. Effects of amplitude and frequency of mechanical vibration stimulation on cultured osteoblasts. J. Syst. Des. Dyn. 2008, 2, 382–388. [Google Scholar]
- Kulkarni, R.N.; Voglewede, P.A.; Liu, D. Mechanical vibration inhibits osteoclast formation by reducing dc-stamp receptor expression in osteoclast precursor cells. Bone 2013, 57, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Hino, H.; Hashimoto, S.; Takahashi, Y.; Nakajima, H. Effect of ultrasonic vibration on proliferation and differentiation of cells. J. Syst. 2016, 14, 1–7. [Google Scholar]
- Konno, K.-I.; Kosawada, T.; Sato, R.; Feng, Z.; Yasukazu, H.; Goto, K. Three-dimensional micro vibration stage and its application to cell culture. In Proceedings of the 6th World Congress of Biomechanics, Singapore, 1–6 August 2010; Springer: Heidelberg/Berlin, Germany. [Google Scholar]
- Hur, Y.S.; Park, J.H.; Ryu, E.K.; Park, S.J.; Lee, J.H.; Lee, S.H.; Yoon, J.; Yoon, S.H.; Hur, C.Y.; Lee, W.D.; et al. Effect of micro-vibration culture system on embryo development. J. Assist. Reprod. Genet. 2013, 30, 835–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruber, I.; Klein, M. Embryo culture media for human IVF: Which possibilities exist? J. Turk. Ger. Gynecol. Assoc. 2011, 12, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Tsimbouri, P.M.; Childs, P.G.; Pemberton, G.D.; Yang, J.; Jayawarna, V.; Orapiriyakul, W.; Burgess, K.; González-García, C.; Blackburn, G.; Thomas, D.; et al. Stimulation of 3D osteogenesis by mesenchymal stem cells using a nanovibrational bioreactor. Nat. Biomed. Eng. 2017, 1, 758–770. [Google Scholar] [CrossRef] [Green Version]
- Al-Qodah, Z.; Al-Shannag, M.; Al-Busoul, M.; Penchev, I.; Orfali, W. Immobilized enzymes bioreactors utilizing a magnetic field: A review. Biochem. Eng. J. 2017, 121, 94–106. [Google Scholar] [CrossRef]
- Mohammed, T.; Murphy, M.F.; Lilley, F.; Burton, D.R.; Bezombes, F. The effects of acoustic vibration on fibroblast cell migration. Mater. Sci. Eng: C 2016, 69, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Chintavalakorn, R.; Khantachawana, A.; Viravaidya-Pasuwat, K.; Santiwong, P.; Surarit, R. In vitro effects of mechanical stimulation and photobiomodulation on osteoblastic cell function: A proof of concept study. Pediatr. Dent. J. 2017, 27, 29–41. [Google Scholar] [CrossRef]
- Cho, H.; Seo, Y.-K.; Jeon, S.; Yoon, H.-H.; Choi, Y.-K.; Park, J.-K. Neural differentiation of umbilical cord mesenchymal stem cells by sub-sonic vibration. Life Sci. 2012, 90, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Menger, M.D. Life is 3D: Boosting spheroid function for tissue engineering. Trends Biotechnol. 2017, 35, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839. [Google Scholar] [CrossRef] [PubMed]
- Langeberg, L.K.; Scott, J.D. Signalling scaffolds and local organization of cellular behaviour. Nat. Rev. Mol. Cell Biol. 2015, 16, 232–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, A.; Adetiba, O.; Kim, H.; Chen, H.; Jacot, J.G.; Verduzco, R. Stimuli-responsive liquid crystal elastomers for dynamic cell culture. J. Mater. Res. 2015, 30, 453–462. [Google Scholar] [CrossRef]
- Huang, Y.; Luan, H.; Sun, L.; Bi, J.; Wang, Y.; Fan, Y. Local vibration enhanced the efficacy of passive exercise on mitigating bone loss in hindlimb unloading rats. Acta Astronaut. 2017, 137, 373–381. [Google Scholar] [CrossRef]
- Cavallucci, D.; Rousselot, F.; Zanni, C. Linking contradictions and laws of engineering system evolution within the triz framework. Creat. Innov. Manag. 2009, 18, 71–80. [Google Scholar] [CrossRef]
- Dolgin, E. Animal testing alternatives come alive in us. Nat. Med. 2010, 16, 1348. [Google Scholar] [CrossRef] [PubMed]
- Wallin, T.J.; Pikul, J.; Shepherd, R.F. 3D printing of soft robotic systems. Nat. Rev. Mater. 2018, 3, 84–100. [Google Scholar] [CrossRef]
- Kim, F.; Kwon, B.; Eom, Y.; Lee, J.E.; Park, S.; Jo, S.; Park, S.H.; Kim, B.-S.; Im, H.J.; Lee, M.H.; et al. 3D printing of shape-conformable thermoelectric materials using all-inorganic Bi2Te3-based inks. Nat. Energy 2018, 3, 301–309. [Google Scholar] [CrossRef]
- Graham, A.D.; Olof, S.N.; Burke, M.J.; Armstrong, J.P.K.; Mikhailova, E.A.; Nicholson, J.G.; Box, S.J.; Szele, F.G.; Perriman, A.W.; Bayley, H. High-resolution patterned cellular constructs by droplet-based 3D printing. Sci. Rep. 2017, 7, 7004. [Google Scholar] [CrossRef] [PubMed]
- Vovrosh, J.; Voulazeris, G.; Petrov, P.G.; Zou, J.; Gaber, Y.; Benn, L.; Woolger, D.; Attallah, M.M.; Boyer, V.; Bongs, K.; et al. Additive manufacturing of magnetic shielding and ultra-high vacuum flange for cold atom sensors. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crippa, F.; Moore, T.L.; Mortato, M.; Geers, C.; Haeni, L.; Hirt, A.M.; Rothen-Rutishauser, B.; Petri-Fink, A. Dynamic and biocompatible thermo-responsive magnetic hydrogels that respond to an alternating magnetic field. J. Magn. Magn. Mater. 2017, 427, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, Y.B.; Sultan, M.T.; Lee, O.J.; Lee, J.S.; Yoon, S.I.; et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat Commun. 2018, 9. [Google Scholar] [CrossRef]
- Moroni, L.; Burdick, J.A.; Highley, C.; Lee, S.J.; Morimoto, Y.; Takeuchi, S.; Yoo, J.J. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat. Rev. Mater. 2018, 3, 21–37. [Google Scholar] [CrossRef]
- Seleznev, V.A.; Prinz, V.Y. Hybrid 3D-2D printing for bone scaffolds fabrication. Nanotechnology 2017, 28, 064004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Nguyen, P.D.; Shi, S.; Burrell, J.C.; Cullen, D.K.; Le, A.D. 3D bio-printed scaffold-free nerve constructs with human gingiva-derived mesenchymal stem cells promote rat facial nerve regeneration. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ragelle, H.; Tibbitt, M.W.; Wu, S.Y.; Castillo, M.A.; Cheng, G.Z.; Gangadharan, S.P.; Anderson, D.G.; Cima, M.J.; Langer, R. Surface tension-assisted additive manufacturing. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
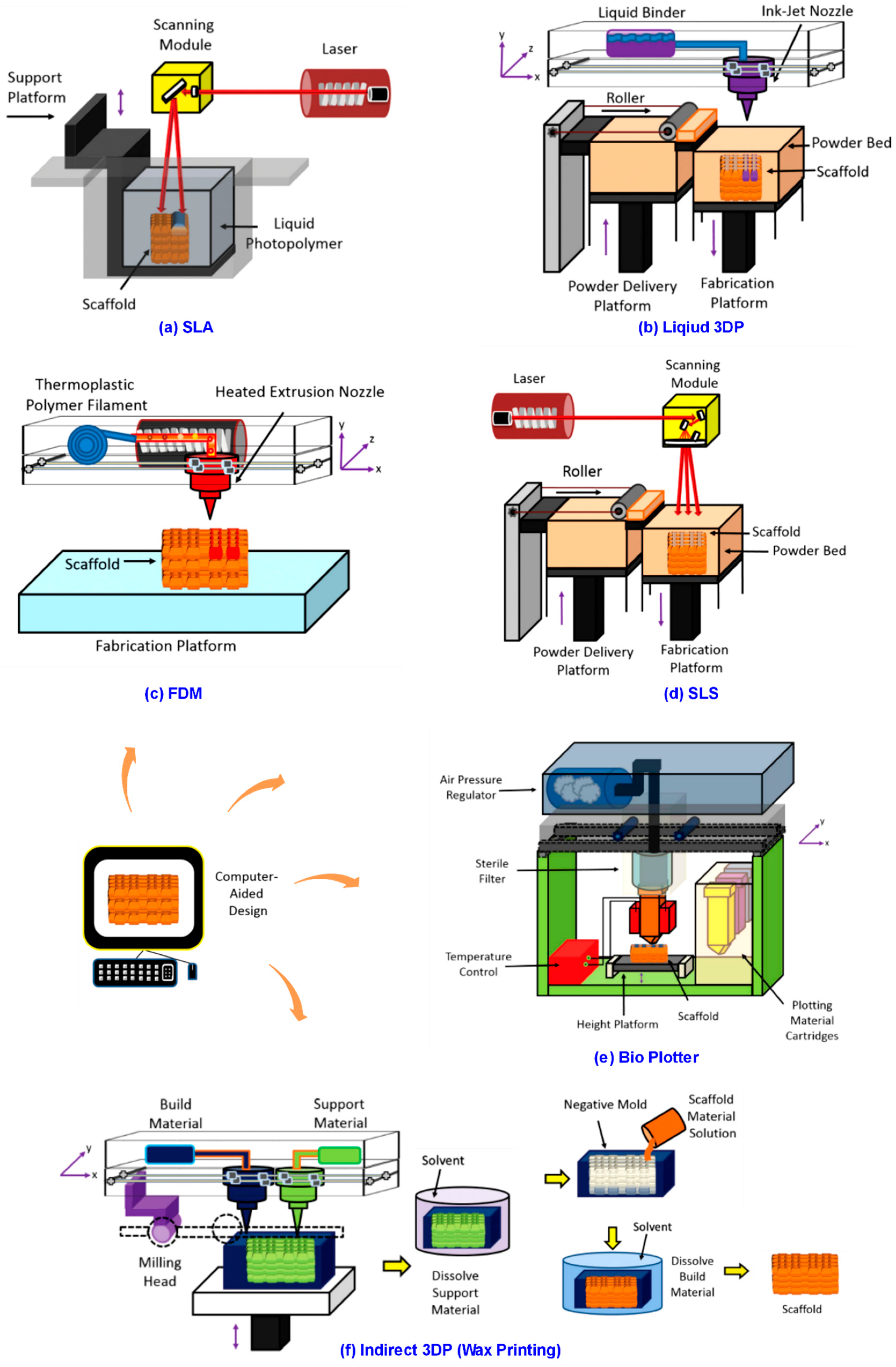
| 3DP Methods | Chief Feature & Mechanism | Materials | Cells Studied | Architecture | Dynamic Structure Appli-Cability | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|---|---|---|---|
| Two-Photon polymerization (2PP) | Laser beam is focused onto a liquid material; CAD | Solidifable fluid: photosensitive materials | Bone cells, human stem cells | Mesh-like, wheel-, pyramid-, cube-like pattern in hydrogel | High | Homogeneous and two-composite polymer | Excess of initially powdered material hard to remove | [2,35,36] |
| Laser Engineered Net Shaping (LENS) | Metal powders used to build or repair scaffold parts | Fine powder: plastic, metal etc. | General tissue cells | Mesh-like network | High | Able to repair old parts and fabricate new; secondary firing process not needed; excellent material properties | Low geometrical control in dimension | [18,37,38,39] |
| Stereolith-ography (SLA) | Laser onto liquid photopolymer to generate scaffold; CAD | Solidifable fluid: photopolymer resins, temperature sensitive polymers, ion cross-linkable hydrogels, ceramic paste, etc. | Rat bone, rabbit trachea, pig tendon cells | Mesh-like, Honeycomb- Wheel-, pyramid-, cube-like; porous cylinder | High | High surface quality, high resolution, high complexity, fast speed. | Limited to specific polymers (photopolymers); need support system; moderate strength; expensive | [36,40,41,42,43,44] |
| Selective Laser Melting (SLM) | Using small diameter wire-frame elements | Fine powder: Plastic, metal, ceramic or composite powders | Mouse bone cells | Mesh-like, Honeycomb- Wheel-, pyramid-, cube-like network | High | Controlled pore interconnectivity and porosity; greater durability of mould; free from temperature-related defects | Low surface quality | [35,40,45] |
| Selective Layer Sintering (SLS) | Laser-based CAD technique; include laser and power bed | Fine powder: Plastic, metal, ceramic or composite powders | Mouse bone, rat heart, rat bone, mouse skin, mouse heart cells | Mesh-like network, porous cylinder | High | Good mechanical strength; complex structures; high resolution; large part size; no support structure needed | High materials requirements (heat, shrinkage resistant); require high processing temperature; powdery surface; costly; time consuming | [2,40,41,42] |
| Laminated Object Manufacturing (LOM) | layers of adhesive-coated laminates being successively glued together and cut to shape with laser | Laminated thin sheet: Ceramics—alumina, silicon nitride, and zirconia and metals | General tissue cells | Mesh-like network | High | Large part size; layer builds quickly; fine accuracy and resolution low cost | Materials limited | [21,40,46] |
| Ink-jet Printing (3DP in traditional terminology) | Liquid binder jetting; drop-on-powder; CAD | Hydroxyapatite, magnesium phosphate, cement, polyurethane | Rat bone, rabbit bone and mouse bone cells | Mesh-like network; porous cylinder | High | Materials versatile; powder can be trapped inside body; don’t need support structure; high speed; cost-efficient | May be toxic; low mechanical strength compared with Laser printing; time consuming in post-processing | [2,21,28,41,42] |
| Fused Deposition Modeling (FDM) | Thermoplastic polymer through heated extrusion Nozzle to create scaffold onto platform; CAD | Non-brittle flament: Thermoplastics like ABS, PLA, and PCL etc. | Rat and Swine Bone cells | Mesh-like network; porous cylinder | High | Relatively inexpensive; low cytotoxicity; good strength; no support structure needed; no power trapped; good mechanical anisotropy; speed control by strand diameter | Limitation on materials (thermoplastics); materials non-biodegradable; support structure required for complex geometrics; post possessing needed; low resolution; low speed | [2,21,28,41,42] |
| 3D Plotting (Bioplotter Printing) | Air pressured system to extrude material from bioink cartridges | Solidifable fluid: ion cross-linkable hydrogels etc. | Rabbit cartilage, rabbit trachea, rat cartilage, mouse cartilage, mouse skin cells etc. | Mesh-like network; dot-like structure | High | Viable cells printable; soft tissue applications; wide variety of natural and synthetic materials; processing at room temperature | Nozzle may be cytotoxic; support structure required when printing complex structure; low dimensional accuracy | [22,28,40] |
| Wax Printing (Indirect 3DP) | Wax being printed as a negative mold where scaffold solution is cast | Wax | Rat bone cells, mouse stem cells | Mesh-like structure | High | Benefit on preproduction; versatility on material casting following obtained mold | Materials may fail to be biocompatible; Low resolution; always need a mold; low speed in fabrication | [41,45] |
| Conventional Methods | Chief Feature & Mechanism | Materials | Cell Studied | Architecture | Dynamic Structure Appli-Cability | Advantages | Disadvantages | Refs. |
| Electrospinning | Polymer solution forced into a capillary to form a jet of solution a tip; high voltage applied between tip and collector | Biodegradable polymers like PCL | Rat bone, mouse bone, rabbit vascular tissue cells | Mesh-like structure; microchannel | Low | Fast speed; cell printing available; soft tissue application; similar to ECM; better mechanical control (shear stress); high aspect ratio and surface area | Fibers printed in random orientation; pore sizes not uniform; high voltage demand; organic solvent needed | [2,41,42] |
| Solvent Casting/Particulate Leaching | Dissolute polymer in an organic solvent and casting into a mould | Composite like PLA/Calcium phosphate | Bone cells | Mesh-like structure | Low | High geometric control; easy processing; fast speed | Organic solvents have to be used | [42,47] |
| Phase Separation | Polymer and solvent mixed pass through a freeze-dryer | Ceramics, i.e., glass | Bone osteoblast cells | Homogeneous and highly porous structures | Low | High porosity; easy to cooperate with other techniques | Possible shrinkage issues; organic solvents used; anisotropic pores | [42,45,48] |
| Gas Forming | Using a process with high-pressure carbon dioxide at room temperature | Polyesters polymers; biodegradable polymers | Bone cells | Mesh-like; microchannel | Low | Organic solvents not needed; room temperature processing; macro-porous scaffold | Poor geometrical and porous control | [23,42,45] |
| Microsphere Sintering | Sintering polymer microspheres thermally or chemically | Polymers | Bone cells | Mesh-like; microchannel | Low | Pore size being gradient; complex shape fabricable | Lack of control in interconnectivity | [42,45,49] |
| Vibration System | Devices Applied | Purpose of System | Scaffold Applicability | Vibration Properties/Frequency | Cells Applications | Effects on cell Culture | Unique Strengths | Limitations | References |
|---|---|---|---|---|---|---|---|---|---|
| Bio-reactor System | A device, like a vessel or container, where cell culturing is carried out | Study the dynamic factors of cells, including oxygen contents, shear, differentiations | Yes, both 2D and 3D | Most frequency 10–200 Hz; amplitude 0–5 mm etc. | bone and cartilage cells, MSCs cells etc. | Increased proliferation; help gene expression etc.; increased cell viability | Tend to be inexpensive, easily establishable | Frequency cannot be precisely controlled | [72,73,74,75,76,77] |
| Loudspeaker-based Vibratory System | A subwoofer loudspeaker, water-proof Mylar speaker etc. | In vitro platform for evaluating cellular responses to vibration | Yes, chiefly for 2D | Frequency 60–1600 Hz, amplitude 0–30 mm etc. | MSCs cells, vocal fold cells | Help proliferation, help release some cell product, like IL-8 | Relatively accurate and stable | Extra tools needed to calibrate the System; limited in cell application | [60,77,78] |
| Mechanical Stimulator System | External device, like piezoelectric actuator or vibratory transducer | Investigate the frequency-dependent effect from vibration | Yes, both 2D and 3D | Frequency 30–200 Hz, amplitude 0–30 mm etc. | Majorly in Bone cells, osteoblasts | Benefit gene expression, proliferation and differentiation | Easily accessible, and widely applied | Limited cell application; inflexibility of frequency control | [79,80,81] |
| Ultrasonic vibration System | Piezoelectric element, Ultrasonic generator etc. | Study cell behavior under vibration stimulation with higher frequencies | Yes, both 2D and 3D | Frequency 100 Hz–1 MHz, amplitude 5–50 μm etc. | Myoblast cells etc. | Increase the proliferation of cells | Capability of generating high frequency | May damage cells and hinder normal proliferation | [69,82] |
| 3D Micro-vibration Stage | A micro-vibrator stage basically consists embedded vibrator | Study the cell behaviors in dynamic culture morphologically | Yes, chiefly for 3D | Frequency 10–50 Hz, amplitude 30–50 μm | human osteoblast cells etc. | Non-invasive and three-dimensional vibration | Affects gene expression pattern and makes the cells remain younger | Limited frequency range; May damage cells | [60,83] |
| Mechanical Micro-vibrator System | A micro-vibrator electric device | Mimic dynamically mechanical forces in vivo, evaluate vibration responses | Yes, both 2D and 3D | Frequency 10–100 Hz, amplitude 0–5 mm | mouse and human embryo etc. | Precious frequency and time control | Benefits cell’s in vitro fertilization and development rates | Limited frequency range; special device needed | [84,85] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Xing, K.; Hsu, H.-Y. Trinity of Three-Dimensional (3D) Scaffold, Vibration, and 3D Printing on Cell Culture Application: A Systematic Review and Indicating Future Direction. Bioengineering 2018, 5, 57. https://doi.org/10.3390/bioengineering5030057
Yuan H, Xing K, Hsu H-Y. Trinity of Three-Dimensional (3D) Scaffold, Vibration, and 3D Printing on Cell Culture Application: A Systematic Review and Indicating Future Direction. Bioengineering. 2018; 5(3):57. https://doi.org/10.3390/bioengineering5030057
Chicago/Turabian StyleYuan, Haobo, Ke Xing, and Hung-Yao Hsu. 2018. "Trinity of Three-Dimensional (3D) Scaffold, Vibration, and 3D Printing on Cell Culture Application: A Systematic Review and Indicating Future Direction" Bioengineering 5, no. 3: 57. https://doi.org/10.3390/bioengineering5030057