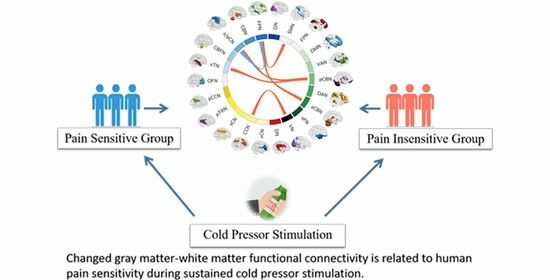
Functional Changes of White Matter Are Related to Human Pain Sensitivity during Sustained Nociception
Abstract
:1. Introduction
2. Study Design
2.1. Participants
2.2. Experimental Procedure
2.3. Data Acquisition
2.4. Data Preprocessing
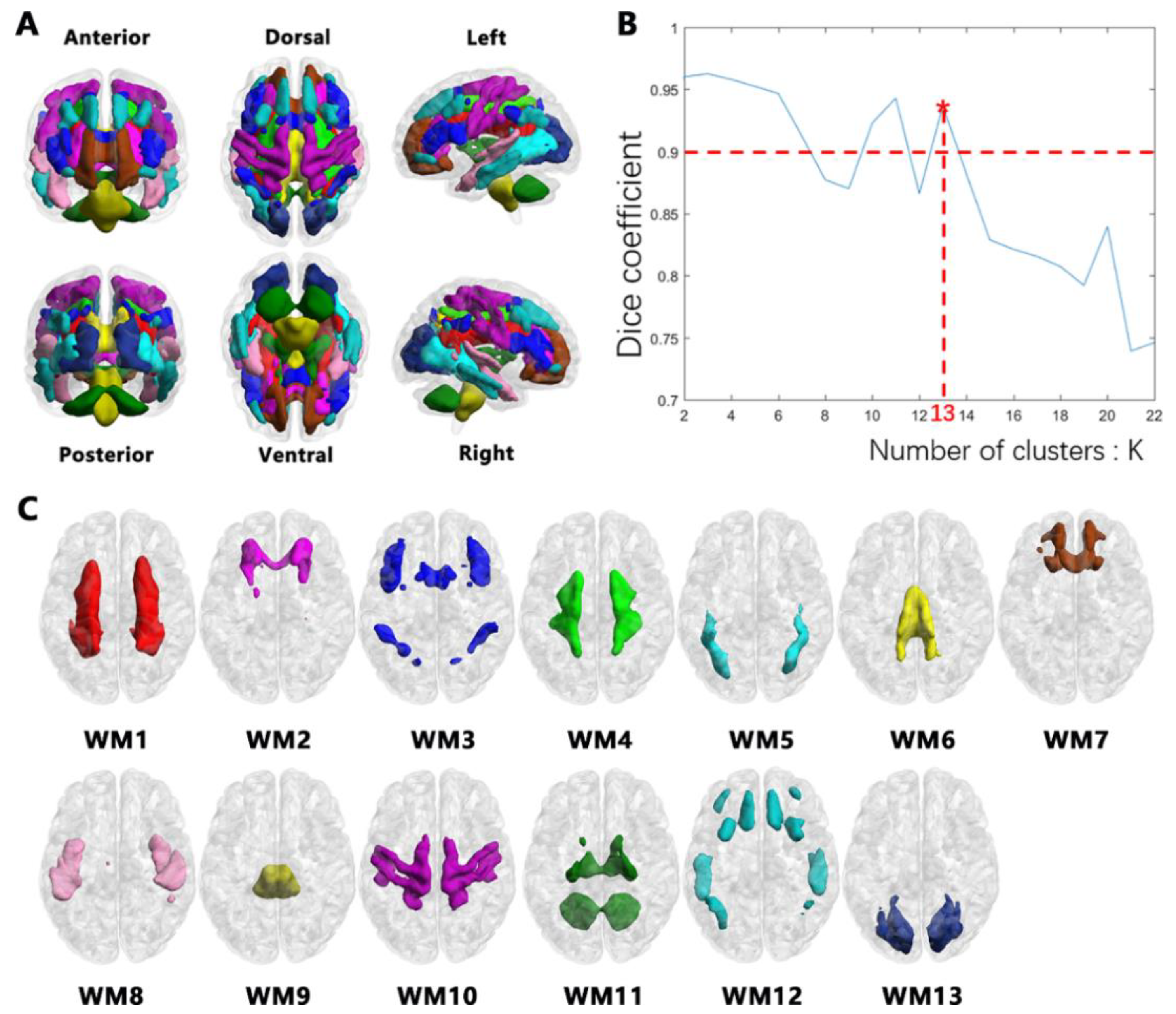
2.5. Clustering WM Networks
2.6. FC Analysis of WM Networks
2.7. Statistical Analysis
2.8. Correlations with Emotion Scales
2.9. Validation Analysis
3. Results
3.1. Demographics and Psychological Characteristics
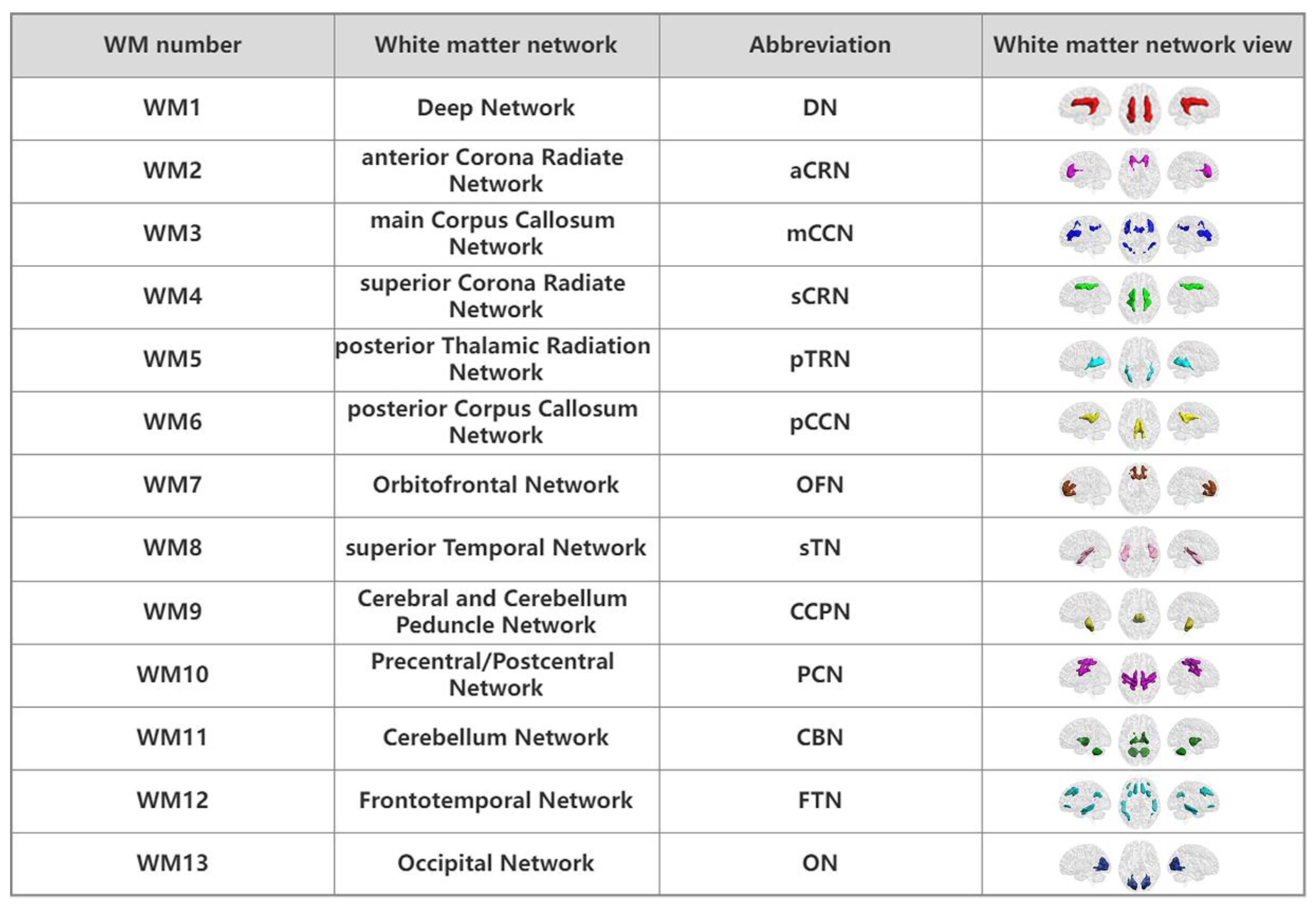
3.2. WM Networks
3.3. Alterations in FC between WM Networks
3.4. Alterations in FC between WM and GM Networks
3.5. Correlation between FC and Scale Scores
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Damascelli, M.; Woodward, T.S.; Sanford, N.; Zahid, H.B.; Lim, R.; Scott, A.; Kramer, J.K. Multiple Functional Brain Networks Related to Pain Perception Revealed by fMRI. Neuroinformatics 2022, 20, 155–172. [Google Scholar] [CrossRef]
- Villemure, C.; Bushnell, M.C. Mood Influences Supraspinal Pain Processing Separately from Attention. J. Neurosci. 2009, 29, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, P.; Kalso, E.; Petersson, K.M.; Ingvar, M. Placebo and Opioid Analgesia—Imaging a Shared Neuronal Network. Science 2002, 295, 1737–1740. [Google Scholar] [CrossRef]
- Wager, T.D.; Rilling, J.K.; Smith, E.E.; Sokolik, A.; Casey, K.L.; Davidson, R.J.; Kosslyn, S.M.; Rose, R.M.; Cohen, J.D. Placebo-Induced Changes in fMRI in the Anticipation and Experience of Pain. Science 2004, 303, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Wiech, K.; Ploner, M.; Tracey, I. Neurocognitive aspects of pain perception. Trends Cogn. Sci. 2008, 12, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, F.; Yang, H.; Jin, Y.; Lai, W.; Roberts, N.; Jia, Z.; Gong, Q. Effect of experimental orthodontic pain on gray and white matter functional connectivity. CNS Neurosci. Ther. 2021, 27, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Tracey, I.; Mantyh, P.W. The Cerebral Signature for Pain Perception and Its Modulation. Neuron 2007, 55, 377–391. [Google Scholar] [CrossRef]
- Cerminara, N.L.; Koutsikou, S.; Lumb, B.M.; Apps, R. The periaqueductal grey modulates sensory input to the cerebellum: A role in coping behaviour? Eur. J. Neurosci. 2009, 29, 2197–2206. [Google Scholar] [CrossRef]
- Moulton, E.A.; Schmahmann, J.D.; Becerra, L.; Borsook, D. The cerebellum and pain: Passive integrator or active participator? Brain Res. Rev. 2010, 65, 14–27. [Google Scholar] [CrossRef]
- Saab, C.Y.; Willis, W.D. Cerebellar stimulation modulates the intensity of a visceral nociceptive reflex in the rat. Exp. Brain Res. 2002, 146, 117–121. [Google Scholar] [CrossRef]
- Slutsky-Ganesh, A.B.; Diekfuss, J.A.; Grooms, D.R.; Simon, J.E.; Anand, M.; Lamplot, J.D.; Jayanthi, N.; Wong, P.K.; Lyle, M.A.; Myer, G.D. A preliminary investigation of the effects of patellar displacement on brain activation and perceived pain in young females with patellofemoral pain. J. Sci. Med. Sport 2022, 25, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Liang, H.-B.; Li, M.; Hu, Y.; Wu, J.; Qiao, Y.; Liu, J.-R.; Du, X. Disrupted White Matter Functional Connectivity with the Cerebral Cortex in Migraine Patients. Front. Neurosci. 2021, 15, 799854. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, G.; Shpaner, M.; Watts, R.; Andrews, T.; Filippi, C.G.; Davis, M.; Naylor, M.R. White Matter Involvement in Chronic Musculoskeletal Pain. J. Pain 2014, 15, 1110–1119. [Google Scholar] [CrossRef]
- Warbrick, T.; Fegers-Stollenwerk, V.; Maximov, I.I.; Grinberg, F.; Shah, N.J. Using Structural and Functional Brain Imaging to Inves-tigate Responses to Acute Thermal Pain. J. Pain 2016, 17, 836–844. [Google Scholar] [CrossRef]
- Stein, N.; Sprenger, C.; Scholz, J.; Wiech, K.; Bingel, U. White matter integrity of the descending pain modulatory system is associated with interindividual differences in placebo analgesia. Pain 2012, 153, 2210–2217. [Google Scholar] [CrossRef]
- Schmelz, M.; Schmid, R.; Handwerker, H.O.; Torebjörk, H.E. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain 2000, 123 Pt 3, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.; Barr, R.G.; Young, S.N. The cold pressor test in children: Methodological aspects and the analgesic effect of intraoral sucrose. Pain 1994, 56, 175–183. [Google Scholar] [CrossRef]
- Dahlquist, L.M.; McKenna, K.D.; Jones, K.K.; Dillinger, L.; Weiss, K.E.; Ackerman, C.S. Active and passive distraction using a head-mounted display helmet: Effects on cold pressor pain in children. Health Psychol. 2007, 26, 794–801. [Google Scholar]
- Eckhardt, K.; Ammon, S.; Hofmann, U.; Riebe, A.; Gugeler, N.; Mikus, G. Gabapentin enhances the analgesic effect of morphine in healthy volunteers. Anesth Analg. 2000, 91, 185–191. [Google Scholar] [CrossRef]
- King, F.J.; Heinrich, D.L.; Stephenson, R.S.; Spielberger, C.D. An investigation of the causal influence of trait and state anxiety on academic achievement. J. Educ. Psychol. 1976, 68, 330–334. [Google Scholar] [CrossRef]
- Shek, D.T. The Chinese version of the State-Trait Anxiety Inventory: Its relationship to different measures of psychological well-being. J. Clin. Psychol. 1993, 49, 349–358. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observa-tional Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Yan, C.-G.; Craddock, R.C.; He, Y.; Milham, M.P. Addressing head motion dependencies for small-world topologies in functional connectomics. Front. Hum. Neurosci. 2013, 7, 910. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and mo-tion correction of brain images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Craddock, R.C.; James, G.A.; Holtzheimer, P.E., 3rd; Hu, X.P.; Mayberg, H.S. A whole brain fMRI atlas generated via spatially con-strained spectral clustering. Hum. Brain Mapp. 2012, 33, 1914–1928. [Google Scholar] [CrossRef] [PubMed]
- Lange, T.; Roth, V.; Braun, M.L.; Buhmann, J.M. Stability-Based Validation of Clustering Solutions. Neural Comput. 2004, 16, 1299–1323. [Google Scholar] [CrossRef]
- Yeo, B.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R.; et al. The organization of the human cerebral cortex estimated by intrinsic functional connec-tivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [PubMed]
- Peer, M.; Nitzan, M.; Bick, A.S.; Levin, N.; Arzy, S. Evidence for Functional Networks within the Human Brain’s White Matter. J. Neurosci. 2017, 37, 6394–6407. [Google Scholar] [CrossRef] [PubMed]
- Adamaszek, M.; D’agata, F.; Ferrucci, R.; Habas, C.; Keulen, S.; Kirkby, K.C.; Leggio, M.; Mariën, P.; Molinari, M.; Moulton, E.; et al. Consensus Paper: Cerebellum and Emotion. Cerebellum 2017, 16, 552–576. [Google Scholar] [CrossRef]
- Blanton, H.; Armin, S.; Muenster, S.; Abood, M.; Benamar, K. Contribution of G Protein-Coupled Receptor 55 to Periaqueductal Gray-Mediated Antinociception in the Inflammatory Pain. Cannabis Cannabinoid Res. 2022, 7, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Heinricher, M.; Tavares, I.; Leith, J.; Lumb, B. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res. Rev. 2009, 60, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Porreca, F.; Ossipov, M.H.; Gebhart, G.F. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002, 25, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.D.; Moayedi, M. Central Mechanisms of Pain Revealed Through Functional and Structural MRI. J. Neuroimmune Pharmacol. 2013, 8, 518–534. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.E.; Mayer, A.R.; Teshiba, T.M.; Ling, J.; Smith, B.W.; Wilcox, G.L.; Mullins, P.G. The Subjective Experience of Pain: An FMRI Study of Percept-Related Models and Functional Connectivity. Pain Med. 2015, 16, 2121–2133. [Google Scholar] [CrossRef]
- Fransson, P.; Marrelec, G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage 2008, 42, 1178–1184. [Google Scholar] [CrossRef]
- Guo, W.; Liu, F.; Zhang, Z.; Liu, G.; Liu, J.; Yu, L.; Xiao, C.; Zhao, J. Increased Cerebellar Functional Connectivity with the Default-Mode Network in Unaffected Siblings of Schizophrenia Patients at Rest. Schizophr. Bull. 2015, 41, 1317–1325. [Google Scholar] [CrossRef]
- Kucyi, A.; Salomons, T.V.; Davis, K.D. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc. Natl. Acad. Sci. USA 2013, 110, 18692–18697. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R.; Smallwood, J.; Spreng, R.N. The default network and self-generated thought: Component processes, dy-namic control, and clinical relevance. Ann. N. Y. Acad. Sci. 2014, 1316, 29–52. [Google Scholar] [CrossRef]
- Agosta, F.; Canu, E.; Inuggi, A.; Chiò, A.; Riva, N.; Silani, V.; Calvo, A.; Messina, S.; Falini, A.; Comi, G.; et al. Resting state functional connectivity alterations in primary lateral sclerosis. Neurobiol. Aging 2014, 35, 916–925. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Mima, T.; Shimazu, H.; Isomura, Y. Directional organization of sensorimotor oscillatory activity related to the elec-tromyogram in the monkey. Clin. Neurophysiol. 2009, 120, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Bruurmijn, M.; Raemaekers, M.; Branco, M.P.; Ramsey, N.F.; Vansteensel, M.J. Distinct representation of ipsilateral hand movements in sensorimotor areas. Eur. J. Neurosci. 2021, 54, 7599–7608. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Liu, B.; Zhang, X.; Li, J.; Qin, W.; Yu, C.; Jiang, T. The Structural Connectivity Pattern of the Default Mode Network and Its Association with Memory and Anxiety. Front. Neuroanat. 2015, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Connelly, M.; Bromberg, M.H.; Anthony, K.K.; Gil, K.M.; Franks, L.; Schanberg, L.E. Emotion Regulation Predicts Pain and Functioning in Children with Juvenile Idiopathic Arthritis: An Electronic Diary Study. J. Pediatr. Psychol. 2012, 37, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Lucchetti, G.; Oliveira, A.B.; Mercante, J.P.P.; Peres, M.F.P. Anxiety and Fear-Avoidance in Musculoskeletal Pain. Curr. Pain Headache Rep. 2012, 16, 399–406. [Google Scholar] [CrossRef]
- Kapoor, S.; White, J.; Thorn, B.E.; Block, P. Patients Presenting to the Emergency Department with Acute Pain: The Significant Role of Pain Catastrophizing and State Anxiety. Pain Med. 2016, 17, 1069–1078. [Google Scholar] [CrossRef]


| Pain Sensitive Group (PS) | Pain Insensitive Group (PIS) | p-Value | |
|---|---|---|---|
| Age (years) | 22.96 ± 1.59 | 22.48 ± 1.93 | 0.3282 a |
| Gender (male/female) | 26/0 | 27/0 | - |
| Head Motion (FD) | cold: 0.035 ± 0.016 normal: 0.038 ± 0.015 | cold: 0.040 ± 0.018 normal: 0.038 ± 0.011 | 0.5530 b1 0.9050 b2 0.1460 b3 |
| STAI-SA score | 36.58 ± 6.81 | 34.93 ± 6.87 | 0.3929 a |
| STAI-TA score | 39.50 ± 6.46 | 38.41 ± 5.59 | 0.5208 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Hu, L.; Tan, S.; Tang, Y.; Duan, M.; Yao, D.; Zhao, G.; Luo, C. Functional Changes of White Matter Are Related to Human Pain Sensitivity during Sustained Nociception. Bioengineering 2023, 10, 988. https://doi.org/10.3390/bioengineering10080988
He H, Hu L, Tan S, Tang Y, Duan M, Yao D, Zhao G, Luo C. Functional Changes of White Matter Are Related to Human Pain Sensitivity during Sustained Nociception. Bioengineering. 2023; 10(8):988. https://doi.org/10.3390/bioengineering10080988
Chicago/Turabian StyleHe, Hui, Lan Hu, Saiying Tan, Yingjie Tang, Mingjun Duan, Dezhong Yao, Guocheng Zhao, and Cheng Luo. 2023. "Functional Changes of White Matter Are Related to Human Pain Sensitivity during Sustained Nociception" Bioengineering 10, no. 8: 988. https://doi.org/10.3390/bioengineering10080988