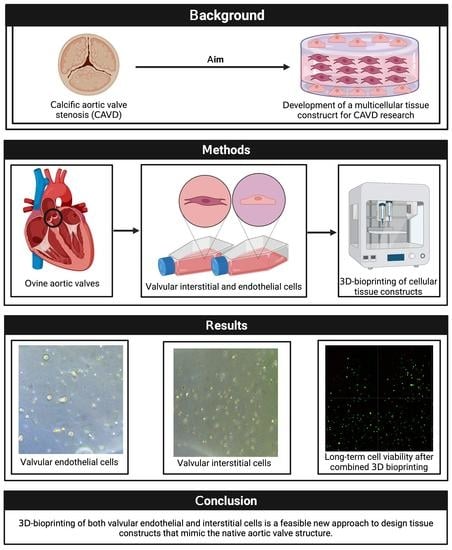
Three-Dimensional Bioprinting of Ovine Aortic Valve Endothelial and Interstitial Cells for the Development of Multicellular Tissue Engineered Tissue Constructs
Abstract
:1. Introduction
2. Materials and Methods
3. Results
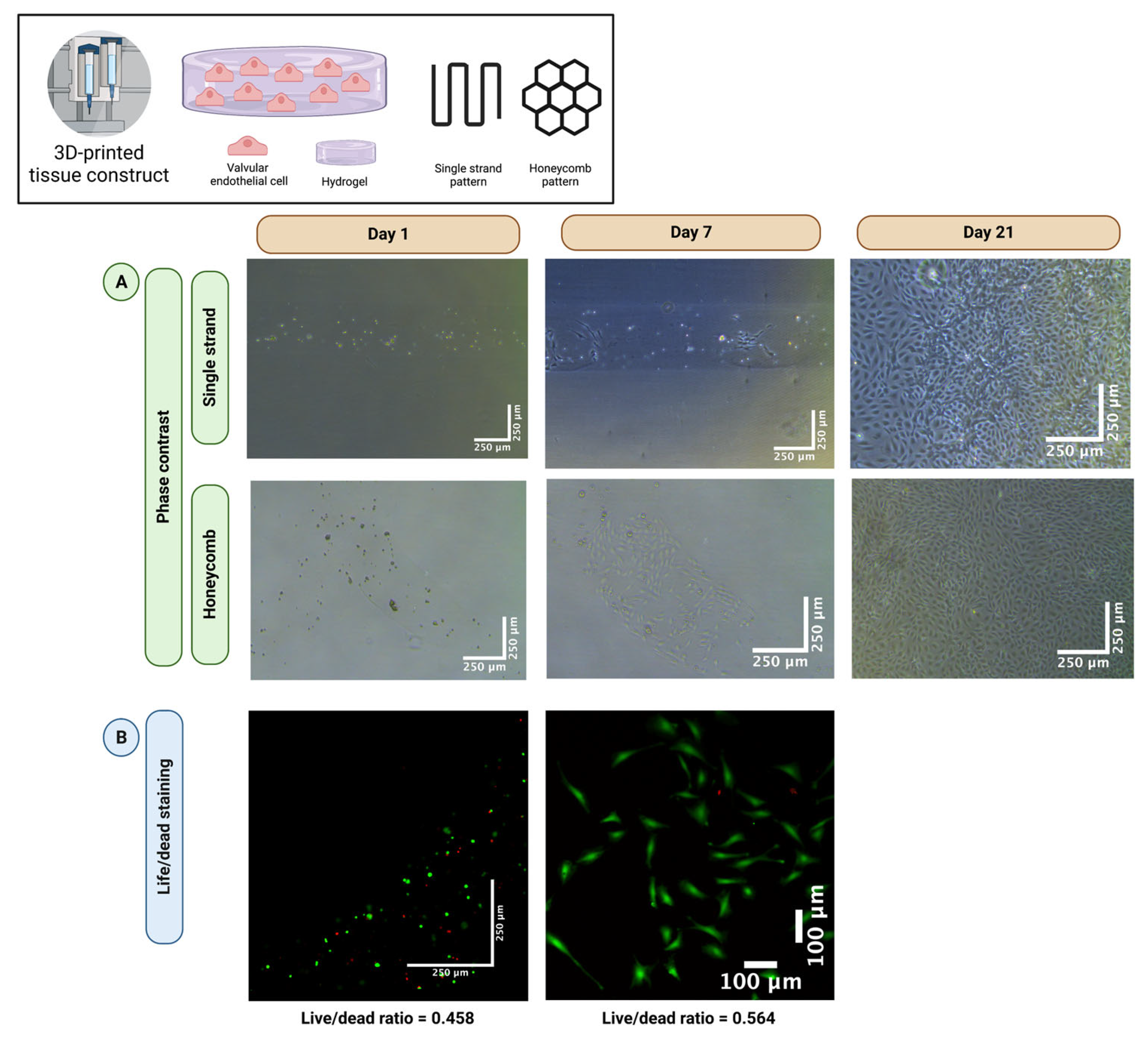
3.1. Valvular Endothelial Cells Retain Good Long-Term Cell Viability after 3D-Bioprinting
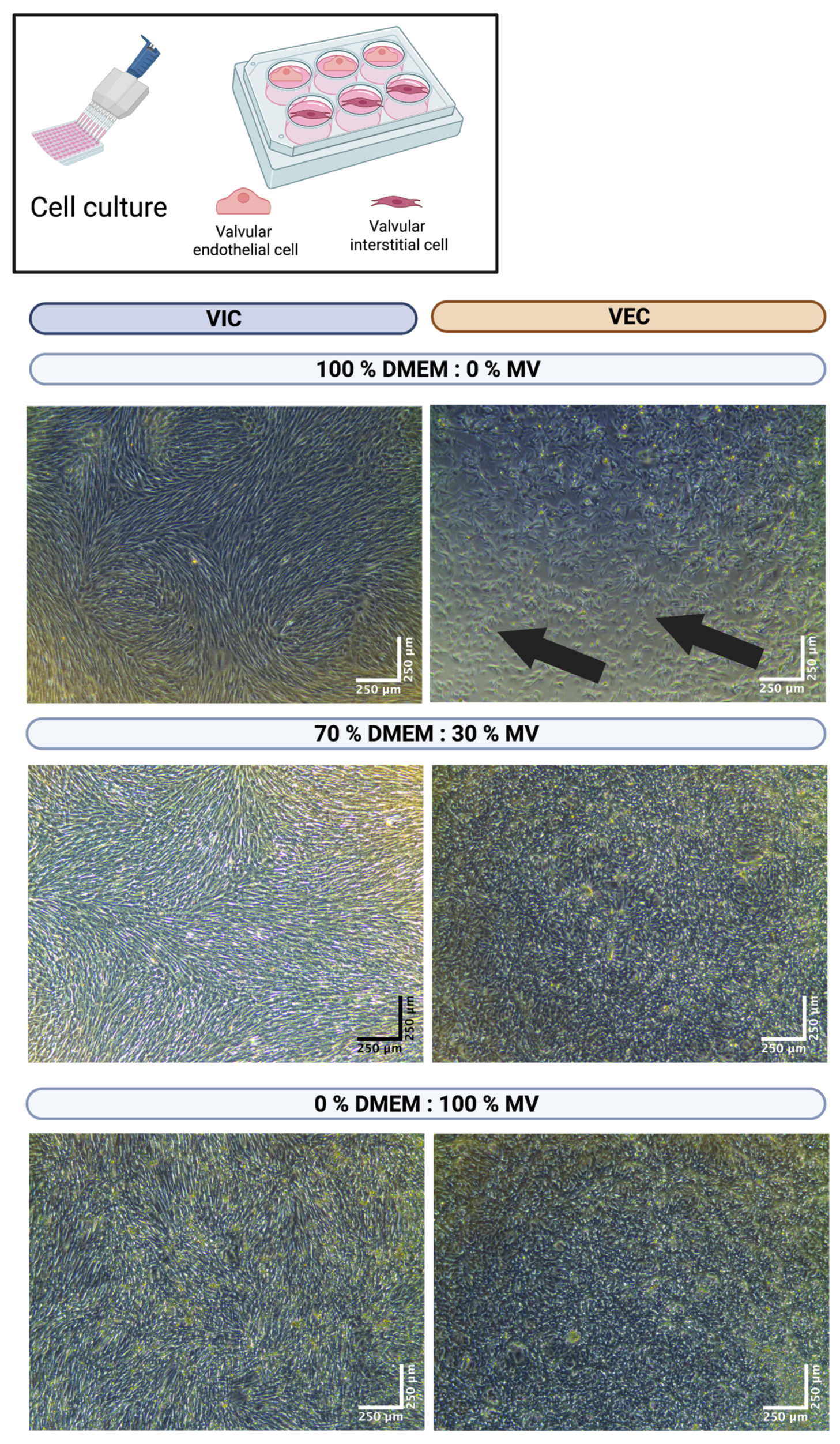
3.2. The DEMEM/MV Ratio of 70:30 Is Best for Both Valvular Interstitial and Endothelial Cells
3.3. The DEMEM/MV Ratio of 70:30 Is Suitable for 3D-Bioprinted Valvular Interstitial and Endothelial Cell Monoculture
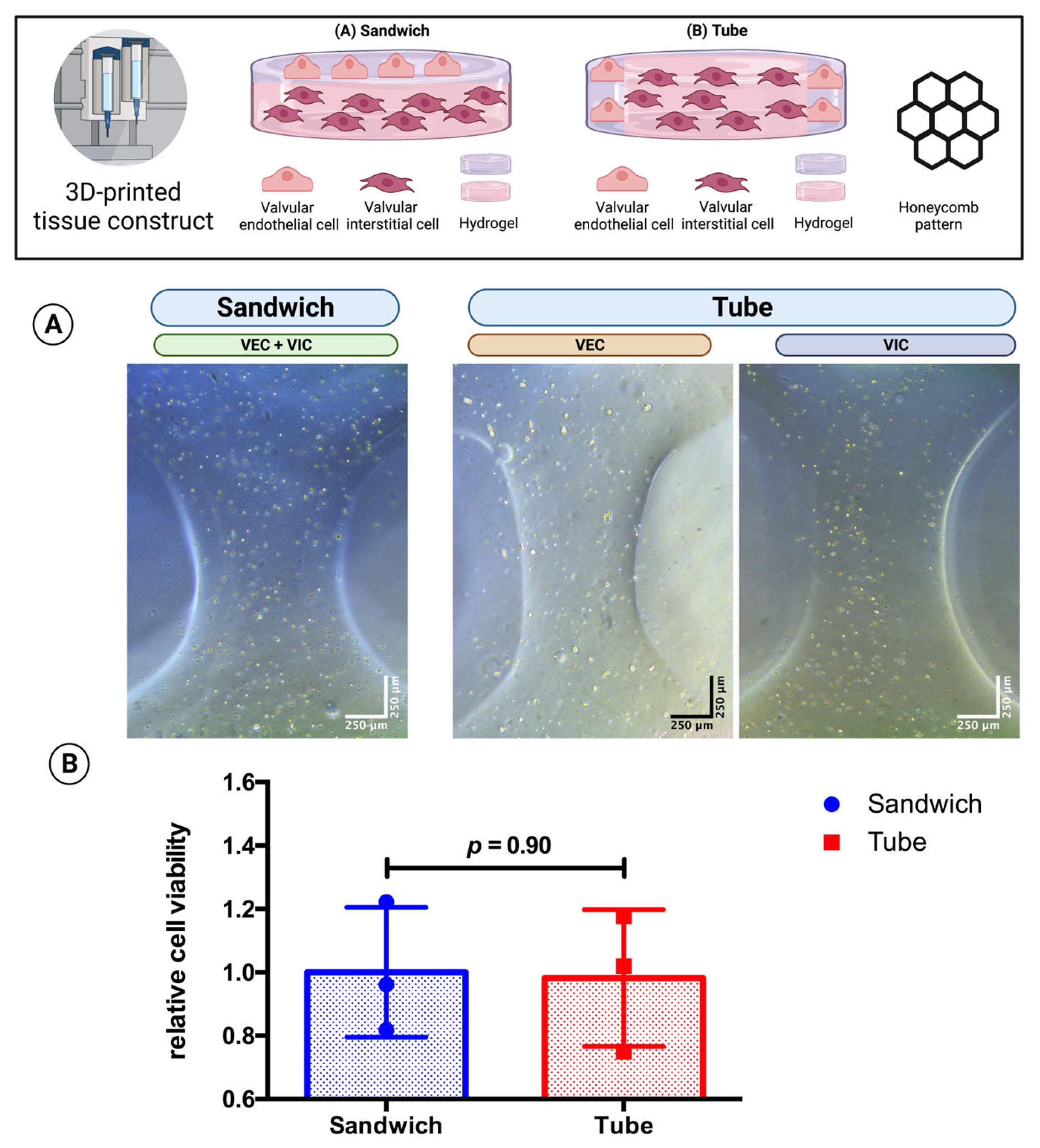
3.4. The DEMEM/MV Ratio of 70:30 Is Suitable for 3D-Bioprinted Multicellular Valvular Interstitial and Endothelial Cell Co-Culture
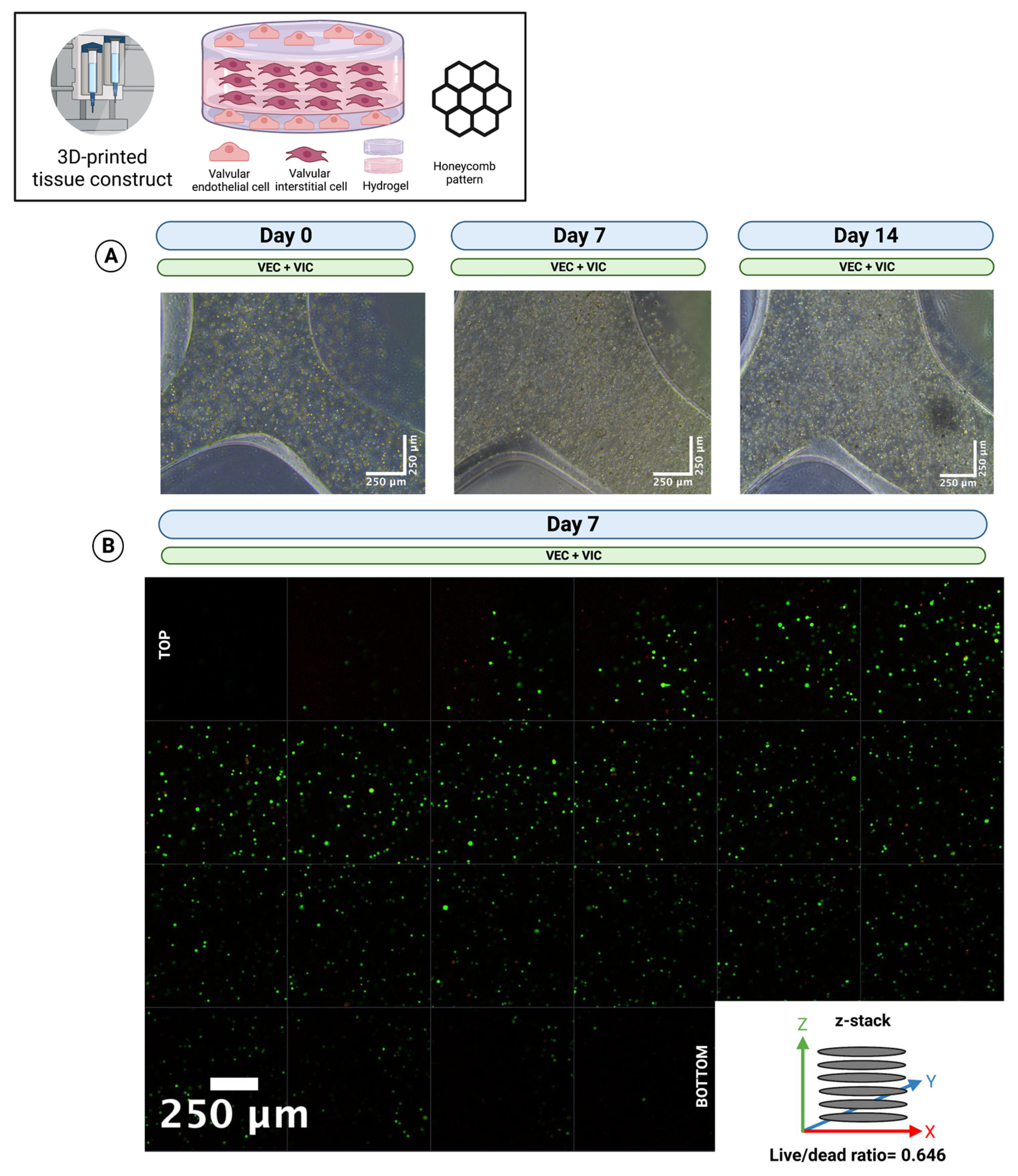
3.5. Multicellular Valvular Interstitial and Endothelial Cell-Laden Tissue Constructs Retain Cell Viability in Different 3D-Architectures
3.6. The Printing of Thick Multilayer Multicellular Valvular Interstitial and Endothelial Cell-Laden Tissue Construct Is Feasible
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAVD | Calcific aortic valve disease |
| DMEM | Dulbecco’s modified eagle’s medium |
| DPBS | Dulbecco’s balanced salt solution |
| ECM | Extracellular matrix |
| HUVEC | Human umbilical vein endothelial cell |
| MV | Endothelial cell growth medium |
| TGF-β | Transforming growth factor beta |
| VEC | Valvular endothelial cell |
| VIC | Valvular interstitial cell |
References
- Lindman, B.R.; Clavel, M.A.; Mathieu, P.; Iung, B.; Lancellotti, P.; Otto, C.M.; Pibarot, P. Calcific aortic stenosis. Nat. Rev. Dis. Primers 2016, 2, 16006. [Google Scholar] [CrossRef] [Green Version]
- Peeters, F.; Meex, S.J.R.; Dweck, M.R.; Aikawa, E.; Crijns, H.; Schurgers, L.J.; Kietselaer, B. Calcific aortic valve stenosis: Hard disease in the heart: A biomolecular approach towards diagnosis and treatment. Eur. Heart J. 2018, 39, 2618–2624. [Google Scholar] [CrossRef] [Green Version]
- Bowler, M.A.; Merryman, W.D. In vitro models of aortic valve calcification: Solidifying a system. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2015, 24, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, M.E.; Gonzalez Rodriguez, A.; Speckl, K.F.; Walker, C.J.; Midekssa, F.S.; Grim, J.C.; Weiss, R.M.; Anseth, K.S. Collagen networks within 3D PEG hydrogels support valvular interstitial cell matrix mineralization. Acta Biomater. 2021, 119, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Hjortnaes, J.; Goettsch, C.; Hutcheson, J.D.; Camci-Unal, G.; Lax, L.; Scherer, K.; Body, S.; Schoen, F.J.; Kluin, J.; Khademhosseini, A.; et al. Simulation of early calcific aortic valve disease in a 3D platform: A role for myofibroblast differentiation. J. Mol. Cell Cardiol. 2016, 94, 13–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabry, K.M.; Payne, S.Z.; Anseth, K.S. Transcriptional profiles of valvular interstitial cells cultured on tissue culture polystyrene, on 2D hydrogels, or within 3D hydrogels. Data Brief 2015, 5, 959–962. [Google Scholar] [CrossRef] [Green Version]
- Mabry, K.M.; Payne, S.Z.; Anseth, K.S. Microarray analyses to quantify advantages of 2D and 3D hydrogel culture systems in maintaining the native valvular interstitial cell phenotype. Biomaterials 2016, 74, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Jenke, A.; Kistner, J.; Saradar, S.; Chekhoeva, A.; Yazdanyar, M.; Bergmann, A.K.; Rötepohl, M.V.; Lichtenberg, A.; Akhyari, P. Transforming growth factor-β1 promotes fibrosis but attenuates calcification of valvular tissue applied as a three-dimensional calcific aortic valve disease model. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1123–H1141. [Google Scholar] [CrossRef]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. A 2013, 101, 1255–1264. [Google Scholar] [CrossRef] [Green Version]
- Van der Valk, D.C.; van der Ven, C.F.T.; Blaser, M.C.; Grolman, J.M.; Wu, P.J.; Fenton, O.S.; Lee, L.H.; Tibbitt, M.W.; Andresen, J.L.; Wen, J.R.; et al. Engineering a 3D-Bioprinted Model of Human Heart Valve Disease Using Nanoindentation-Based Biomechanics. Nanomaterials 2018, 8, 296. [Google Scholar] [CrossRef] [Green Version]
- Duan, B.; Kapetanovic, E.; Hockaday, L.A.; Butcher, J.T. Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater. 2014, 10, 1836–1846. [Google Scholar] [CrossRef] [Green Version]
- Immohr, M.B.; Dos Santos Adrego, F.; Teichert, H.L.; Schmidt, V.; Sugimura, Y.; Bauer, S.J.; Barth, M.; Lichtenberg, A.; Akhyari, P. 3D-Bioprinting of Aortic Valve Interstitial Cells: Impact of Hydrogel and Printing Parameters on Cell Viability. Biomed. Mater. 2022, 18, 015004. [Google Scholar] [CrossRef] [PubMed]
- Deo, K.A.; Singh, K.A.; Peak, C.W.; Alge, D.L.; Gaharwar, A.K. Bioprinting 101: Design, Fabrication, and Evaluation of Cell-Laden 3D Bioprinted Scaffolds. Tissue Eng. Part A 2020, 26, 318–338. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, A.; Pagan-Diaz, G.J.; Grant, L.; Cvetkovic, C.; Bramlet, M.; Vozenilek, J.; Kesavadas, T.; Bashir, R. 3D printing for preoperative planning and surgical training: A review. Biomed Microdevices 2018, 20, 65. [Google Scholar] [CrossRef] [PubMed]
- Blaeser, A.; Duarte Campos, D.F.; Puster, U.; Richtering, W.; Stevens, M.M.; Fischer, H. Controlling Shear Stress in 3D Bioprinting is a Key Factor to Balance Printing Resolution and Stem Cell Integrity. Adv Healthc. Mater. 2016, 5, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Yang, B.; Mohabatpour, F.; Betancourt, N.; Sarker, M.D.; Papagerakis, P.; Chen, X. Process-induced cell damage: Pneumatic versus screw-driven bioprinting. Biofabrication 2020, 12, 025011. [Google Scholar] [CrossRef]
- Deb, N.; Lacerda, C.M.R. The Individual and Combined Effects of Shear, Tension, and Flexure on Aortic Heart Valve Endothelial Cells in Culture. Cardiovasc. Eng. Technol. 2022, 13, 443–451. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.S.; Kwon, Y.W.; Kong, J.S.; Park, G.T.; Gao, G.; Han, W.; Kim, M.B.; Lee, H.; Kim, J.H.; Cho, D.W. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53. [Google Scholar] [CrossRef]
- Duong, V.T.; Dang, T.T.; Hwang, C.H.; Back, S.H.; Koo, K.I. Coaxial printing of double-layered and free-standing blood vessel analogues without ultraviolet illumination for high-volume vascularised tissue. Biofabrication 2020, 12, 045033. [Google Scholar] [CrossRef]
- Agarwal, T.; Tan, S.-A.; Onesto, V.; Law, J.X.; Agrawal, G.; Pal, S.; Lim, W.L.; Sharifi, E.; Moghaddam, F.D.; Maiti, T.K. Engineered herbal scaffolds for tissue repair and regeneration: Recent trends and technologies. Biomed. Eng. Adv. 2021, 2, 100015. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Yeleswarapu, S.; Heinrich, M.A.; Prakash, J.; Pati, F. Mimicking tumor microenvironment by 3D bioprinting: 3D cancer modeling. Biofabrication 2022, 14, 032002. [Google Scholar] [CrossRef] [PubMed]
- Bedir, T.; Ulag, S.; Ustundag, C.B.; Gunduz, O. 3D bioprinting applications in neural tissue engineering for spinal cord injury repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110741. [Google Scholar] [CrossRef] [PubMed]
- Weng, T.; Zhang, W.; Xia, Y.; Wu, P.; Yang, M.; Jin, R.; Xia, S.; Wang, J.; You, C.; Han, C.; et al. 3D bioprinting for skin tissue engineering: Current status and perspectives. J. Tissue Eng. 2021, 12, 20417314211028574. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.L.; Merryman, W.D. Side-specific valvular endothelial-interstitial cell mechano-communication via cadherin-11. J. Biomech. 2021, 119, 110253. [Google Scholar] [CrossRef]
- Hjortnaes, J.; Shapero, K.; Goettsch, C.; Hutcheson, J.D.; Keegan, J.; Kluin, J.; Mayer, J.E.; Bischoff, J.; Aikawa, E. Valvular interstitial cells suppress calcification of valvular endothelial cells. Atherosclerosis 2015, 242, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Tseng, H.; Balaoing, L.R.; Grigoryan, B.; Raphael, R.M.; Killian, T.C.; Souza, G.R.; Grande-Allen, K.J. A three-dimensional co-culture model of the aortic valve using magnetic levitation. Acta Biomater. 2014, 10, 173–182. [Google Scholar] [CrossRef]
- Vadana, M.; Cecoltan, S.; Ciortan, L.; Macarie, R.D.; Tucureanu, M.M.; Mihaila, A.C.; Droc, I.; Butoi, E.; Manduteanu, I. Molecular mechanisms involved in high glucose-induced valve calcification in a 3D valve model with human valvular cells. J. Cell Mol. Med. 2020, 24, 6350–6361. [Google Scholar] [CrossRef]
- Stadelmann, K.; Weghofer, A.; Urbanczyk, M.; Maulana, T.I.; Loskill, P.; Jones, P.D.; Schenke-Layland, K. Development of a bi-layered cryogenic electrospun polylactic acid scaffold to study calcific aortic valve disease in a 3D co-culture model. Acta Biomater. 2022, 140, 364–378. [Google Scholar] [CrossRef]
- Puperi, D.S.; Balaoing, L.R.; O’Connell, R.W.; West, J.L.; Grande-Allen, K.J. 3-Dimensional spatially organized PEG-based hydrogels for an aortic valve co-culture model. Biomaterials 2015, 67, 354–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gee, T.W.; Richards, J.M.; Mahmut, A.; Butcher, J.T. Valve endothelial-interstitial interactions drive emergent complex calcific lesion formation in vitro. Biomaterials 2021, 269, 120669. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.; El-Hamamsy, I.; Chen, S.; Sarang, Z.; Sarathchandra, P.; Yacoub, M.H.; Chester, A.H.; Butcher, J.T. Side-specific endothelial-dependent regulation of aortic valve calcification: Interplay of hemodynamics and nitric oxide signaling. Am. J. Pathol. 2013, 182, 1922–1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.R.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell’Erba, V.; et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016, 110, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Saini, G.; Segaran, N.; Mayer, J.L.; Saini, A.; Albadawi, H.; Oklu, R. Applications of 3D Bioprinting in Tissue Engineering and Regenerative Medicine. J. Clin. Med. 2021, 10, 4966. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [Green Version]
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Hann, S.Y.; Cui, H.; Esworthy, T.; Zhou, X.; Lee, S.J.; Plesniak, M.W.; Zhang, L.G. Dual 3D printing for vascularized bone tissue regeneration. Acta Biomater. 2021, 123, 263–274. [Google Scholar] [CrossRef]
- Xie, R.; Zheng, W.; Guan, L.; Ai, Y.; Liang, Q. Engineering of Hydrogel Materials with Perfusable Microchannels for Building Vascularized Tissues. Small 2020, 16, e1902838. [Google Scholar] [CrossRef]
- Puluca, N.; Lee, S.; Doppler, S.; Munsterer, A.; Dressen, M.; Krane, M.; Wu, S.M. Bioprinting Approaches to Engineering Vascularized 3D Cardiac Tissues. Curr. Cardiol. Rep. 2019, 21, 90. [Google Scholar] [CrossRef]
- Kovacic, J.C.; Dimmeler, S.; Harvey, R.P.; Finkel, T.; Aikawa, E.; Krenning, G.; Baker, A.H. Endothelial to Mesenchymal Transition in Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 190–209. [Google Scholar] [CrossRef]
- Wirrig, E.E.; Yutzey, K.E. Conserved transcriptional regulatory mechanisms in aortic valve development and disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 737–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yutzey, K.E.; Demer, L.L.; Body, S.C.; Huggins, G.S.; Towler, D.A.; Giachelli, C.M.; Hofmann-Bowman, M.A.; Mortlock, D.P.; Rogers, M.B.; Sadeghi, M.M.; et al. Calcific aortic valve disease: A consensus summary from the Alliance of Investigators on Calcific Aortic Valve Disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2387–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ten Dijke, P.; Egorova, A.D.; Goumans, M.J.; Poelmann, R.E.; Hierck, B.P. TGF-beta signaling in endothelial-to-mesenchymal transition: The role of shear stress and primary cilia. Sci. Signal 2012, 5, pt2. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepanovska, J.; Supova, M.; Hanzalek, K.; Broz, A.; Matejka, R. Collagen Bioinks for Bioprinting: A Systematic Review of Hydrogel Properties, Bioprinting Parameters, Protocols, and Bioprinted Structure Characteristics. Biomedicines 2021, 9, 1137. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, J.; Mu, X.; Pramanick, A.; Kaplan, D.L.; Ghosh, S. Recent advances in bioprinting using silk protein-based bioinks. Biomaterials 2022, 287, 121672. [Google Scholar] [CrossRef]
- Di Vito, A.; Donato, A.; Presta, I.; Mancuso, T.; Brunetti, F.S.; Mastroroberto, P.; Amorosi, A.; Malara, N.; Donato, G. Extracellular Matrix in Calcific Aortic Valve Disease: Architecture, Dynamic and Perspectives. Int. J. Mol. Sci. 2021, 22, 913. [Google Scholar] [CrossRef]
- Sung, D.C.; Bowen, C.J.; Vaidya, K.A.; Zhou, J.; Chapurin, N.; Recknagel, A.; Zhou, B.; Chen, J.; Kotlikoff, M.; Butcher, J.T. Cadherin-11 Overexpression Induces Extracellular Matrix Remodeling and Calcification in Mature Aortic Valves. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1627–1637. [Google Scholar] [CrossRef] [Green Version]
- Hutson, H.N.; Marohl, T.; Anderson, M.; Eliceiri, K.; Campagnola, P.; Masters, K.S. Calcific Aortic Valve Disease Is Associated with Layer-Specific Alterations in Collagen Architecture. PLoS ONE 2016, 11, e0163858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinton, R.B., Jr.; Lincoln, J.; Deutsch, G.H.; Osinska, H.; Manning, P.B.; Benson, D.W.; Yutzey, K.E. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ. Res. 2006, 98, 1431–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Immohr, M.B.; Teichert, H.L.; dos Santos Adrego, F.; Schmidt, V.; Sugimura, Y.; Bauer, S.J.; Barth, M.; Lichtenberg, A.; Akhyari, P. Three-Dimensional Bioprinting of Ovine Aortic Valve Endothelial and Interstitial Cells for the Development of Multicellular Tissue Engineered Tissue Constructs. Bioengineering 2023, 10, 787. https://doi.org/10.3390/bioengineering10070787
Immohr MB, Teichert HL, dos Santos Adrego F, Schmidt V, Sugimura Y, Bauer SJ, Barth M, Lichtenberg A, Akhyari P. Three-Dimensional Bioprinting of Ovine Aortic Valve Endothelial and Interstitial Cells for the Development of Multicellular Tissue Engineered Tissue Constructs. Bioengineering. 2023; 10(7):787. https://doi.org/10.3390/bioengineering10070787
Chicago/Turabian StyleImmohr, Moritz Benjamin, Helena Lauren Teichert, Fabió dos Santos Adrego, Vera Schmidt, Yukiharu Sugimura, Sebastian Johannes Bauer, Mareike Barth, Artur Lichtenberg, and Payam Akhyari. 2023. "Three-Dimensional Bioprinting of Ovine Aortic Valve Endothelial and Interstitial Cells for the Development of Multicellular Tissue Engineered Tissue Constructs" Bioengineering 10, no. 7: 787. https://doi.org/10.3390/bioengineering10070787