Characterization of Postural Sway in Women with Osteoporosis and a Control Group by Means of Linear and Nonlinear Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Center-of-Pressure Recording and Data Analyses
2.3. Statistical Analysis
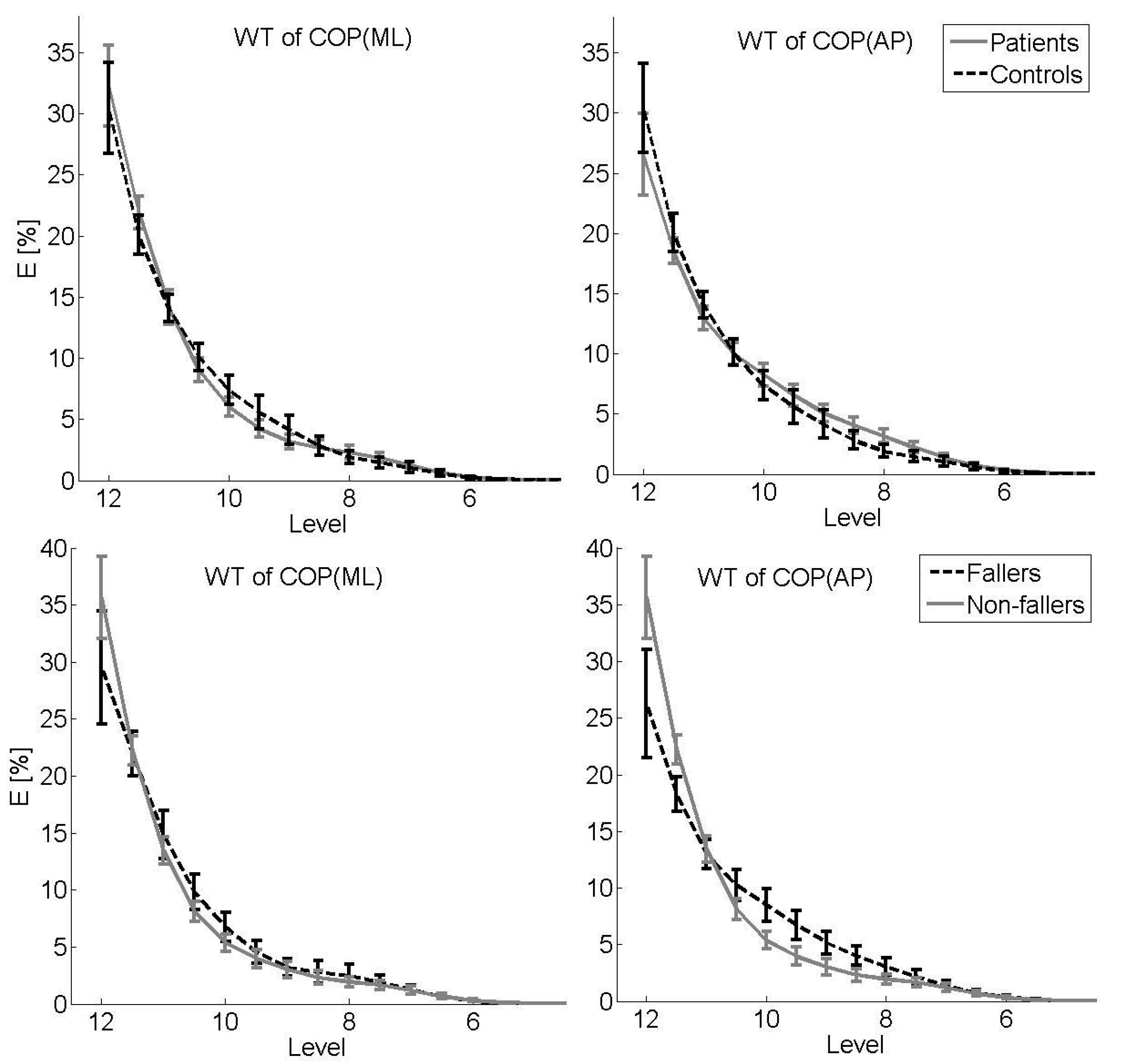
3. Results
3.1. Primary Analysis: Patients vs. Controls
3.2. Secondary Analysis: Fallers vs. Non-Fallers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Input Parameters
| Method | Details and Input Parameter |
|---|---|
| SD(x) (mm) | , data point of signal x, = mean value of x |
| ROM(x) (mm) | max(x) − min(x) |
| V(x) (mm/s) | , data point of signal x |
| PL (mm²) | with data point of ML sway and data point of AP sway |
| PSDWelch’s method | Hamming window of 2000 samples, 50% overlap, nfft =, fs = 100 Hz |
| WT | Mother wavelet = Coiflet with central frequency Hz, levels , which corresponds to the frequency range pf Hz to Hz , fs = 100 Hz |
| MSE | Radius , , scale , fs = 20 Hz (downsampling to 20 Hz) |
Appendix B. Wavelet Transformation and Multiscale Entropy
References
- Cummings, S.R.; Melton, L.J. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002, 359, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, P.; Cooper, C. Osteoporosis. Lancet 2006, 367, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Ganz, D.A.; Bao, Y.; Shekelle, P.G.; Rubenstein, L.Z. Will my patient fall? JAMA 2007, 297, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Stief, F.; Schäfer, A.; Vogt, L.; Kirchner, M.; Hübscher, M.; Thiel, C.; Banzer, W.; Meurer, A. Differences in gait performance, quadriceps strength, and fear of falling between fallers and non-fallers in women with osteoporosis. J. Aging Phys. Act. 2016, 24, 430–434. [Google Scholar] [CrossRef]
- Nardone, A.; Schieppati, M. The role of instrumental assessment of balance in clinical decision making. Eur. J. Phys. Rehabil. Med. 2010, 46, 221–237. [Google Scholar]
- Mancini, M.; Horak, F.B. The relevance of clinical balance assessment tools to differentiate balance deficits. Eur. J. Phys. Rehabil. Med. 2010, 46, 239–248. [Google Scholar]
- Piirtola, M.; Era, P. Force platform measurements as predictors of falls among older people—A review. Gerontology 2006, 52, 1–16. [Google Scholar] [CrossRef]
- Fujita, T.; Nakamura, S.; Ohue, M.; Fujii, Y.; Miyauchi, A.; Takagi, Y.; Tsugeno, H. Effect of age on body sway assessed by computerized posturography. J. Bone Miner. Metab. 2005, 23, 152–156. [Google Scholar] [CrossRef]
- Prado, J.M.; Stoffregen, T.A.; Duarte, M. Postural sway during dual tasks in young and elderly adults. Gerontology 2007, 53, 274–281. [Google Scholar] [CrossRef]
- Maki, B.E.; McIlroy, W.E. Control of rapid limb movements for balance recovery: Age-related changes and implications for fall prevention. Age Ageing 2006, 35, ii12–ii18. [Google Scholar] [CrossRef] [Green Version]
- Abreu, D.; Trevisan, D.; Costa, G.; Vasconcelos, F.; Gomes, M.; Cameiro, A. The association between osteoporosis and static balance in elderly women. Osteoporos. Int. 2010, 21, 1487–1491. [Google Scholar] [CrossRef]
- Liu-Ambrose, T.; Eng, J.J.; Khan, K.M.; Carter, N.D.; McKay, H.A. Older Women with Osteoporosis Have Increased Postural Sway and Weaker Quadriceps Strength than Counterparts with Normal Bone Mass: Overlooked Determinants of Fracture Risk? J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, M862–M866. [Google Scholar] [CrossRef] [Green Version]
- Campbell, A.J.; Borrie, M.J.; Spears, G.F. Risk factors for falls in a community-based prospective study of people 70 years and older. J. Gerontol. 1989, 44, M112–M117. [Google Scholar] [CrossRef]
- Kucznski, M.; Ostrowska, B. Understanding falls in osteoporosis: The viscoelastic modeling perspective. Gait Posture 2006, 23, 51–58. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Watts, N.B.; Dwivedi, A.; Shukla, R.; Mani, A.; Diab, D. Combined Measures of Dynamic Bone Quality and Postural Balance—A Fracture Risk Assessment Approach in Osteoporosis. J. Clin. Densitom. 2016, 19, 154–164. [Google Scholar] [CrossRef]
- Hsu, W.L.; Chen, C.Y.; Tsauo, J.Y.; Yang, R.S. Balance control in elderly people with osteoporosis. J. Formos. Med. Assoc. 2014, 113, 334–339. [Google Scholar] [CrossRef]
- Sinaki, M.; Brey, R.H.; Hughes, C.A.; Larson, D.R.; Kaufman, K.R. Balance disorder and increased risk of falls in osteoporosis and kyphosis: Significance of kyphotic posture and muscle strength. Osteoporos. Int. 2005, 16, 1004–1010. [Google Scholar] [CrossRef]
- Harbourne, R.T.; Stergiou, N. Movement variability and the use of nonlinear tools: Principles to guide physical therapist practice. Phys. Ther. 2009, 89, 267–282. [Google Scholar] [CrossRef] [Green Version]
- Stergiou, N.; Harbourne, R.; Cavanaugh, J. Optimal movement variability: A new theoretical perspective for neurologic physical therapy. J. Neurol. Phys. Ther. 2006, 30, 120–130. [Google Scholar] [CrossRef] [Green Version]
- Van Emmerik, R.; Van Wegen, E. On the functional aspects of variability in postural control. Exerc. Sport Sci. Rev. 2002, 30, 177–183. [Google Scholar] [CrossRef]
- Herman, T.; Giladi, N.; Gurevich, T.; Hausdorff, J.M. Gait instability and fractal dynamics of older adults with a “cautious” gait: Why do certain older adults walk fearfully? Gait Posture 2005, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, N.; Decker, L.M. Human movement variability, nonlinear dynamics, and pathology: Is there a connection? Hum. Mov. Sci. 2011, 30, 869–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ness, K.K.; Gurney, J.G.; Ice, G.H. Screening, education, and associated behavioral responses to reduce risk for falls among people over age 65 years attending a community health fair. Phys. Ther. 2003, 83, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002, 359, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, L.; Byles, J.; D’Este, C. Validation of self-reported fall events in intervention studies. Clin. Rehabil. 2006, 20, 331–339. [Google Scholar] [CrossRef]
- Booth, M. Assessment of physical activity: An international perspective. Res. Q. Exerc. Sport 2000, 71, 114–120. [Google Scholar] [CrossRef]
- Blaszczyk, J.W.; Prince, F.; Raiche, M.; Hebert, R. Effect of aeging and vision on limb load asymmetry during quiet stance. J. Biomech. 2000, 33, 1243–1248. [Google Scholar] [CrossRef]
- Ruhe, A.; Fejer, R.; Walker, B. The test-retest reliability of centre of pressure measures in bipedal static task conditions—A systematic review of the literature. Gait Posture 2010, 32, 436–445. [Google Scholar] [CrossRef] [Green Version]
- Winter, D.A. Biomechanics and Motor Control of Human Movement, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Hayes, M. Statistical Digital Signal Processing and Modeling; John Wiley & Sons, Inc.: New York, NY, USA, 1996. [Google Scholar]
- Mertins, A. Signaltheorie: Grundlagen der Signalbeschreibung, Filterbänke, Wavelets, Zeit-Frequenz-Analyse, Parameter- und Signalschätzung, 2nd ed.; Springer Vieweg: Wiesbaden, Germany, 2010. [Google Scholar]
- Baratto, L.; Morasso, P.G.; Re, C.; Spada, G. A new look at posturographic analysis in the clinical context: Sway-density versus other parameterization techniques. Motor Control 2002, 6, 246–270. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of biological signals. Phys. Rev. E 2005, 71, 021906. [Google Scholar] [CrossRef] [Green Version]
- Addison, P. The Illustrated Wavelet Transform Handbook, 1st ed.; Taylor & Francis Ltd.: London, UK, 2002. [Google Scholar]
- Chagdes, J.R.; Rietdyk, S.; Haddad, J.M.; Zelaznik, H.N.; Raman, A.; Rhea, C.K.; Silver, T.A. Multiple timescales in postural dynamics associated with vision and a secondary task are revealed by wavelet analysis. Exp. Brain Res. 2009, 197, 297–310. [Google Scholar] [CrossRef]
- Thurner, S.; Mittermaier, C.; Hanel, R.; Ehrenberger, K. Scaling-violation phenomena and fractality in the human posture control systems. Phys. Rev. E 2000, 62, 4018–4024. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H. Use of Statistical Methods to Assess the Effects of Localized Muscle Fatigue on Stability during Upright Stance. Master’s Thesis, Faculty of the Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2006. [Google Scholar]
- Smulders, E.; van Lankveld, W.; Laan, R.; Duysens, J.; Weerdesteyn, V. Does osteoporosis predispose falls? A study on obstacle avoidance and balance confidence. BMC Musculoskelet. Disorder. 2011, 12, 1. [Google Scholar] [CrossRef] [Green Version]
- Winter, D.A.; Prince, F.; Frank, J.S.; Powel, C.; Zabjek, K.F. Unified Theory Regarding A/P and M/L Balance in Quiet Stance. J. Neurophysiol. 1996, 75, 2334–2343. [Google Scholar] [CrossRef]
- Lord, S.R.; Rogers, M.W.; Howland, A.; Fitzpatrick, R. Lateral stability, sensorimotor function and falls in older people. J. Am. Geriatr. Soc. 1999, 47, 1077–1081. [Google Scholar] [CrossRef]
- Maki, B.E.; McIlroy, W.E. The control of foot placement during compensatory stepping reactions: Does speed of response take precedence over stability? IEEE Trans. Rehabil. Eng. 1999, 7, 80–90. [Google Scholar] [CrossRef]
- Mille, M.L.; Johnson, M.E.; Martinez, K.M.; Rogers, M.W. Age-dependent differences in lateral balance recovery through protective stepping. Clin. Biomech. 2005, 20, 607–616. [Google Scholar] [CrossRef]
- Blaszczyk, J.W. Sway ratio—A new measure for quantifying postural stability. Acta Neurobiol. Exp. 2008, 68, 51–57. [Google Scholar]
- Lacour, M.; Bernard-Demanze, L.; Dumitrescu, M. Posture control, aging, and attention resources: Models and posture-analysis methods. Neurophysiol. Clin. 2008, 38, 411–421. [Google Scholar] [CrossRef]
- Donker, S.F.; Roerding, M.; Greven, A.J.; Beek, P.J. Regularity of center-of-pressure trajectories depends on the amount of attention invested in postural control. Exp. Brain Res. 2007, 181, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kirchner, M.; Schubert, P.; Schmidtbleicher, D.; Haas, C.T. Evaluation of the temporal structure of postural sway fluctuations based on a comprehensive set of analysis tools. Phys. A 2012, 391, 4692–4703. [Google Scholar] [CrossRef]
- Kirchner, M.; Schubert, P.; Getrost, T.; Haas, C.T. Effect of altered surfaces on postural sway characteristics in elderly subjects. Hum. Mov. Sci. 2013, 32, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Solomon, I.; Chon, K. Comparison of the use of approximate entropy and sample entropy: Applications to neural respiratory signal. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2005, 4, 4212–4215. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [Green Version]
- Rhea, C.K.; Silver, T.A.; Hong, S.L.; Ryu, J.H.; Studenka, B.E.; Hughes, C.M.; Haddad, J.M. Noise and complexity in human postural control: Interpreting the different estimations of entropy. PLoS ONE 2011, 6, e17696. [Google Scholar] [CrossRef]

| Parameter | Controls (n = 19) | Patients (Women with Osteoporosis) | ||
|---|---|---|---|---|
| All (n = 41) | Fallers (n = 17) | Non-Fallers (n = 24) | ||
| Age (years) | 68.3 ± 5.3 | 70.0 ± 5.4 | 70.8 ± 4.3 | 69.4 ± 6.1 |
| Height (m) | 1.61 ± 0.05 | 1.61 ± 0.06 | 1.62 ± 0.06 | 1.61 ± 0.07 |
| Body mass (kg) | 63.5 ± 10.1 | 65.1 ± 10.0 | 68.2 ± 10.0 | 62.9 ± 9.6 |
| BMI (kg/m2) | 24.5 ± 3.2 | 25.1 ± 3.9 | 26.3 ± 4.0 | 24.2 ± 3.7 |
| Maximum T-score | −3.28 ± 0.61 | −3.44 ± 0.64 | −3.19 ± 0.59 | |
| IPAQ (MET-min/week) | 4593 ± 3908 | 4100 ± 3264 | 4545 ± 3687 | 3855 ± 3104 |
| Parameter | Controls (n = 19) | Patients (n = 41) | Test Statistic | p-Value | Fallers (n = 17) | Non-Fallers (n = 24) | Test Statistic | p-Value |
|---|---|---|---|---|---|---|---|---|
| SD (ML) (mm) | 2.00 ± 0.58 | 2.63 ± 1.00 | U = −2.30 | 0.021 | 2.62 ± 1.16 | 2.64 ± 0.19 | U = −0.20 | 0.843 |
| SD (AP) (mm) | 4.82 ± 0.86 | 4.92 ± 1.46 | T = 0.34 | 0.734 | 5.03 ± 1.37 | 4.84 ± 1.54 | T = −0.40 | 0.690 |
| ROM (ML) | 10.86 ± 3.14 | 15.33 ± 5.58 | U = −3.08 | 0.002 | 15.11 ± 6.68 | 15.49 ± 4.80 | U = −0.64 | 0.525 |
| (mm) | ||||||||
| ROM (AP)(mm) | 25.39 ± 5.23 | 28.50 ± 8.26 | U = −1.33 | 0.185 | 28.85 ± 8.58 | 28.24 ± 8.19 | U = −0.19 | 0.853 |
| V (ML) (mm/s) | 10.79 ± 1.72 | 11.30 ± 3.00 | U = −0.41 | 0.685 | 10.62 ± 1.41 | 11.78 ± 3.69 | U = −1.05 | 0.296 |
| V (AP) (mm/s) | 13.83 ± 2.90 | 14.78 ± 2.90 | U = −0.93 | 0.353 | 14.76 ± 3.70 | 14.78 ± 2.26 | U = −0.70 | 0.483 |
| PL (mm²) | 1170.8 ± 191.4 | 1241.3 ± 246.5 | U = −0.83 | 0.404 | 1209.5 ± 229.5 | 1263.9 ± 260.2 | U = −0.79 | 0.427 |
| f80 (ML) (Hz) | 0.37 ± 0.17 | 0.42 ± 0.16 | T = 0.97 | 0.335 | 0.42 ± 0.16 | 0.42 ± 0.17 | T = −0.03 | 0.976 |
| f80 (AP) (Hz) | 0.36 ± 0.23 | 0.42 ± 0.15 | U = −1.80 | 0.072 | 0.43 ± 0.18 | 0.41 ± 0.14 | U = −0.32 | 0.750 |
| CI (ML) | 11.24 ± 3.31 | 12.47 ± 2.62 | T = 1.56 | 0.123 | 12.45 ± 2.44 | 12.49 ± 1.78 | T = 0.05 | 0.964 |
| CI (AP) | 11.18 ± 4.44 | 13.75 ± 2.19 | U = −2.22 | 0.027 | 13.59 ± 2.71 | 13.86 ± 1.78 | U = −0.23 | 0.822 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stief, F.; Sohn, A.; Vogt, L.; Meurer, A.; Kirchner, M. Characterization of Postural Sway in Women with Osteoporosis and a Control Group by Means of Linear and Nonlinear Methods. Bioengineering 2023, 10, 403. https://doi.org/10.3390/bioengineering10040403
Stief F, Sohn A, Vogt L, Meurer A, Kirchner M. Characterization of Postural Sway in Women with Osteoporosis and a Control Group by Means of Linear and Nonlinear Methods. Bioengineering. 2023; 10(4):403. https://doi.org/10.3390/bioengineering10040403
Chicago/Turabian StyleStief, Felix, Anna Sohn, Lutz Vogt, Andrea Meurer, and Marietta Kirchner. 2023. "Characterization of Postural Sway in Women with Osteoporosis and a Control Group by Means of Linear and Nonlinear Methods" Bioengineering 10, no. 4: 403. https://doi.org/10.3390/bioengineering10040403








