The Effects of Negative Pressure Induced by Flow Separation Vortices on Vocal Fold Dynamics during Voice Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Computational Methodology
2.2. Simulation Setup
3. Results and Discussion
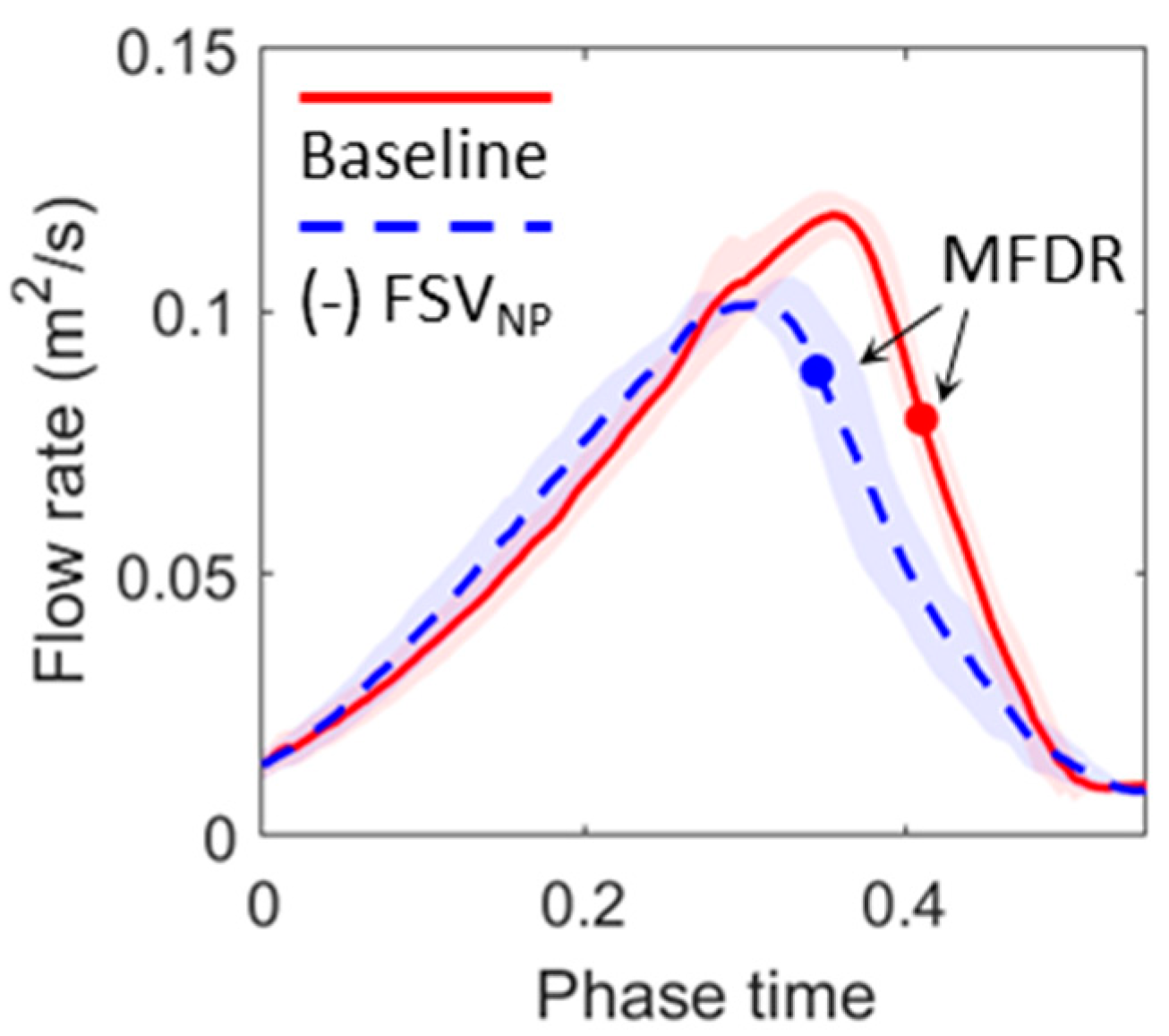
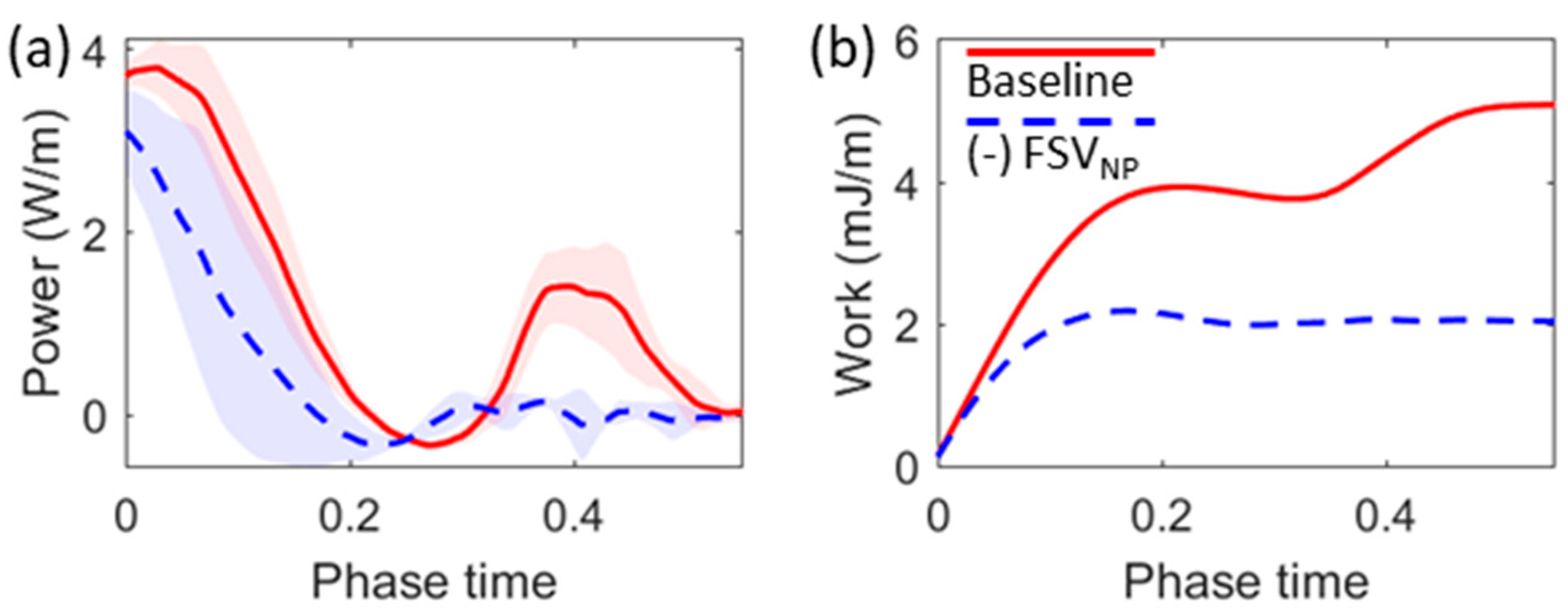
3.1. Flow Dynamics
3.2. Effects of Eliminating FSVNP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alipour, F.; Jaiswal, S.; Finnegan, E. Aerodynamic and Acoustic Effects of False Vocal Folds and Epiglottis in Excised Larynx Models. Ann. Otol. Rhinol. Laryngol. 2007, 116, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Saraniti, C.; Speciale, R.; Santangelo, M.; Massaro, N.; Maniaci, A.; Gallina, S.; Serra, A.; Cocuzza, S. Functional Outcomes after Supracricoid Modified Partial Laryngectomy. J. Biol. Regul. Homeost. Agents 2019, 33, 1903–1907. [Google Scholar]
- Oren, L.; Khosla, S.; Gutmark, E. Medial Surface Dynamics as a Function of Subglottal Pressure in a Canine Larynx Model. J. Voice 2019, 35, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Schickhofer, L.; Mihaescu, M. Analysis of the Aerodynamic Sound of Speech through Static Vocal Tract Models of Various Glottal Shapes. J. Biomech. 2020, 99, 109484. [Google Scholar] [CrossRef]
- Zhang, Z. Mechanics of Human Voice Production and Control. J. Acoust. Soc. Am. 2016, 140, 2614–2635. [Google Scholar] [CrossRef] [PubMed]
- Lasota, M.; Šidlof, P.; Maurerlehner, P.; Kaltenbacher, M.; Schoder, S. Anisotropic Minimum Dissipation Subgrid-Scale Model in Hybrid Aeroacoustic Simulations of Human Phonation. J. Acoust. Soc. Am. 2023, 153, 1052–1063. [Google Scholar] [CrossRef]
- Oren, L.; Khosla, S.; Gutmark, E. Intraglottal Geometry and Velocity Measurements in Canine Larynges. J. Acoust. Soc. Am. 2014, 135, 380–388. [Google Scholar] [CrossRef]
- Sundström, E.; Oren, L.; Farbos de Luzan, C.; Gutmark, E.; Khosla, S. Fluid-Structure Interaction Analysis of Aerodynamic and Elasticity Forces during Vocal Fold Vibration. J. Voice 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Li, S.; Scherer, R.C.; Wan, M.X.; Wang, S.P.; Song, B. Intraglottal Pressure: A Comparison between Male and Female Larynxes. J. Voice 2019, 34, 813–822. [Google Scholar] [CrossRef]
- Alipour, F.; Scherer, R.C. Flow Separation in a Computational Oscillating Vocal Fold Model. J. Acoust. Soc. Am. 2004, 116, 1710–1719. [Google Scholar] [CrossRef]
- Pelorson, X.; Hirschberg, A.; Wijnands, A.; Bailliet, H. Description of the Flow through In-Vitro Models of the Glottis during Phonation. Acta Acust. 1995, 3, 191–202. [Google Scholar]
- Scherer, R.C.; Torkaman, S.; Kucinschi, B.R.; Afjeh, A.A. Intraglottal Pressures in a Three-Dimensional Model with a Non-Rectangular Glottal Shape. J. Acoust. Soc. Am. 2010, 128, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Scherer, R.C.; Shinwari, D.; De Witt, K.J.; Zhang, C.; Kucinschi, B.R.; Afjeh, A.A. Intraglottal Pressure Profiles for a Symmetric and Oblique Glottis with a Divergence Angle of 10 Degrees. J. Acoust. Soc. Am. 2001, 109, 1616–1630. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.J.; Titze, I.R. Measurement of Vocal Fold Intraglottal Pressure and Impact Stress. J. Voice 1994, 8, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Alipour, F.; Scherer, R.C. Dynamic Glottal Pressures in an Excised Hemilarynx Model. J. Voice 2000, 14, 443–454. [Google Scholar] [CrossRef] [PubMed]
- DeJonckere, P.H.; Lebacq, J.; Titze, I.R. Dynamics of the Driving Force During the Normal Vocal Fold Vibration Cycle. J. Voice 2017, 31, 649–661. [Google Scholar] [CrossRef]
- Motie-Shirazi, M.; Zañartu, M.; Peterson, S.D.; Erath, B.D. Vocal Fold Dynamics in a Synthetic Self-Oscillating Model: Intraglottal Aerodynamic Pressure and Energy. J. Acoust. Soc. Am. 2021, 150, 1332–1345. [Google Scholar] [CrossRef]
- Farbos de Luzan, C.; Oren, L.; Gutmark, E.; Khosla, S.M. Quantification of the Intraglottal Pressure Induced by Flow Separation Vortices Using Large Eddy Simulation. J. Voice 2021, 35, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Kreiman, J.; Gerratt, B.R. Perceptual Interaction of the Harmonic Source and Noise in Voice. J. Acoust. Soc. Am. 2012, 131, 492–500. [Google Scholar] [CrossRef]
- Sapienza, C.M.; Stathopoulos, E.T. Comparison of Maximum Flow Declination Rate: Children versus Adults. J. Voice 1994, 8, 240–247. [Google Scholar] [CrossRef]
- Gauffin, J.; Sundberg, J. Spectral Correlates of Glottal Voice Source Waveform Characteristics. J. Speech Lang. Heart Res. 1989, 32, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Influence of Flow Separation Location on Phonation Onset. J. Acoust. Soc. Am. 2008, 124, 1689–1694. [Google Scholar] [CrossRef] [PubMed]
- Pelorson, X.; Hirschberg, A.; van Hassel, R.R.; Wijnands, A.P.J.; Auregan, Y. Theoretical and Experimental Study of Quasisteady-Flow Separation within the Glottis during Phonation. Application to a Modified Two-Mass Model. J. Acoust. Soc. Am. 1994, 96, 3416–3431. [Google Scholar] [CrossRef]
- Pirnia, A.; Browning, E.A.; Peterson, S.D.; Erath, B.D. Discrete and Periodic Vortex Loading on a Flexible Plate; Application to Energy Harvesting and Voiced Speech Production. J. Sound Vib. 2018, 433, 476–492. [Google Scholar] [CrossRef]
- Alipour, F.; Vigmostad, S. Measurement of Vocal Folds Elastic Properties for Continuum Modeling. J. Voice 2012, 26, 816.e21–816.e29. [Google Scholar] [CrossRef]
- Chhetri, D.K.; Zhang, Z.; Neubauer, J. Measurement of Young’s Modulus of Vocal Folds by Indentation. J. Voice 2011, 25, 1–7. [Google Scholar] [CrossRef]
- Farahani, M.H.; Zhang, Z. A Computational Study of the Effect of Intraglottal Vortex-Induced Negative Pressure on Vocal Fold Vibration. J. Acoust. Soc. Am. 2014, 136, EL369–EL375. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Mittal, R. A High-Order Immersed Boundary Method for Acoustic Wave Scattering and Low-Mach Number Flow-Induced Sound in Complex Geometries. J. Comput. Phys. 2011, 230, 1000–1019. [Google Scholar] [CrossRef]
- Seo, J.H.; Moon, Y.J. Linearized Perturbed Compressible Equations for Low Mach Number Aeroacoustics. J. Comput. Phys. 2006, 218, 702–719. [Google Scholar] [CrossRef]
- Bodaghi, D.; Jiang, W.; Xue, Q.; Zheng, X. Effect of Supraglottal Acoustics on Fluid-Structure Interaction during Human Voice Production. J. Biomech. Eng. 2021, 143, 041010. [Google Scholar] [CrossRef]
- Mittal, R.; Dong, H.; Bozkurttas, M.; Najjar, F.M.M.; Vargas, A.; von Loebbecke, A. A Versatile Sharp Interface Immersed Boundary Method for Incompressible Flows with Complex Boundaries. J. Comput. Phys. 2008, 227, 4825–4852. [Google Scholar] [CrossRef] [PubMed]
- Story, B.H.; Titze, I.R. Voice Simulation with a Body-Cover Model of the Vocal Folds. J. Acoust. Soc. Am. 1995, 97, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Farbos De Luzan, C.; Wang, X.; Oren, L.; Khosla, S.M.; Xue, Q.; Zheng, X. Computational Modeling of Voice Production Using Excised Canine Larynx. J. Biomech. Eng. 2022, 144, 021003. [Google Scholar] [CrossRef] [PubMed]
- Titze, I.R. Principles of Voice Production; National Center for Voice and Speech: Iowa City, IA, USA, 2000. [Google Scholar]
- Mihaescu, M.; Khosla, S.M.; Murugappan, S.; Gutmark, E.J. Unsteady Laryngeal Airflow Simulations of the Intra-Glottal Vortical Structures. J. Acoust. Soc. Am. 2010, 127, 435–444. [Google Scholar] [CrossRef]
- Li, S.; Scherer, R.C.; Wan, M.; Wang, S.; Wu, H. The Effect of Glottal Angle on Intraglottal Pressure. J. Acoust. Soc. Am. 2006, 119, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S.L.; Mongeau, L.; Frankel, S.H. Aerodynamic Transfer of Energy to the Vocal Folds. J. Acoust. Soc. Am. 2005, 118, 1689. [Google Scholar] [CrossRef]
- Döllinger, M.; Zhang, Z.; Schoder, S.; Šidlof, P.; Tur, B.; Kniesburges, S. Overview on State-of-the-Art Numerical Modeling of the Phonation Process. Acta Acust. 2023, 7, 25. [Google Scholar] [CrossRef]
- Ishizaka, K.; Flanagan, J.L. Synthesis of Voiced Sounds From a Two-Mass Model of the Vocal Cords. Bell Syst. Technol. J. 1972, 51, 1233–1268. [Google Scholar] [CrossRef]
- Story, B.H. An Overview of the Physiology, Physics and Modeling of the Sound Source for Vowels. Acoust. Sci. Technol. 2002, 23, 195–206. [Google Scholar] [CrossRef]
- Towns, J.; Cockerill, T.; Dahan, M.; Foster, I.; Gaither, K.; Grimshaw, A.; Hazlewood, V.; Lathrop, S.; Lifka, D.; Peterson, G.D.; et al. XSEDE: Accelerating Scientific Discovery. Comput. Sci. Eng. 2014, 16, 62–74. [Google Scholar] [CrossRef]
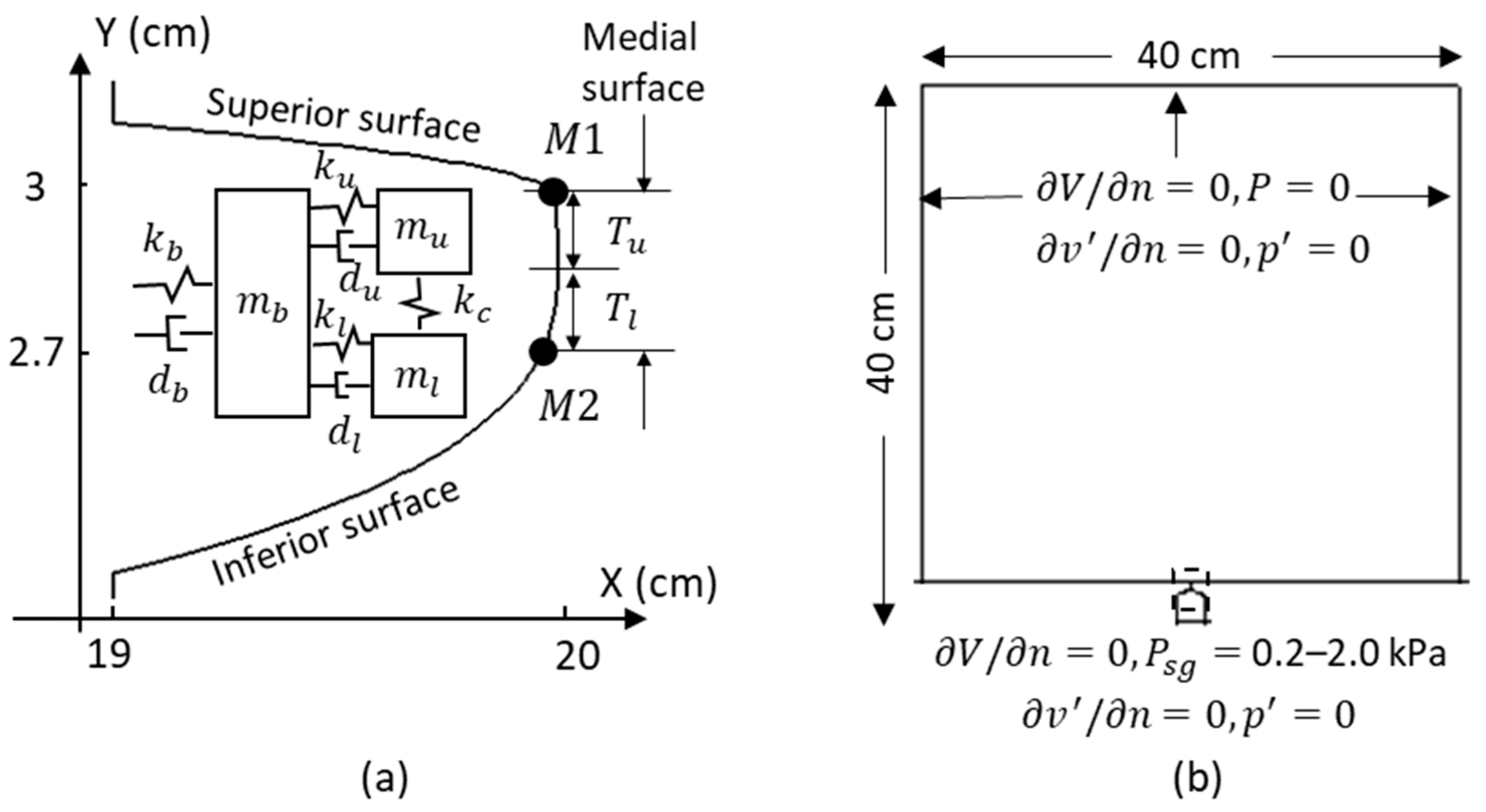



(g) | (g) | (g) | (N/m) | (N/m) | (N/m) | (N/m) | (cm) | (cm) | |
|---|---|---|---|---|---|---|---|---|---|
| Value | 0.04 | 0.01 | 0.05 | 5 | 7 | 100 | 1 | 0.15 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Zheng, X.; Farbos de Luzan, C.; Oren, L.; Gutmark, E.; Xue, Q. The Effects of Negative Pressure Induced by Flow Separation Vortices on Vocal Fold Dynamics during Voice Production. Bioengineering 2023, 10, 1215. https://doi.org/10.3390/bioengineering10101215
Jiang W, Zheng X, Farbos de Luzan C, Oren L, Gutmark E, Xue Q. The Effects of Negative Pressure Induced by Flow Separation Vortices on Vocal Fold Dynamics during Voice Production. Bioengineering. 2023; 10(10):1215. https://doi.org/10.3390/bioengineering10101215
Chicago/Turabian StyleJiang, Weili, Xudong Zheng, Charles Farbos de Luzan, Liran Oren, Ephraim Gutmark, and Qian Xue. 2023. "The Effects of Negative Pressure Induced by Flow Separation Vortices on Vocal Fold Dynamics during Voice Production" Bioengineering 10, no. 10: 1215. https://doi.org/10.3390/bioengineering10101215






