A New Gas Analysis Method Based on Single-Beam Excitation Stimulated Raman Scattering in Hollow Core Photonic Crystal Fiber Enhanced Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the FERS System Using a Single-Beam Pulsed Pump
2.2. Description of Gas Raman Measurements
2.3. Raman Spectral Analysis
3. Results
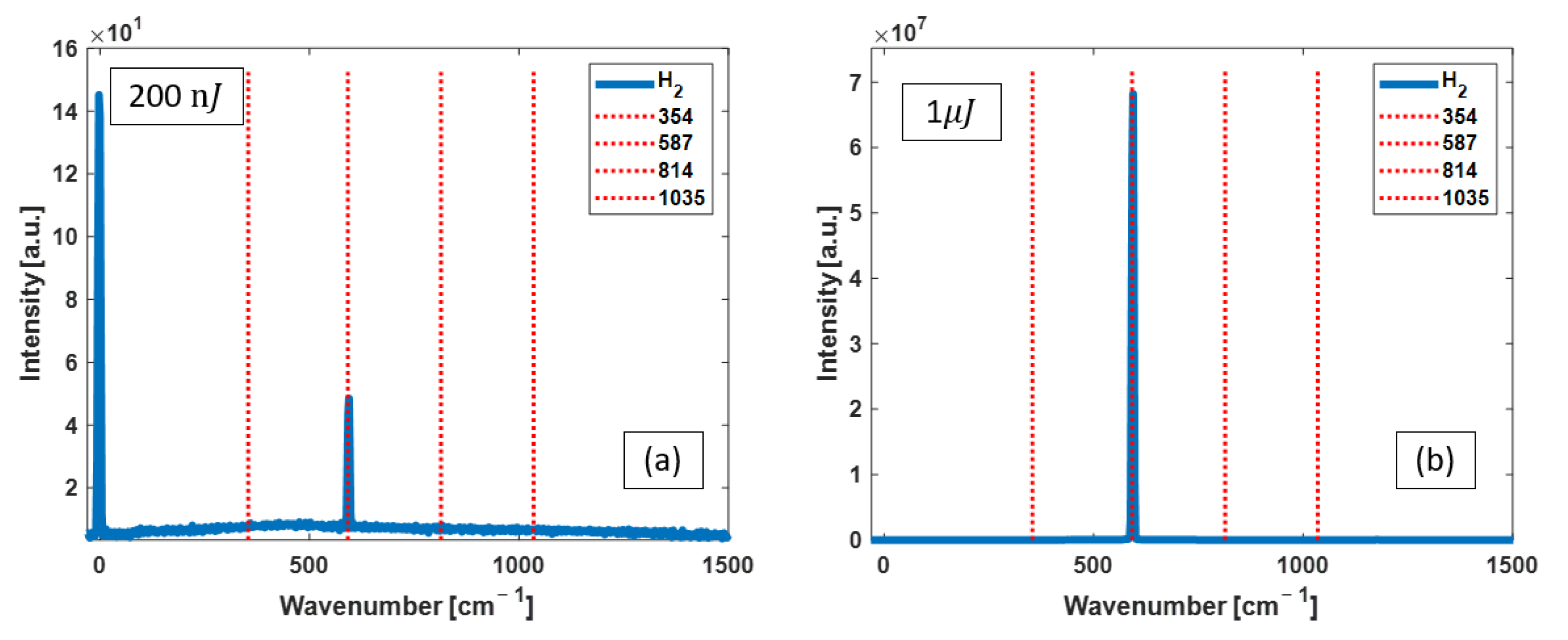
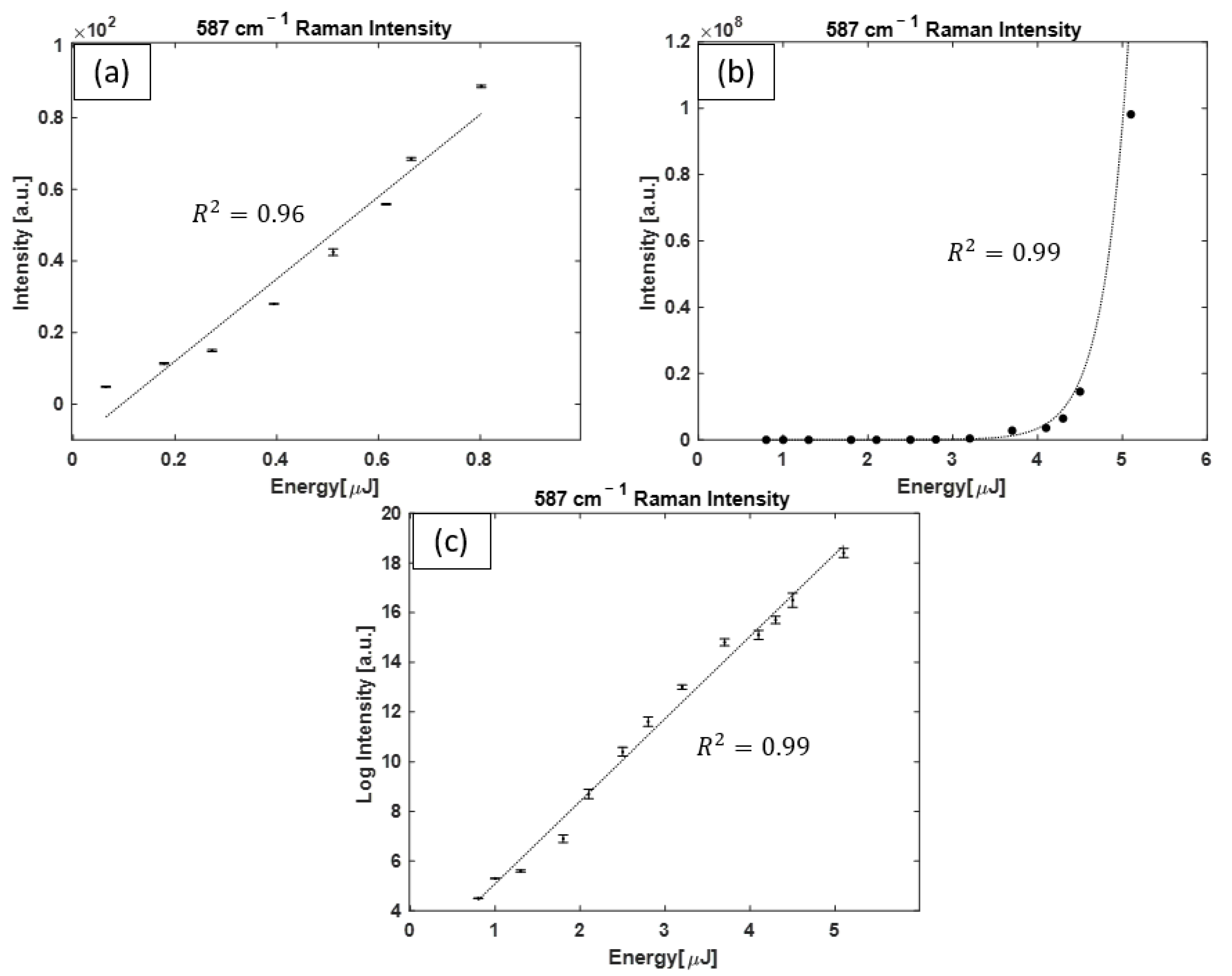
3.1. H2 Raman Measurements
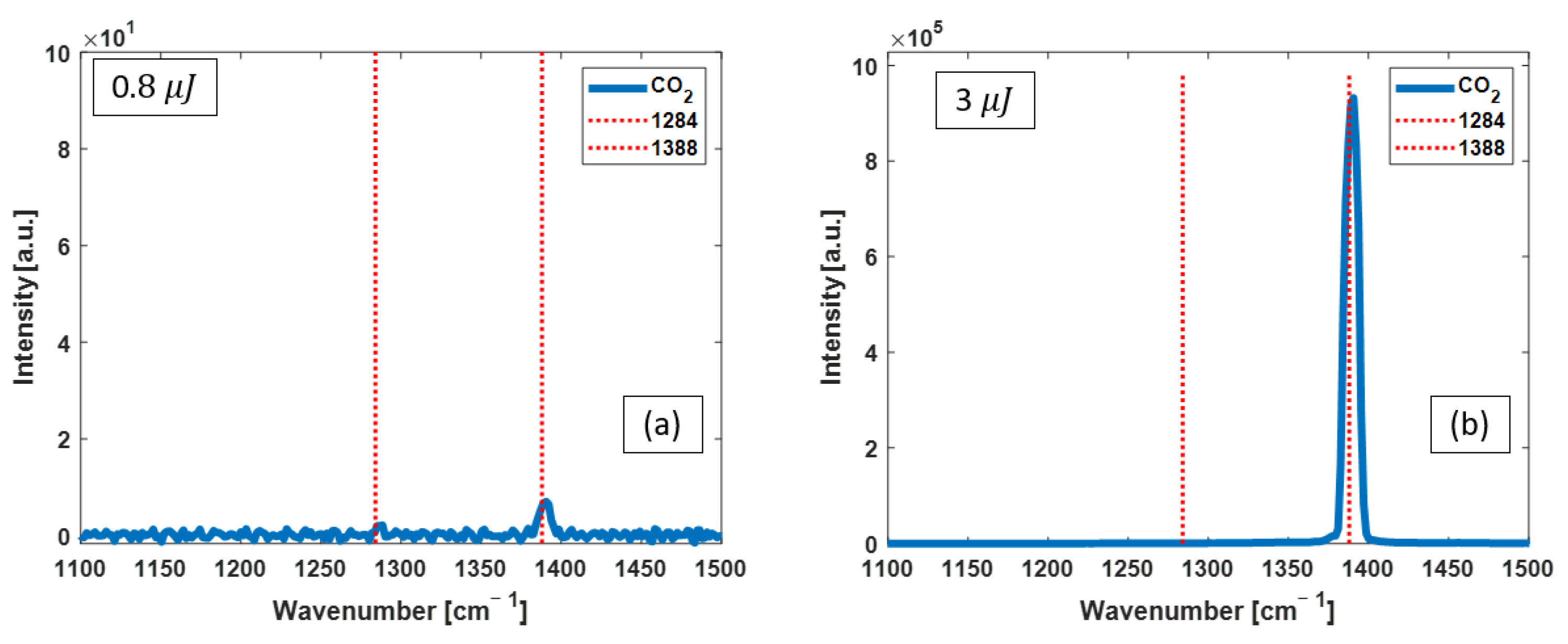
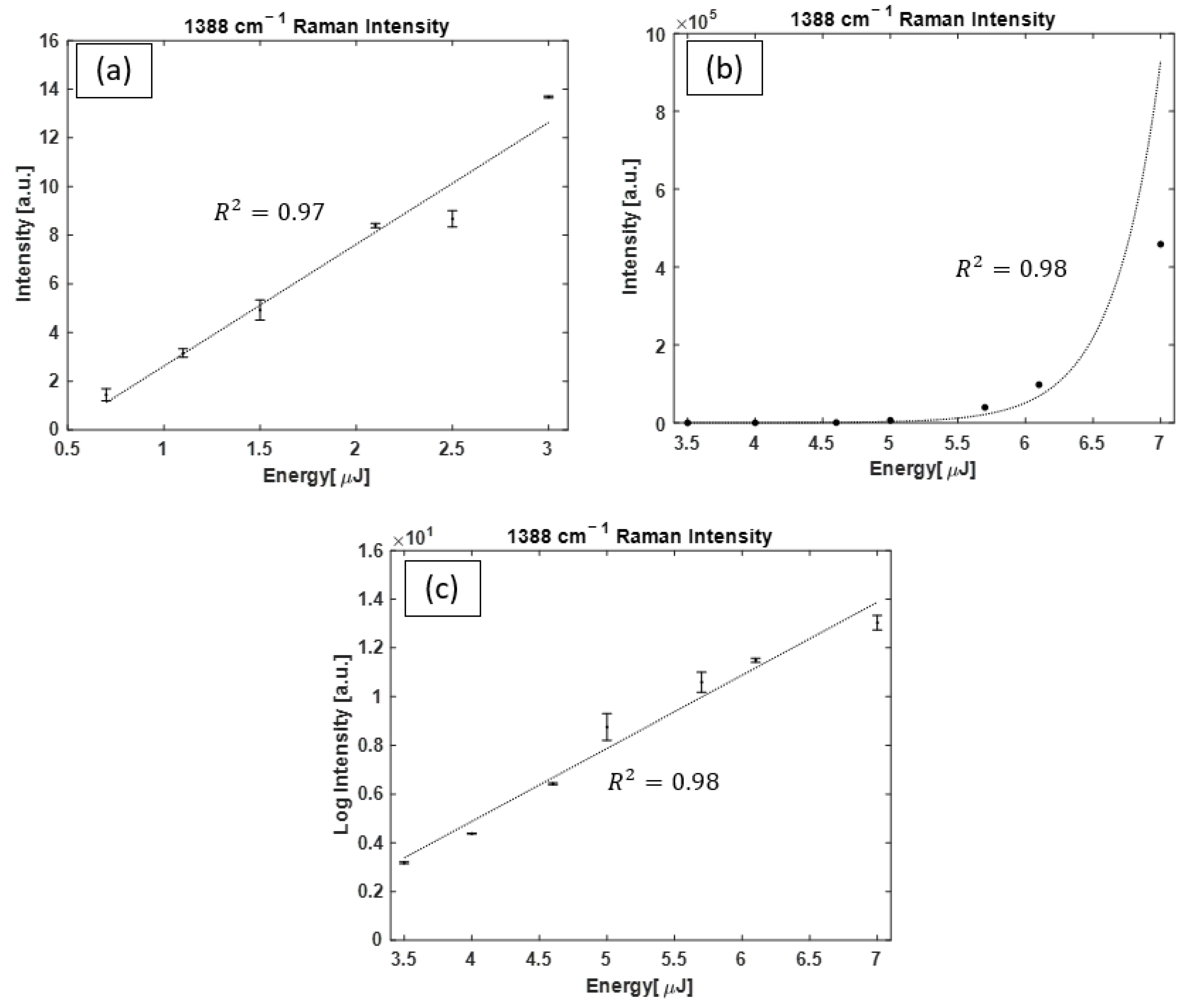
3.2. CO2 Raman Measurements
3.3. Propene Raman Measurements
3.4. Raman Spectral Analysis
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hippler, M. Cavity-Enhanced Raman Spectroscopy of Natural Gas with Optical Feedback cw-Diode Lasers. Anal. Chem. 2015, 87, 7803–7809. [Google Scholar] [CrossRef]
- Niklas, C.; Wackerbarth, H.; Ctistis, G. A Short Review of Cavity-Enhanced Raman Spectroscopy for Gas Analysis. Sensors 2021, 21, 1698. [Google Scholar] [CrossRef]
- Wang, P.; Chen, W.; Wang, J.; Lu, Y.; Tang, Z.; Tan, Y. Cavity-Enhanced Raman Spectroscopy for Detection of Trace Gaseous Impurities in Hydrogen for Fuel Cells. Anal. Chem. 2023, 95, 6894–6904. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, P.; Chen, W.; Wan, F.; Lu, Y.; Tang, Z.; Dong, A.; Lei, Z.; Zhang, Z. Highly sensitive multi-pass cavity enhanced Raman spectroscopy with novel polarization filtering for quantitative measurement of SF6 decomposed components in gas-insulated power equipment. Sens. Actuators B Chem. 2023, 380, 133350. [Google Scholar] [CrossRef]
- Eichmann, S.C.; Kiefer, J.; Benz, J.; Kempf, T.; Leipertz, A.; Seeger, T. Determination of gas composition in a biogas plant using a Raman-based sensorsystem. Meas. Sci. Technol. 2014, 25, 075503. [Google Scholar] [CrossRef]
- Sharma, R.; Poonacha, S.; Bekal, A.; Vartak, S.; Weling, A.; Tilak, V.; Mitra, C. Raman analyzer for sensitive natural gas composition analysis. Opt. Eng. 2016, 55, 104103. [Google Scholar] [CrossRef]
- Knebl, A.; Domes, R.; Wolf, S.; Domes, C.; Popp, J.; Frosch, T. Fiber-Enhanced Raman Gas Spectroscopy for the Study of Microbial Methanogenesis. Anal. Chem. 2020, 92, 12564–12571. [Google Scholar] [CrossRef]
- Knebl, A.; Domes, R.; Yan, D.; Popp, J.; Trumbore, S.; Frosch, T. Fiber-Enhanced Raman Gas Spectroscopy for 18O−13C-Labeling Experiments. Anal. Chem 2019, 91, 7569. [Google Scholar] [CrossRef] [PubMed]
- Okita, Y.; Katagiri, T.; Matsuura, Y. A Raman cell based on hollow optical fibers for breath analysis. In Proceedings of the Optical Fibers and Sensors for Medical Diagnostics and Treatment Applications X, San Francisco, CA, USA, 23–24 January 2010; p. 755908. [Google Scholar]
- Chow, K.K.; Short, M.; Lam, S.; McWilliams, A.; Zeng, H. A Raman cell based on hollow core photonic crystal fiber for human breath analysis. Med. Phys. 2014, 41, 092701. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Pan, F.; Liu, J.; Wang, K.; Zhang, C.; Cheng, S.; Lu, L.; Zhang, W.; Zhang, Z.; et al. Breath Analysis Based on Surface-Enhanced Raman Scattering Sensors Distinguishes Early and Advanced Gastric Cancer Patients from Healthy Persons. ACS Nano 2016, 10, 8169–8179. [Google Scholar] [CrossRef]
- Bruderer, T.; Gaisl, T.; Gaugg, M.T.; Nowak, N.; Streckenbach, B.; Müller, S.; Moeller, A.; Kohler, M.; Zenobi, R. On-Line Analysis of Exhaled Breath. Chem. Rev. 2019, 119, 10803–10828. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zeng, L.; Li, Z.; Li, D. Review of optical fibre probes for enhanced Raman sensing. J. Raman Spectrosc. 2017, 48, 1040–1055. [Google Scholar] [CrossRef]
- Cregan, R.F.; Mangan, B.J.; Knight, J.C.; Birks, T.A.; Russell, P.S.J.; Roberts, P.J.; Allan, D.C. Single-Mode Photonic Band Gap Guidance of Light in Air. Science 1999, 285, 1537–1539. [Google Scholar] [CrossRef]
- Mangan, B.J.; Farr, L.; Langford, A.; Roberts, P.J.; Williams, D.P.; Couny, F.; Lawman, M.; Mason, M.; Coupland, S.; Flea, R.; et al. Low loss (1.7 dB/km) hollow core photonic bandgap fiber. In Proceedings of the Optical Fiber Communication Conference, Los Angeles, CA, USA, 22 February 2004; p. PD24. [Google Scholar]
- Habib, M.S.; Antonio-Lopez, J.E.; Markos, C.; Schülzgen, A.; Amezcua-Correa, R. Single-mode, low loss hollow-core anti-resonant fiber designs. Opt. Express 2019, 27, 3824–3836. [Google Scholar] [CrossRef]
- Belardi, W.; Knight, J.C. Hollow antiresonant fibers with reduced attenuation. Opt. Lett. 2014, 39, 1853–1856. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.C.; Russell, P.S.J. New ways to guide light. Science 2002, 296, 276. [Google Scholar] [CrossRef]
- Bögözi, T.; Popp, J.; Frosch, T. Fiber-enhanced Raman multi-gas spectroscopy: What is the potential of its application to breath analysis? Bioanalysis 2015, 7, 281–284. [Google Scholar] [CrossRef]
- Knebl, A.; Yan, D.; Popp, J.; Frosch, T. Fiber enhanced Raman gas spectroscopy. TrAC Trends Anal. Chem. 2018, 103, 230–238. [Google Scholar] [CrossRef]
- Qian, G.; Wan, F.; Zhou, F.; Wang, J.; Kong, W.; Chen, W. Fiber-Enhanced Raman Spectroscopy for Trace-Gas Sensing in the High-Concentration Gas Background with an Anti-Resonant Hollow Core Fiber. Front. Phys. 2022, 10, 917688. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.; Wang, P.; Zhang, Z.; Wan, F.; Zhou, F.; Song, R.; Wang, Y.; Gao, S. Fiber-enhanced Raman spectroscopy for highly sensitive H2 and SO2 sensing with a hollow-core anti-resonant fiber. Opt. Express 2021, 29, 32296–32311. [Google Scholar] [CrossRef]
- Kelly, T.W.; Davidson, I.A.; Warren, C.; Brooks, W.S.M.; Foster, M.J.; Poletti, F.; Richardson, D.J.; Horak, P.; Wheeler, N.V. Sub-ppm gas phase Raman spectroscopy in an anti-resonant hollow core fiber. Opt. Express 2022, 30, 43317–43329. [Google Scholar] [CrossRef] [PubMed]
- Knebl, A.; Domes, C.; Domes, R.; Wolf, S.; Popp, J.; Frosch, T. Hydrogen and C2–C6 Alkane Sensing in Complex Fuel Gas Mixtures with Fiber-Enhanced Raman Spectroscopy. Anal. Chem. 2021, 93, 10546–10552. [Google Scholar] [CrossRef] [PubMed]
- Brooks, W.S.M.; Partridge, M.; Davidson, I.A.K.; Warren, C.; Rushton, G.; Large, J.; Wharton, M.; Storey, J.; Wheeler, N.V.; Foster, M.J. Development of a gas-phase Raman instrument using a hollow core anti-resonant tubular fibre. J. Raman Spectrosc. 2021, 52, 1772–1782. [Google Scholar] [CrossRef]
- Buric, M.P.; Chen, K.P.; Falk, J.; Woodruff, S.D. Enhanced spontaneous Raman scattering and gas composition analysis using a photonic crystal fiber. Appl. Opt. 2008, 47, 4255–4261. [Google Scholar] [CrossRef] [PubMed]
- Buric, M.P.; Chen, K.P.; Falk, J.; Woodruff, S.D. Improved sensitivity gas detection by spontaneous Raman scattering. Appl. Opt. 2009, 48, 4424. [Google Scholar] [CrossRef]
- Sieburg, A.; Knebl, A.; Jacob, J.M.; Frosch, T. Characterization of fuel gases with fiber-enhanced Raman spectroscopy. Anal. Bioanal. Chem. 2019, 411, 7399–7408. [Google Scholar] [CrossRef] [PubMed]
- Sandfort, V.; Trabold, B.M.; Abdolvand, A.; Bolwien, C.; Russell, P.S.J.; Wöllenstein, J.; Palzer, S. Monitoring the wobbe index of natural gas using fiber-enhanced Raman spectroscopy. Sensors 2017, 17, 2714. [Google Scholar] [CrossRef]
- Hanf, S.; Keiner, R.; Yan, D.; Popp, J.; Frosch, T. Fiber-Enhanced Raman Multigas Spectroscopy: A Versatile Tool for Environmental Gas Sensing and Breath Analysis. Anal. Chem. 2014, 86, 5278–5285. [Google Scholar] [CrossRef]
- Hanf, S.; Bögözi, T.; Keiner, R.; Frosch, T.; Rgen Popp, J. Fast and Highly Sensitive Fiber-Enhanced Raman Spectroscopic Monitoring of Molecular H2 and CH4 for Point-of-Care Diagnosis of Malabsorption Disorders in Exhaled Human Breath. Anal. Chem 2015, 87, 45. [Google Scholar] [CrossRef]
- Prince, R.C.; Frontiera, R.R.; Potma, E.O. Stimulated Raman Scattering: From Bulk to Nano. Chem. Rev. 2017, 117, 5070–5094. [Google Scholar] [CrossRef]
- Benabid, F.; Knight, J.C.; Antonopoulos, G.; Russell, P.S.J.; Russell, J. Stimulated Raman Scattering in Hydrogen-Filled Hollow-Core Photonic Crystal Fiber. Science 2002, 298, 399–402. [Google Scholar] [CrossRef]
- Benabid, F.; Couny, F.; Knight, J.C.; Birks, T.A.; Russell, P.S.J. Compact, stable and efficient all-fibre gas cells using hollow-core photonic crystal fibres. Nature 2005, 434, 488–491. [Google Scholar] [CrossRef]
- Benabid, F.; Antonopoulos, G.; Knight, J.C.; Russell, P.S.J. Stokes Amplification Regimes in Quasi-cw Pumped Hydrogen-Filled Hollow-Core Photonic Crystal Fiber. Phys. Rev. Lett. 2005, 95, 213903. [Google Scholar] [CrossRef] [PubMed]
- Debord, B.; Amrani, F.; Vincetti, L.; Gérôme, F.; Benabid, F. Hollow-Core Fiber Technology: The Rising of “Gas Photonics”. Fibers 2019, 7, 16. [Google Scholar] [CrossRef]
- Boyd, R.W. Chapter 10—Stimulated Raman Scattering and Stimulated Rayleigh-Wing Scattering. In Nonlinear Optics, 3rd ed.; Academic Press: Singapore, 2008; pp. 473–509. [Google Scholar]
- Fenner, W.R.; Hyatt, H.A.; Kellam, J.M.; Porto, S.P.S. Raman cross section of some simple gases. JOSA 1973, 63, 73–77. [Google Scholar] [CrossRef]
- Couny, F.; Benabid, F.; Light, P.S. Subwatt Threshold cw Raman Fiber-Gas Laser Based on H2-Filled Hollow-Core Photonic Crystal Fiber. Phys. Rev. Lett. 2007, 99, 143903. [Google Scholar] [CrossRef]
- Trabold, B.M.; Abdolvand, A.; Euser, T.G.; Walser, A.M.; Russell, P.S.J. Amplification of higher-order modes by stimulated Raman scattering in H2-filled hollow-core photonic crystal fiber. Opt. Lett. 2013, 38, 600. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Herrera, J.; Krishnan, S.; Zain, M.; Greenberg, J.; Cataneo, R.N. Variation in volatile organic compounds in the breath of normal humans. J. Chromatogr. B Biomed. Sci. Appl. 1999, 729, 75–88. [Google Scholar] [CrossRef]
- De Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef]
- Ding, H.; Hu, D.J.J.; Yu, X.; Liu, X.; Zhu, Y.; Wang, G. Review on All-Fiber Online Raman Sensor with Hollow Core Microstructured Optical Fiber. Photonics 2022, 9, 134. [Google Scholar] [CrossRef]
- Taylor, D.G.; Strauss, H.L. The rotational Raman spectrum of H2 in water. J. Chem. Phys. 1989, 90, 768–772. [Google Scholar] [CrossRef]
- Veirs, D.K.; Rosenblatt, G.M. Raman line positions in molecular hydrogen: H2, HD, HT, D2, DT, and T2. J. Mol. Spectrosc. 1987, 121, 401–419. [Google Scholar] [CrossRef]
- Ananthakrishnan, R. The Raman spectra of propylene and ISO-Butane. Proc. Indian Acad. Sci. Sect. A 1936, 3, 527–532. [Google Scholar] [CrossRef]
- Lord, R.C.; Venkateswarlu, P. The Infrared Spectra of Propylene and Propylene-d6. J. Opt. Soc. Am. 1953, 43, 1079. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirmohammad, M.; Short, M.A.; Zeng, H. A New Gas Analysis Method Based on Single-Beam Excitation Stimulated Raman Scattering in Hollow Core Photonic Crystal Fiber Enhanced Raman Spectroscopy. Bioengineering 2023, 10, 1161. https://doi.org/10.3390/bioengineering10101161
Shirmohammad M, Short MA, Zeng H. A New Gas Analysis Method Based on Single-Beam Excitation Stimulated Raman Scattering in Hollow Core Photonic Crystal Fiber Enhanced Raman Spectroscopy. Bioengineering. 2023; 10(10):1161. https://doi.org/10.3390/bioengineering10101161
Chicago/Turabian StyleShirmohammad, Maryam, Michael A. Short, and Haishan Zeng. 2023. "A New Gas Analysis Method Based on Single-Beam Excitation Stimulated Raman Scattering in Hollow Core Photonic Crystal Fiber Enhanced Raman Spectroscopy" Bioengineering 10, no. 10: 1161. https://doi.org/10.3390/bioengineering10101161




