Methylamine Activates Glucose Uptake in Human Adipocytes Without Overpassing Action of Insulin or Stimulating its Secretion in Pancreatic Islets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Subjects and Preparation of Adipose Samples
2.3. Animals
2.4. Methylamine Oxidation by Human ScAT
2.5. Adipocyte Preparation, Glucose Transport, and Lipolysis Assays
2.6. Insulin Secretion by Isolated Pancreatic Islets
2.7. Oxidation of Radiolabeled Benzylamine by Adipose Tissue Homogenates
2.8. Statistical Analysis
3. Results
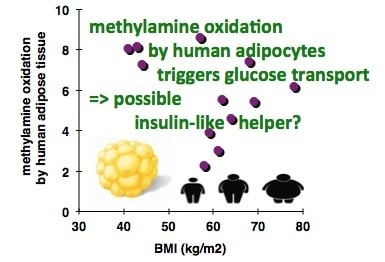
3.1. In Vitro Oxidation of Methylamine by Adipose Tissue Preparations from High-Risk Obese Patients
3.2. Stimulation by Methylamine of Glucose Transport in Adipose Cells from Non-Obese, Overweight, and Obese Patients
3.3. Comparative Examinations of Methylamine Actions in Human Adipocytes
3.4. Lack of Methylamine-Induced Insulin Secretion in Pancreatic Islets
3.5. Methylamine is Interacting with Rabbit Amine Oxidases and Adipocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Tang, W.H.; Hazen, S.L. Microbiome, trimethylamine N-oxide, and cardiometabolic disease. Transl. Res. 2017, 179, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.C.; Zhang, A.Q. Methylamine in human urine. Clin. Chim. Acta 2001, 312, 107–114. [Google Scholar] [CrossRef]
- Poortmans, J.R.; Kumps, A.; Duez, P.; Fofonka, A.; Carpentier, A.; Francaux, M. Effect of oral creatine supplementation on urinary methylamine, formaldehyde, and formate. Med. Sci. Sports Exerc. 2005, 37, 1717–1720. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.Y. Methylamine: A vital nitrogen (and carbon) source for marine microbes. Environ. Microbiol. 2017, 19, 2117–2118. [Google Scholar] [CrossRef] [PubMed]
- Taubert, M.; Grob, C.; Howat, A.M.; Burns, O.J.; Pratscher, J.; Jehmlich, N.; von Bergen, M.; Richnow, H.H.; Chen, Y.; Murrell, J.C. Methylamine as a nitrogen source for microorganisms from a coastal marine environment. Environ. Microbiol. 2017, 19, 2246–2257. [Google Scholar] [CrossRef] [Green Version]
- Nayak, D.D.; Agashe, D.; Lee, M.C.; Marx, C.J. Selection Maintains Apparently Degenerate Metabolic Pathways due to Tradeoffs in Using Methylamine for Carbon versus Nitrogen. Curr. Biol. 2016, 26, 1416–1426. [Google Scholar] [CrossRef] [Green Version]
- McTaggart, T.L.; Beck, D.A.; Setboonsarng, U.; Shapiro, N.; Woyke, T.; Lidstrom, M.E.; Kalyuzhnaya, M.G.; Chistoserdova, L. Genomics of Methylotrophy in Gram-Positive Methylamine-Utilizing Bacteria. Microorganisms 2015, 3, 94–112. [Google Scholar] [CrossRef] [Green Version]
- Sannino, F.; Parrilli, E.; Apuzzo, G.A.; de Pascale, D.; Tedesco, P.; Maida, I.; Perrin, E.; Fondi, M.; Fani, R.; Marino, G.; et al. Pseudoalteromonas haloplanktis produces methylamine, a volatile compound active against Burkholderia cepacia complex strains. N. Biotechnol. 2017, 35, 13–18. [Google Scholar] [CrossRef]
- Pfundstein, B.; Tricker, A.R.; Theobald, E.; Spiegelhalder, B.; Preussmann, R. Mean daily intake of primary and secondary amines from foods and beverages in West Germany in 1989–1990. Food Chem. Toxicol. 1991, 29, 733–739. [Google Scholar] [CrossRef]
- Atawodi, S.E.; Mende, P.; Pfundstein, B.; Preussmann, R.; Spiegelhalder, B. Nitrosatable amines and nitrosamide formation in natural stimulants: Cola acuminata, C. nitida and Garcinia cola. Food Chem. Toxicol. 1995, 33, 625–630. [Google Scholar] [CrossRef]
- Woodworth-Hobbs, M.E.; Hudson, M.B.; Rahnert, J.A.; Zheng, B.; Franch, H.A.; Price, S.R. Docosahexaenoic acid prevents palmitate-induced activation of proteolytic systems in C2C12 myotubes. J. Nutr. Biochem. 2014, 25, 868–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cioni, L.; De Siena, G.; Ghelardini, C.; Sernissi, O.; Alfarano, C.; Pirisino, R.; Raimondi, L. Activity and expression of semicarbazide-sensitive benzylamine oxidase in a rodent model of diabetes: Interactive effects with methylamine and alpha-aminoguanidine. Eur. J. Pharmacol. 2006, 529, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, L.; Alfarano, C.; Pacini, A.; Livi, S.; Ghelardini, C.; DeSiena, G.; Pirisino, R. Methylamine-dependent release of nitric oxide and dopamine in the CNS modulates food intake in fasting rats. Br. J. Pharmacol. 2007, 150, 1003–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahzouni, M.; Zendehdel, M.; Babapour, V.; Charkhkar, S. Methylamine induced hypophagia is mediated via dopamine D1 and D2 receptors in neonatal meat chicks. Vet. Res. Commun. 2016, 40, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Huber, K.W.; Lutz, W.K. Methylation of DNA in stomach and small intestine of rats after oral administration of methylamine and nitrite. Carcinogenesis 1984, 5, 1729–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Precious, E.; Gunn, C.E.; Lyles, G.A. Deamination of methylamine by semicarbazide-sensitive amine oxidase in human umbilical artery and rat aorta. Biochem. Pharmacol. 1988, 37, 707–713. [Google Scholar] [CrossRef]
- Salek, R.M.; Maguire, M.L.; Bentley, E.; Rubtsov, D.V.; Hough, T.; Cheeseman, M.; Nunez, D.; Sweatman, B.C.; Haselden, J.N.; Cox, R.D.; et al. A metabolomic comparison of urinary changes in type 2 diabetes in mouse, rat, and human. Physiol. Genomics 2007, 29, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Iglesias-Osma, M.C.; Bour, S.; Garcia-Barrado, M.J.; Visentin, V.; Pastor, M.F.; Testar, X.; Marti, L.; Enrique-Tarancon, G.; Valet, P.; Moratinos, J.; et al. Methylamine but not mafenide mimics insulin-like activity of the semicarbazide-sensitive amine oxidase-substrate benzylamine on glucose tolerance and on human adipocyte metabolism. Pharmacol. Res. 2005, 52, 475–484. [Google Scholar] [CrossRef]
- Salmi, M.; Jalkanen, S. Vascular Adhesion Protein-1: A Cell Surface Amine Oxidase in Translation. Antioxid. Redox Signal. 2019, 30, 314–332. [Google Scholar] [CrossRef]
- Stolen, C.M.; Yegutkin, G.G.; Kurkijarvi, R.; Bono, P.; Alitalo, K.; Jalkanen, S. Origins of serum semicarbazide-sensitive amine oxidase. Circ. Res. 2004, 95, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Meszaros, Z.; Szombathy, T.; Raimondi, L.; Karadi, I.; Romics, L.; Magyar, K. Elevated serum semicarbazide-sensitive amine oxidase activity in non-insulin-dependent diabetes mellitus: Correlation with body mass index and serum triglyceride. Metabolism 1999, 48, 113–117. [Google Scholar] [CrossRef]
- Li, H.Y.; Wei, J.N.; Lin, M.S.; Smith, D.J.; Vainio, J.; Lin, C.H.; Chiang, F.T.; Shih, S.R.; Huang, C.H.; Wu, M.Y.; et al. Serum vascular adhesion protein-1 is increased in acute and chronic hyperglycemia. Clin. Chim. Acta 2009, 404, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Pannecoeck, R.; Serruys, D.; Benmeridja, L.; Delanghe, J.R.; van Geel, N.; Speeckaert, R.; Speeckaert, M.M. Vascular adhesion protein-1: Role in human pathology and application as a biomarker. Crit. Rev. Clin. Lab. Sci. 2015, 52, 284–300. [Google Scholar] [CrossRef] [PubMed]
- Visentin, V.; Bour, S.; Boucher, J.; Prévot, D.; Valet, P.; Ordener, C.; Parini, A.; Carpéné, C. Glucose handling in streptozotocin-induced diabetic rats is improved by tyramine but not by the amine oxidase inhibitor semicarbazide. Eur. J. Pharmacol. 2005, 522, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.H.; Wei, J.N.; Yang, C.Y.; Ou, H.Y.; Wu, H.T.; Fan, K.C.; Wang, S.H.; Hua, C.H.; Hsiao, C.H.; Lee, M.K.; et al. Serum vascular adhesion protein-1 is up-regulated in hyperglycemia and is associated with incident diabetes negatively. Int J. Obes. 2018, 43, 512–522. [Google Scholar] [CrossRef]
- Shen, S.H.; Wertz, D.L.; Klinman, J.P. Implication for functions of the ectopic adipocyte copper amine oxidase (AOC3) from purified enzyme and cell-based kinetic studies. PLoS ONE 2012, 7, e29270. [Google Scholar] [CrossRef]
- Carpéné, C.; Les, F.; Hasnaoui, M.; Biron, S.; Marceau, P.; Richard, D.; Galitzky, J.; Joanisse, D.R.; Mauriège, P. Anatomical distribution of primary amine oxidase activity in four adipose depots and plasma of severely obese women with or without a dysmetabolic profile. J. Physiol. Biochem. 2016, 73, 475–486. [Google Scholar] [CrossRef]
- Enrique-Tarancon, G.; Marti, L.; Morin, N.; Lizcano, J.M.; Unzeta, M.; Sevilla, L.; Camps, M.; Palacin, M.; Testar, X.; Carpéné, C.; et al. Role of semicarbazide-sensitive amine oxidase on glucose transport and GLUT4 recruitment to the cell surface in adipose cells. J. Biol. Chem. 1998, 273, 8025–8032. [Google Scholar] [CrossRef]
- Mercader, J.; Iffiu-Soltesz, Z.; Brenachot, X.; Foldi, A.; Dunkel, P.; Balogh, B.; Attané, C.; Valet, P.; Matyus, P.; Carpéné, C. SSAO substrates exhibiting insulin-like effects in adipocytes as a promising treatment option for metabolic disorders. Future Med. Chem. 2010, 2, 1735–1749. [Google Scholar] [CrossRef]
- Dunkel, P.; Balogh, B.; Meleddu, R.; Maccioni, E.; Gyires, K.; Matyus, P. Semicarbazide-sensitive amine oxidase/vascular adhesion protein-1: A patent survey. Expert Opin. Ther. Pat. 2011, 21, 1453–1471. [Google Scholar] [CrossRef] [PubMed]
- Carpéné, C.; Boulet, N.; Chaplin, A.; Mercader, J. Past, Present and Future Anti-Obesity Effects of Flavin-Containing and/or Copper-Containing Amine Oxidase Inhibitors. Medicines 2019, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, A.; Rico, D.; Khiari, Z.; Henehan, G.; O’Sullivan, J.; Tipton, K. From caffeine to fish waste: Amine compounds present in food and drugs and their interactions with primary amine oxidase. J. Neural Transm. 2011, 118, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Marceau, P.; Hould, F.S.; Potvin, M.; Lebel, S.; Biron, S. Biliopancreatic diversion (duodenal switch procedure). Eur. J. Gastroenterol. Hepatol. 1999, 11, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Carpéné, C.; Garcia-Vicente, S.; Serrano, M.; Marti, L.; Belles, C.; Royo, M.; Galitzky, J.; Zorzano, A.; Testar, X. Insulin-mimetic compound hexaquis (benzylammonium) decavanadate is antilipolytic in human fat cells. World J. Diabetes 2017, 8, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Panchuk-Voloshina, N. A one-step fluorometric method for the continuous measurement of monoamine oxidase activity. Anal. Biochem. 1997, 253, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Deleruyelle, S.; Cassagnes, L.E.; Boutin, J.A.; Balogh, B.; Arbones-Mainar, J.M.; Biron, S.; Marceau, P.; Richard, D.; Nepveu, F.; et al. Piceatannol and resveratrol share inhibitory effects on hydrogen peroxide release, monoamine oxidase and lipogenic activities in adipose tissue, but differ in their antilipolytic properties. Chem. Biol. Interact. 2016, 258, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Carpéné, C.; Galitzky, J.; Belles, C.; Zakaroff-Girard, A. Mechanisms of the antilipolytic response of human adipocytes to tyramine, a trace amine present in food. J. Physiol. Biochem. 2018, 74, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Carpéné, C.; Les, F.; Esteve, D.; Galitzky, J. Short-term effects of obestatin on hexose uptake and triacylglycerol breakdown in human subcutaneous adipocytes. World J. Diabetes 2018, 9, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Morin, N.; Lizcano, J.M.; Fontana, E.; Marti, L.; Smih, F.; Rouet, P.; Prévot, D.; Zorzano, A.; Unzeta, M.; Carpéné, C. Semicarbazide-sensitive amine oxidase substrates stimulate glucose transport and inhibit lipolysis in human adipocytes. J. Pharmacol. Exp. Ther. 2001, 297, 563–572. [Google Scholar] [PubMed]
- Castan, I.; Valet, P.; Quideau, N.; Voisin, T.; Ambid, L.; Laburthe, M.; Lafontan, M.; Carpéné, C. Antilipolytic effects of alpha 2-adrenergic agonists, neuropeptide Y, adenosine, and PGE1 in mammal adipocytes. Am. J. Physiol. 1994, 266, R1141–R1147. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Osma, M.C.; Garcia-Barrado, M.J.; Visentin, V.; Pastor-Mansilla, M.F.; Bour, S.; Prevot, D.; Valet, P.; Moratinos, J.; Carpéné, C. Benzylamine exhibits insulin-like effects on glucose disposal, glucose transport, and fat cell lipolysis in rabbits and diabetic mice. J. Pharmacol. Exp. Ther. 2004, 309, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.C.; Plant, T.D.; Angel, I.; Langer, S.Z.; Henquin, J.C. In vitro stimulation of insulin release by SL 84.0418, a new alpha2-adrenoceptor antagonist. Eur. J. Pharmacol. 1994, 254, 27–33. [Google Scholar] [CrossRef]
- Garcia Barrado, M.J.; Pastor, M.F.; Iglesias-Osma, M.C.; Carpéné, C.; Moratinos, J. Comparative effects of idazoxan, efaroxan, and BU 224 on insulin secretion in the rabbit: Not only interaction with pancreatic imidazoline I2 binding sites. Health 2010, 2, 112–123. [Google Scholar] [CrossRef] [Green Version]
- Carpéné, C.; Abello, V.; Iffiu-Soltesz, Z.; Mercier, N.; Fève, B.; Valet, P. Limitation of adipose tissue enlargement in rats chronically treated with semicarbazide-sensitive amine oxidase and monoamine oxidase inhibitors. Pharmacol. Res. 2008, 57, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Enrique-Tarancon, G.; Castan, I.; Morin, N.; Marti, L.; Abella, A.; Camps, M.; Casamitjana, R.; Palacin, M.; Testar, X.; Degerman, E.; et al. Substrates of semicarbazide-sensitive amine oxidase co-operate with vanadate to stimulate tyrosine phosphorylation of insulin-receptor-substrate proteins, phosphoinositide 3-kinase activity and GLUT4 translocation in adipose cells. Biochem. J. 2000, 350, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Bour, S.; Prévot, D.; Guigne, C.; Stolen, C.; Jalkanen, S.; Valet, P.; Carpéné, C. Semicarbazide-sensitive amine oxidase substrates fail to induce insulin-like effects in fat cells from AOC3 knockout mice. J. Neural Transm. 2007, 114, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Grès, S.; Bour, S.; Valet, P.; Carpéné, C. Benzylamine antihyperglycemic effect is abolished by AOC3 gene invalidation in mice but not rescued by semicarbazide-sensitive amine oxidase expression under the control of aP2 promoter. J. Physiol. Biochem. 2012, 68, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Bour, S.; Daviaud, D.; Gres, S.; Lefort, C.; Prevot, D.; Zorzano, A.; Wabitsch, M.; Saulnier-Blache, J.S.; Valet, P.; Carpene, C. Adipogenesis-related increase of semicarbazide-sensitive amine oxidase and monoamine oxidase in human adipocytes. Biochimie 2007, 89, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Conklin, D.J.; Cowley, H.R.; Wiechmann, R.J.; Johnson, G.H.; Trent, M.B.; Boor, P.J. Vasoactive effects of methylamine in isolated human blood vessels: Role of semicarbazide-sensitive amine oxidase, formaldehyde, and hydrogen peroxide. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H667–H676. [Google Scholar] [CrossRef]
- Aalto, K.; Maksimow, M.; Juonala, M.; Viikari, J.; Jula, A.; Kahonen, M.; Jalkanen, S.; Raitakari, O.T.; Salmi, M. Soluble vascular adhesion protein-1 correlates with cardiovascular risk factors and early atherosclerotic manifestations. Arter. Thromb. Vasc. Biol. 2012, 32, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Carpéné, C.; Gomez-Zorita, S.; Gupta, R.; Gres, S.; Rancoule, C.; Cadoudal, T.; Mercader, J.; Gomez, A.; Bertrand, C.; Iffiu-Soltesz, Z. Combination of low dose of the anti-adipogenic agents resveratrol and phenelzine in drinking water is not sufficient to prevent obesity in very-high-fat diet-fed mice. Eur. J. Nutr. 2014, 53, 1625–1635. [Google Scholar] [CrossRef] [PubMed]
- Carpéné, C.; Visentin, V.; Morin, N.; Prevot, D.; Smih, F.; Rouet, P.; Jayat, D.; Fontana, E.; Lizcano, J.M. Characterization of semicarbazide-sensitive amine oxidase in human subcutaneous adipocytes and search for novel functions. Inflammopharmacology 2003, 11, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Taesuwan, S.; Cho, C.E.; Malysheva, O.V.; Bender, E.; King, J.H.; Yan, J.; Thalacker-Mercer, A.E.; Caudill, M.A. The metabolic fate of isotopically labeled trimethylamine-N-oxide (TMAO) in humans. J. Nutr. Biochem. 2017, 45, 77–82. [Google Scholar] [CrossRef] [PubMed]







| Parameter | Mean Values |
|---|---|
| Age (y) | 36 ± 2 (28–46) |
| Body weight (kg) | 154.0 ± 9.3 |
| Body mass index (kg/m2) | 58.67 ± 3.24 |
| Fasting glucose (mmol/L) | 6.65 ± 1.01 |
| Fasting insulin (µU/mL) | 43.56 ± 8.55 |
| HOMA (insulin resistance) | 15.3 ± 5.4 |
| Condition | Glycerol Release µmoles/100 mg lipids/90 min | p < |
|---|---|---|
| Baseline | 0.10 ± 0.01 | 0.000001 |
| Isoprenaline 10 nM (Iso, β-agonist) | 0.40 ± 0.08 | 0.02 |
| Isoprenaline 100 nM | 0.67 ± 0.09 | Control |
| Isoprenaline 1 µM | 0.73 ± 0.09 | NS |
| Iso 100 nM + benzylamine 1 mM | 0.36 ± 0.05 | 0.003 |
| Iso 100 nM + methylamine 1 mM | 0.38 ± 0.06 | 0.009 |
| Iso 100 nM + brimonidine 1 µM (α2-agonist) | 0.27 ± 0.05 | 0.0004 |
| Iso 100 nM + bupranolol 10 µM (β-antagonist) | 0.09 ± 0.02 | 0.00009 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpéné, C.; Mauriège, P.; Boulet, N.; Biron, S.; Grolleau, J.-L.; Garcia-Barrado, M.J.; Iglesias-Osma, M.C. Methylamine Activates Glucose Uptake in Human Adipocytes Without Overpassing Action of Insulin or Stimulating its Secretion in Pancreatic Islets. Medicines 2019, 6, 89. https://doi.org/10.3390/medicines6030089
Carpéné C, Mauriège P, Boulet N, Biron S, Grolleau J-L, Garcia-Barrado MJ, Iglesias-Osma MC. Methylamine Activates Glucose Uptake in Human Adipocytes Without Overpassing Action of Insulin or Stimulating its Secretion in Pancreatic Islets. Medicines. 2019; 6(3):89. https://doi.org/10.3390/medicines6030089
Chicago/Turabian StyleCarpéné, Christian, Pascale Mauriège, Nathalie Boulet, Simon Biron, Jean-Louis Grolleau, Maria José Garcia-Barrado, and Mari Carmen Iglesias-Osma. 2019. "Methylamine Activates Glucose Uptake in Human Adipocytes Without Overpassing Action of Insulin or Stimulating its Secretion in Pancreatic Islets" Medicines 6, no. 3: 89. https://doi.org/10.3390/medicines6030089