Clinical Safety of Combined Targeted and Viscum album L. Therapy in Oncological Patients
Abstract
:1. Introduction
2. Materials and Methods
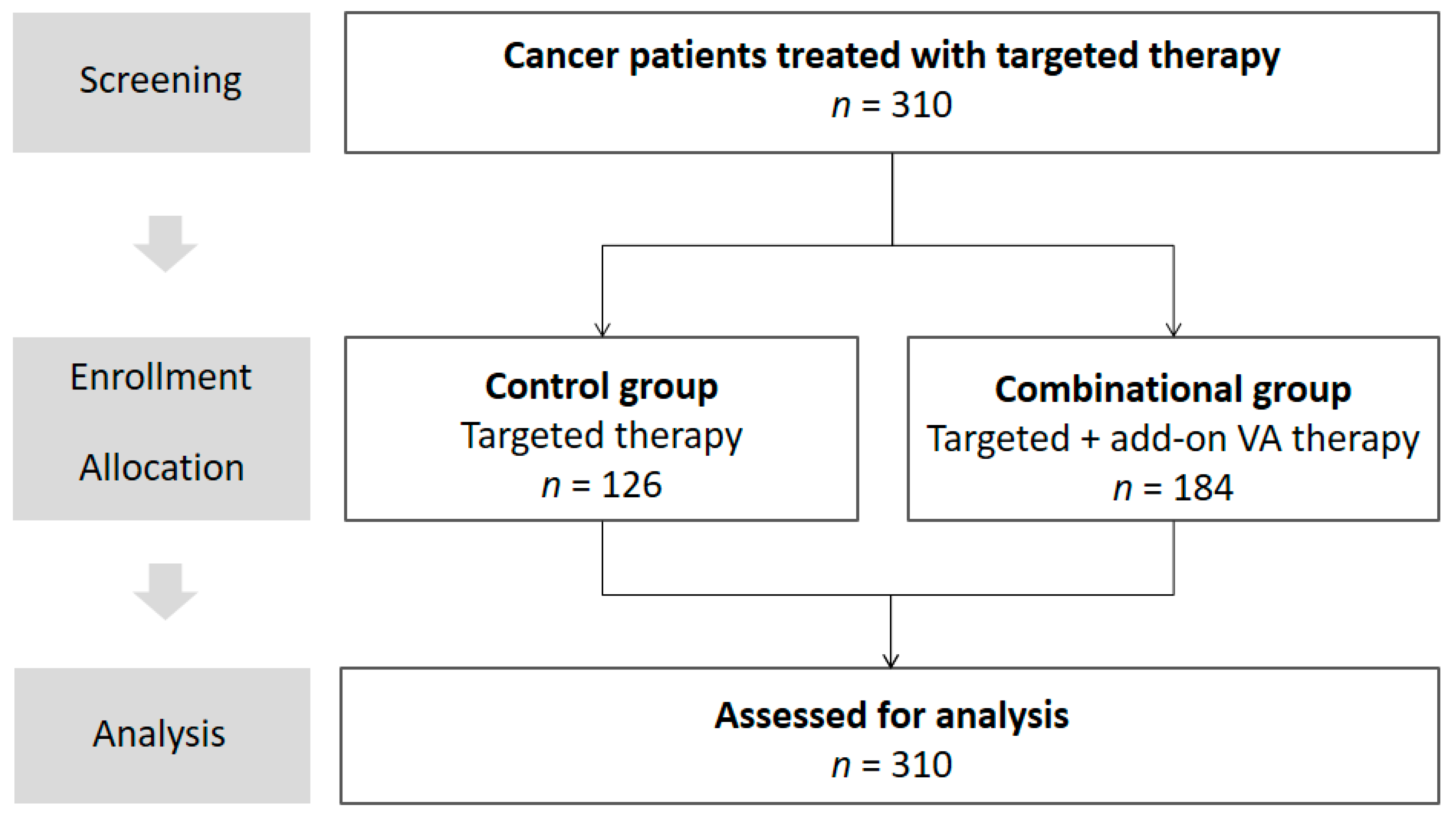
2.1. Study Design
2.2. Description of Study Participants
2.3. Data Source and Assessment
2.4. Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. Oncological Pharmacological and Non-Pharmacological Treatment
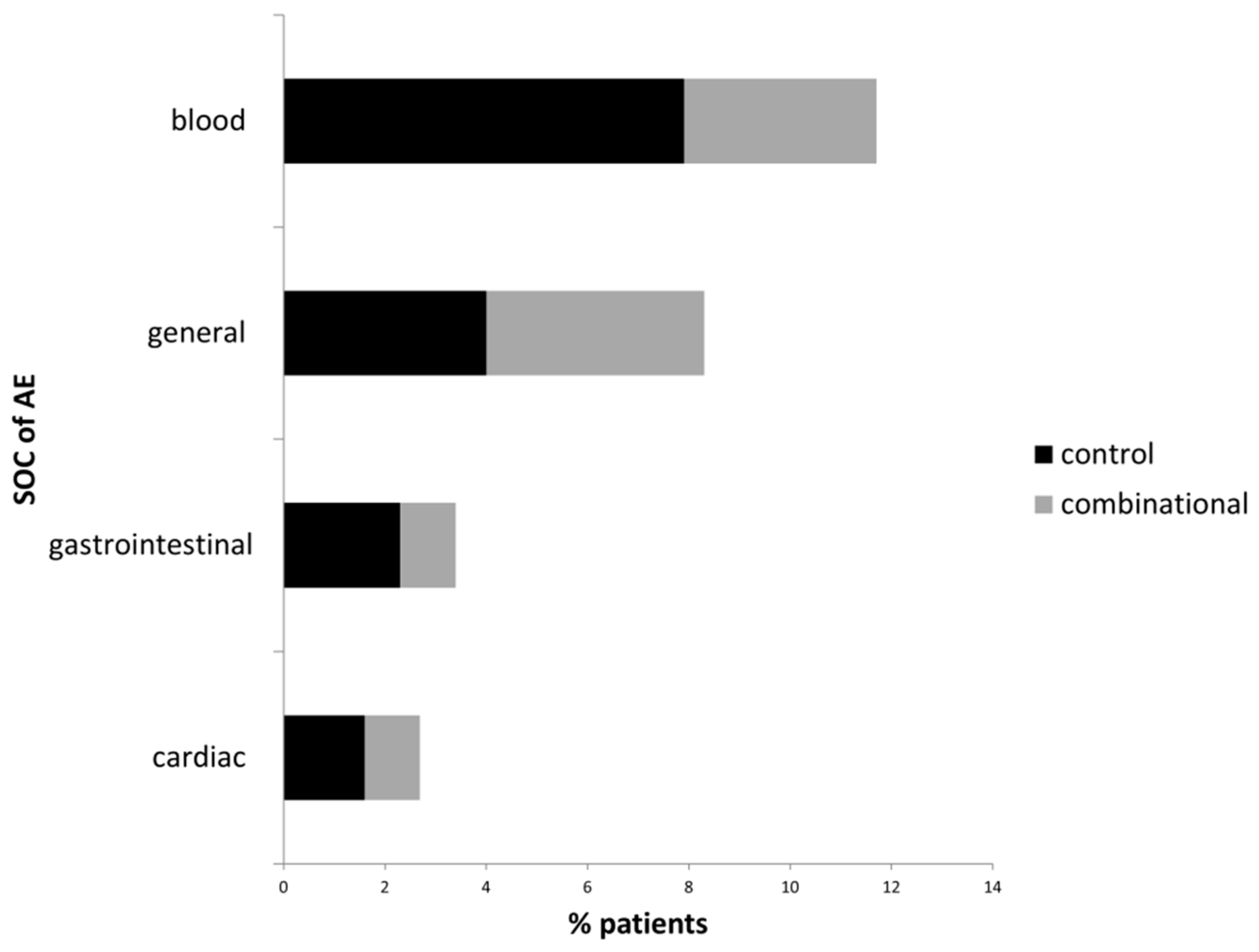
3.3. Adverse Events Related to Targeted and to Combinational Treatment
3.4. Factors Associated with Occurrence of AE
3.5. Treatment Discontinuation in Patients Experiencing Adverse Events
3.6. Factors Associated with Oncological Treatment Discontinuation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kim, B.J.; Kim, J.H.; Kim, H.S. Survival benefit of immune checkpoint inhibitors according to the histology in non-small-cell lung cancer: A meta-analysis and review. Oncotarget 2017, 8, 51779–51785. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, O.; Khairul-Asri, M.G.; Stenzl, A.; Gakis, G. The current status of checkpoint inhibitors in metastatic bladder cancer. Clin. Exp. Metastasis 2016, 33, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Colli, L.M.; Machiela, M.J.; Zhang, H.; Myers, T.A.; Jessop, L.; Delattre, O.; Yu, K.; Chanock, S.J. Landscape of combination immunotherapy and targeted therapy to improve cancer management. Cancer Res. 2017, 77, 3666–3671. [Google Scholar] [CrossRef] [PubMed]
- Ottaiano, A.; Capozzi, M.; De Divitiis, C.; De Stefano, A.; Botti, G.; Avallone, A.; Tafuto, S. Gemcitabine mono-therapy versus gemcitabine plus targeted therapy in advanced pancreatic cancer: A meta-analysis of randomized phase III trials. Acta Oncol. 2017, 56, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Partridge, A.H.; Rumble, R.B.; Carey, L.A.; Come, S.E.; Davidson, N.E.; Di Leo, A.; Gralow, J.; Hortobagyi, G.N.; Moy, B.; Yee, D.; et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2014, 32, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Naci, H.; Gurpinar, E.; Poplavska, E.; Pinto, A.; Aggarwal, A. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: Retrospective cohort study of drug approvals 2009-13. BMJ 2017, 359, j4530. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Prasad, V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival. JAMA Intern. Med. 2015, 175, 1992–1994. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Qin, J.; Pan, S.; Li, X.; Pan, Y.; Ma, S. Maintenance therapy in ovarian cancer with targeted agents improves PFS and OS: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0139026. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Hellmann, M.D.; Brahmer, J.R.; Juergens, R.A.; Borghaei, H.; Gettinger, S.; Chow, L.Q.; Gerber, D.E.; Laurie, S.A.; Goldman, J.W.; et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J. Clin. Oncol. 2016, 34, 2969–2979. [Google Scholar] [CrossRef] [PubMed]
- Botrel, T.E.A.; Clark, L.G.O.; Paladini, L.; Clark, O.A.C. Efficacy and safety of bevacizumab plus chemotherapy compared to chemotherapy alone in previously untreated advanced or metastatic colorectal cancer: A systematic review and meta-analysis. BMC Cancer 2016, 16, 677. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.; Shen, J.; Tong, J.L.; Xu, X.T.; Zhu, M.M.; Ran, Z.H. Meta-analysis: The efficacy and safety of monoclonal antibody targeted to epidermal growth factor receptor in the treatment of patients with metastatic colorectal cancer. J. Dig. Dis. 2009, 10, 247–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, J.S.; Kähler, K.C.; Hauschild, A. Management of immune-related adverse events and kinetics of response with ipilimumab. J. Clin. Oncol. 2012, 30, 2691–2697. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Carneiro, B.A.; Agulnik, M.; Rademaker, A.W.; Pai, S.G.; Villaflor, V.M.; Cristofanilli, M.; Sosman, J.A.; Giles, F.J. Toxicity profile of approved anti-PD-1 monoclonal antibodies in solid tumors: A systematic review and meta-analysis of randomized clinical trials. Oncotarget 2017, 8, 8910–8920. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.T.; Xu, H.; Xu, H.M.; Wang, Z.N.; Xu, Y.Y.; Song, Y.X.; Yin, S.-C.; Liu, X.-Y.; Miao, Z.-F. The efficacy and safety of targeted therapy with or without chemotherapy in advanced gastric cancer treatment: A network meta-analysis of well-designed randomized controlled trials. Gastric Cancer 2018, 21, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Soiffer, R.J.; Davids, M.S.; Chen, Y.B. Tyrosine kinase inhibitors and immune checkpoint blockade in allogeneic hematopoietic cell transplantation. Blood 2018, 131, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Z.; Xu, Q.N.; Wang, H.B.; Li, X.Y. Fulvestrant plus targeted agents versus fulvestrant alone for treatment of hormone-receptor positive advanced breast cancer progressed on previous endocrine therapy: A meta-analysis of randomized controlled trials. Breast Cancer 2017, 24, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Lidar, M.; Giat, E.; Garelick, D.; Horowitz, Y.; Amital, H.; Steinberg-Silman, Y.; Schachter, J.; Shapira-Frommer, R.; Markel, G. Rheumatic manifestations among cancer patients treated with immune checkpoint inhibitors. Autoimmun. Rev. 2018, 17, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; He, J.; Yu, M.; Li, T.; Luo, L.; Liu, P. The efficacy and safety of platinum plus gemcitabine (PG) chemotherapy with or without molecular targeted agent (MTA) in first-line treatment of non-small cell lung cancer (NSCLC). Medicine 2016, 95, e5599. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.J.; Grauer, D.W.; Henry, D.W.; Rockey, M.L. Corticosteroids for the management of immune-related adverse events in patients receiving checkpoint inhibitors. J. Oncol. Pharm. Pract. 2017. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.; Rice, T.W.; Cooksley, T. Problem-based review: Immune-mediated complications of ‘Checkpoint Inhibitors’ for the Acute Physician. Acute Med. 2017, 16, 21–24. [Google Scholar] [PubMed]
- Roberts, K.; Culleton, V.; Lwin, Z.; O’Byrne, K.; Hughes, B.G. Immune checkpoint inhibitors: Navigating a new paradigm of treatment toxicities. Asia Pac. J. Clin. Oncol. 2017, 13, 277–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reimschissel, E.; Dela Cruz, B.; Gonzalez, M.; Buitrago, J.; Goodman, C.; Johnston, P.A. Immunotherapy toxicities: A new electronic documentation template to improve patient care. Clin. J. Oncol. Nurs. 2017, 21, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., 3rd; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.; Forbes, S.G.; Fowler, N. Toxicity management: Development of a novel and immune-mediated adverse events algorithm. Clin. J. Oncol. Nurs. 2017, 21, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Cousin, S.; Seneschal, J.; Italiano, A. Toxicity profiles of immunotherapy. Pharmacol. Ther. 2018, 181, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Marone, G.; Mercurio, V.; Galdiero, M.R.; Bonaduce, D.; Tocchetti, C.G. Immune checkpoint inhibitors and cardiac toxicity: An emerging issue. Curr. Med. Chem. 2017, 25, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Galdiero, M.R.; Marone, G.; Criscuolo, G.; Triassi, M.; Bonaduce, D.; Marone, G.; Tocchetti, C.G. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open 2017, 2, e000247. [Google Scholar] [CrossRef] [PubMed]
- Tetzlaff, M.T.; Jazaeri, A.A.; Torres-Cabala, C.A.; Korivi, B.R.; Landon, G.A.; Nagarajan, P.; Choksi, A.; Chen, L.; Uemura, M.; Aung, P.P.; et al. Erythema nodosum-like panniculitis mimicking disease recurrence: A novel toxicity from immune checkpoint blockade therapy—Report of 2 patients. J. Cutan. Pathol. 2017, 44, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Spain, L.; Walls, G.; Julve, M.; O’Meara, K.; Schmid, T.; Kalaitzaki, E.; Turajlic, S.; Gore, M.; Rees, J.; Larkin, J. Neurotoxicity from immune-checkpoint inhibition in the treatment of melanoma: A single centre experience and review of the literature. Ann. Oncol. 2017, 28, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Makarious, D.; Horwood, K.; Coward, J.I.G. Myasthenia gravis: An emerging toxicity of immune checkpoint inhibitors. Eur. J. Cancer 2017, 82, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Coward, J.; McCaffrey, E.; Coucher, J.; Kalokerinos, P.; O’Byrne, K. Pembrolizumab-induced encephalopathy: A review of neurological toxicities with immune checkpoint inhibitors. J. Thorac. Oncol. 2017, 12, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Bock, P.R.; Friedel, W.E.; Hanisch, J.; Karasmann, M.; Schneider, B. Efficacy and safety of long-term complementary treatment with standardized European mistletoe extract (Viscum album L.) in addition to the conventional adjuvant oncologic therapy in patients with primary non-metastasized mammary carcinoma. Results of a multi-center, comparative, epidemiological cohort study in Germany and Switzerland. Arzneimittelforschung 2004, 54, 456–466. [Google Scholar] [PubMed]
- Bar-Sela, G.; Wollner, M.; Hammer, L.; Agbarya, A.; Dudnik, E.; Haim, N. Mistletoe as complementary treatment in patients with advanced non-small-cell lung cancer treated with carboplatin-based combinations: A randomised phase ii study. Eur. J. Cancer 2013, 49, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Büssing, A. Immune modulation using mistletoe (Viscum album L.) extracts Iscador. Arzneimittelforschung 2011, 56, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Schad, F.; Axtner, J.; Kroz, M.; Matthes, H.; Steele, M.L. Safety of combined treatment with monoclonal antibodies and Viscum album L. preparations. Integr. Cancer Ther. 2016, 17, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Thronicke, A.; Steele, M.; Grah, C.; Matthes, B.; Schad, F. Clinical safety of combined therapy of immune checkpoint inhibitors and Viscum album L. therapy in patients with advanced or metastatic cancer. Abstract. J. Thorac. Oncol. 2017, 12, S1295–S1296. [Google Scholar] [CrossRef]
- Thronicke, A.; Oei, S.; Grah, C.; Matthes, B.; Schad, F. Nivolumab-induced toxicity profile in patients with advanced or metastasized lung cancer treated with Viscum album L. extracts. In Proceedings of the Deutscher Krebskongress 2018 (DKK2018), Berlin, Germany, 21–24 February 2018. No. 514. [Google Scholar]
- Steele, M.L.; Axtner, J.; Happe, A.; Kröz, M.; Matthes, H.; Schad, F. Safety of intravenous application of mistletoe (Viscum album L.) preparations in oncology: An observational study. Evid. Based Complement. Altern. Med. 2014, 2014, 236310. [Google Scholar] [CrossRef] [PubMed]
- Steele, M.L.; Axtner, J.; Happe, A.; Kröz, M.; Matthes, H.; Schad, F. Adverse drug reactions and expected effects to therapy with subcutaneous mistletoe extracts (Viscum album L.) in cancer patients. Evid. Based Complement. Alternat. Med. 2014, 2014, 724258. [Google Scholar] [CrossRef] [PubMed]
- Steele, M.L.; Axtner, J.; Happe, A.; Kröz, M.; Matthes, H.; Schad, F. Use and safety of intratumoral application of european mistletoe (Viscum album L.) preparations in oncology. Integr. Cancer Ther. 2015, 14, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Schad, F.; Axtner, J.; Happe, A.; Breitkreuz, T.; Paxino, C.; Gutsch, J.; Matthes, B.; Debus, M.; Kröz, M.; Spahn, G.; et al. Network Oncology (NO)—A clinical cancer register for health services research and the evaluation of integrative therapeutic interventions in anthroposophic medicine. Forsch. Komplementärmed. 2013, 20, 353–360. [Google Scholar] [CrossRef] [PubMed]
- ICH Harmonised Tripartite Guideline. Clinical Safety Data Management: Definitions and Standards for Expedited Reporting E2D. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2D/Step4/E2D_Guideline.pdf (accessed on 5 May 2018).
- National Cancer Institute, Division of Cancer Treatment and Diagnosis. Common Terminology Criteria for Adverse Events (CTCAE) v.4.0. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 5 May 2018).
- Brier, G.W. Verification of forecasts expressed in terms of probability. Mon. Weather. Rev. 1950, 78, 1–3. [Google Scholar] [CrossRef]
- Nagelkerke, N.J.D. A note on a general definition of the coefficient of determination. Biometrika 1991, 78, 691–692. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2016. Available online: https://www.R-project.org/ (accessed on 5 May 2018).
- Perez, E.A. Cardiac toxicity of ErbB2-targeted therapies: What do we know? Clin. Breast Cancer 2008, 8, S114–S120. [Google Scholar] [CrossRef] [PubMed]
- Thronicke, A.; Oei, S.L.; Merkle, A.; Herbstreit, C.; Lemmens, H.-P.; Grah, C.; Kröz, M.; Matthes, H.; Schad, F. Integrative cancer care in a certified Cancer Centre of a German Anthroposophic hospital. Complement. Ther. Med. 2018, in press. [Google Scholar] [CrossRef]
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lupi, C.; Sensi, E.; Lonardi, S.; Mezi, S.; Tomasello, G.; Ronzoni, M.; Zaniboni, A.; et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015, 16, 1306–1315. [Google Scholar] [CrossRef]
- Khattak, M.A.; Martin, H.; Davidson, A.; Phillips, M. Role of first-line anti-epidermal growth factor receptor therapy compared with anti-vascular endothelial growth factor therapy in advanced colorectal cancer: A meta-analysis of randomized clinical trials. Clin. Colorectal Cancer 2015, 14, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Jean, G.W.; Shah, S.R. Epidermal growth factor receptor monoclonal antibodies for the treatment of metastatic colorectal cancer. Pharmacotherapy 2008, 28, 742–754. [Google Scholar] [CrossRef] [PubMed]
- EMA. Herceptin, Annex, I. Summary of Product Characteristics. 2017. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000278/WC500074922.pdf (accessed on 5 May 2018).
- EMA. MabThera, Annex, I. Summary of Product Characterization. 2018. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000165/WC500025821.pdf (accessed on 5 May 2018).
- Thronicke, A.; Steele, M.L.; Grah, C.; Matthes, B.; Schad, F. Clinical safety of combined therapy of immune checkpoint inhibitors and Viscum album L. therapy in patients with advanced or metastatic cancer. BMC Complement. Altern. Med. 2017, 17, 534. [Google Scholar] [CrossRef] [PubMed]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J.T. The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 2010, 9, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Van Helden, E.J.; Menke-van der Houven van Oordt, C.W.; Heymans, M.W.; Ket, J.C.F.; van den Oord, R.; Verheul, H.M.W. Optimal use of anti-EGFR monoclonal antibodies for patients with advanced colorectal cancer: A meta-analysis. Cancer Metastasis Rev. 2017, 36, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Steinborn, C.; Klemd, A.M.; Sanchez-Campillo, A.S.; Rieger, S.; Scheffen, M.; Sauer, B.; Garcia-Käufer, M.; Urech, K.; Follo, M.; Ücker, A.; et al. Viscum album neutralizes tumor-induced immunosuppression in a human in vitro cell model. PLoS ONE 2017, 12, e0181553. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, T.; Heinrichs, N.; Baucom, D.H. “Does one size fit all?” moderators in psychosocial interventions for breast cancer patients: A meta-analysis. Ann. Behav. Med. 2007, 34, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Kienle, G.S.; Kiene, H. Die Mistel in der Onkologie: Fakten und konzeptionelle Grundlagen; Schattauer Verlag: Stuttgart, Germany, 2003. [Google Scholar]
- Thies, A.; Nugel, D.; Pfüller, U.; Moll, I.; Schumacher, U. Influence of mistletoe lectins and cytokines induced by them on cell proliferation of human melanoma cells in vitro. Toxicology 2005, 207, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Weissenstein, U.; Kunz, M.; Urech, K.; Regueiro, U.; Baumgartner, S. Interaction of a standardized mistletoe (Viscum album) preparation with antitumor effects of Trastuzumab in vitro. BMC Complement. Altern. Med. 2016, 16, 271. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, K.C.; Lee, C. Mistletoe (Viscum album) extract targets Axl to suppress cell proliferation and overcome cisplatin- and erlotinib-resistance in non-small cell lung cancer cells. Phytomedicine 2017, 36, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Yance, D.R., Jr.; Sagar, S.M. Targeting angiogenesis with integrative cancer therapies. Integr. Cancer Ther. 2006, 5, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Podlech, O.; Harter, P.N.; Mittelbronn, M.; Pöschel, S.; Naumann, U. Fermented mistletoe extract as a multimodal antitumoral agent in gliomas. Evid. Based Complement. Altern. Med. 2012, 2012, 501796. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, E.; Hajto, T.; Hostanska, K. Improvement of DNA repair in lymphocytes of breast cancer patients treated with Viscum album extract (Iscador). Eur. J. Cancer Clin. Oncol. 1991, 27, 1672–1676. [Google Scholar] [CrossRef]
- Kovacs, E. Effect of iscador on DNA repair after radiation or cyclophosphamide: Correlation with IFN-gamma production. Onkologie 1995, 18, 65. [Google Scholar]
- Kovacs, E.; Kuehn, J.J.; Werner, M.; Hoffmann, J. Die Wirkung von Viscum album (Iscador) auf die DNA-Reparatur in peripheren Lymphozyten nach Gammastrahlen- und Cyclophosphamid-Exposition. Korrelation zur IFN-Gamma-Produktion: in vitro-Ergebnisse. In Grundlagen der Misteltherapie: Aktueller Stand der Forschung und klinische Anwendung; Scheer, R., Becker, H., Berg, A., Eds.; Hippokrates-Verlag: Stuttgart, Germany, 1996; pp. 197–205. [Google Scholar]
- Kuttan, G.; Kuttan, R. Reduction of leukopenia in mice by “Viscum album” administration during radiation and chemotherapy. Tumori 1993, 79, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Narimanov, A.A.; Popova, O.I.; Murav’eva, D.A. Changes in the sensitivity of mice to the action of gamma irradiation by Viscum album L. polysaccharides. Radiobiologiia 1992, 32, 868–872. [Google Scholar] [PubMed]
- Kovacs, E. The in vitro effect of Viscum album (VA) extract on DNA repair of peripheral blood mononuclear cells (PBMC) in cancer patients. Phytother. Res. 2002, 16, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Kienle, G.S.; Berrino, F.; Bussing, A.; Portalupi, E.; Rosenzweig, S.; Kiene, H. Mistletoe in cancer—A systematic review on controlled clinical trials. Eur. J. Med. Res. 2003, 8, 109–119. [Google Scholar] [PubMed]
- Horneber, M.A.; Bueschel, G.; Huber, R.; Linde, K.; Rostock, M. Mistletoe therapy in oncology. Cochrane Database Syst. Rev. 2008. [Google Scholar] [CrossRef] [PubMed]
- Büssing, A.; Raak, C.; Ostermann, T. Quality of life and related dimensions in cancer patients treated with mistletoe extract (iscador): A meta-analysis. Evid. Based Complement. Altern. Med. 2012, 2012, 219402. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ma, J.-T.; Zhang, S.-L.; Zou, H.-W.; Han, C.-B. Efficacy and safety of chemotherapy or tyrosine kinase inhibitors combined with bevacizumab versus chemotherapy or tyrosine kinase inhibitors alone in the treatment of non-small cell lung cancer: A systematic review and meta-analysis. Med. Oncol. 2015, 32, 34. [Google Scholar] [CrossRef] [PubMed]
- Ching, L.C.; Devi, M.K.; DNurs, A.N.K.E. Anxiety in patients with breast cancer undergoing treatment: A systematic review. JBI Libr. Syst. Rev. 2010, 8, 1016–1057. [Google Scholar] [CrossRef] [PubMed]


| Patient Characteristics | Total Cohort | Control Group | Combinational Group | Significance |
|---|---|---|---|---|
| n = 310 | n = 126 | n = 184 | ||
| Age at first diagnosis, years, median (IQR) | 59.0 (49.0–68.0) | 59.0 (48.0–67.0) | 60.0 (50.0–69.0) | — |
| Gender | ||||
| Male, n (%) | 95 (30.6) | 33 (26.2) | 62 (33.7) | — |
| Female, n (%) | 215 (67.4) | 93 (73.9) | 122 (66.3) | — |
| Cancer by body location, n (%) | ||||
| Breast, n (%) | 113 (36.5) | 59 (46.8) | 54 (29.4) | ** a) |
| Digestive/Gastrointestinal, n (%) | 84 (27.1) | 17 (13.5) | 67 (36.4) | ** b) |
| Hematologic/Blood, n (%) | 36 (11.6) | 29 (23.0) | 7 (3.8) | ** b) |
| Respiratory/Thoracic, n (%) | 62 (20.0) | 19 (15.1) | 43 (23.4) | — |
| Genitourinary, n (%) | 7 (2.3) | 1 (0.8) | 6 (3.3) | — |
| Gynecologic, n (%) | 4 (1.3) | 1 (0.8) | 3 (1.63) | — |
| Musculoskeletal, n (%) | 1 (0.3) | 0 | 1 (0.5) | — |
| Skin, n (%) | 3 (1.0) | 0 | 3 (1.6) | — |
| UICC stage at first treatment, n (%) | ||||
| 0, n (%) | 1 (0.3) | 0 | 1 (0.5) | — |
| I, n (%) | 22 (7.1) | 10 (7.9) | 12 (6.5) | — |
| II, n (%) | 43 (13.9) | 22 (17.5) | 21 (11.4) | — |
| III, n (%) | 62 (20.0) | 24 (19.0) | 38 (20.7) | — |
| IV, n (%) | 108 (34.8) | 30 (23.8) | 78 (42.4) | ** b) |
| Target Therapy Class | Target | Total Cohort | Control Group | Combinational Group | Significance |
|---|---|---|---|---|---|
| n = 310 | n = 126 | n = 184 | |||
| Monoclonal antibodies | |||||
| bevacizumab, n (%) | VEGFR | 76 (24.5) | 11 (8.7) | 65 (35.3) | *** a) |
| cetuximab, n (%) | EGFR | 23 (7.4) | 5 (4.0) | 18 (9.8) | — |
| panitumumab, n (%) | EGFR | 12 (3.9) | 2 (1.6) | 10 (5.4) | — |
| rituximab, n (%) | CD20 | 33 (10.6) | 27 (21.4) | 6 (3.3) | *** a) |
| trastuzumab, n (%) | HER2 | 107 (34.5) | 61 (48.4) | 46 (25.0) | *** a) |
| Immunotherapy | |||||
| ipilimumab, n (%) | CTLA-4 | 1 (0.3) | — | 1 (0.5) | — |
| nivolumab, n (%) | PD-1 | 3 (1.0) | — | 3 (1.6) | — |
| pembrolizumab, n (%) | PD-1 | 5 (1.6) | 2 (1.6) | 1 (0.5) | — |
| Tyrosine kinase inhibitors | |||||
| erlotinib, n (%) | EGFR | 38 (12.3) | 3 (2.4) | 35 (19.0) | *** a) |
| gefitinib, n (%) | EGFR | 9 (2.9) | 3 (2.4) | 6 (3.3) | — |
| sorafenib, n (%) | Dual Raf-kinase/VEGFR | 7 (2.3) | — | 7 (3.8) | — |
| sunitinib, n (%) | Receptor tyrosine kinases | 7 (2.3) | 1 (0.8) | 6 (3.3) | — |
| VA Remedy | Total | Colorectal Cancer | Lung Cancer | Breast Cancer | Other Cancer |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| VA | 184 | 49 (100) | 40 (100) | 50 (100) | 45 (100) |
| VA abietis | 4 (2.1) | - | 1 (2.0) | 3 (6.0) | - |
| VA aceris | 6 (3.2) | - | 5 (10.2) | - | 1 (2.2) |
| VA craetegi | 2 (1.1) | 1 (2.0) | - | - | 1 (2.2) |
| VA fraxini | 119 (63.6) | 23 (46.9) | 32 (65.3) | 31 (62.0) | 34 (75.6) |
| VA mali | 20 (10.7) | 2 (4.1) | 1 (2.0) | 16 (32.0) | 1 (2.2) |
| VA quercus | 33 (17.6) | 27 (55.1) | 2 (4.1) | - | 4 (8.9) |
| VA pini | 6 (3.2) | 1 (2.0) | 1 (2.0) | 3 (6.0) | 1 (2.2) |
| System Organ Class | Adverse Event | Control Group | Combinational Group |
|---|---|---|---|
| n = 126 | n = 184 | ||
| blood and lymphatic system disorder | bradycardia | 1 (0.8) c | -- |
| chronic renal insufficiency | 1 (0.8) k | -- | |
| circulatory instability (t) | 1 (0.8) z | -- | |
| hemorrhage (c, t) | 1 (0.8) i | -- | |
| neutropenia (c) | 1 (0.8) p | -- | |
| thrombocytopenia | 1 (0.8) j | -- | |
| syncope (t) | 1 (0.8) c | -- | |
| dyspnea (c, t) | 1 (0.8) v | 1 (0.5) a | |
| anemia (c, t) | 3 (2.4) г, j, j | 1 (0.5) l | |
| dyspnea (c, t) | 1 (0.8) v | 1 (0.5) a | |
| agranulocytosis | -- | 1 (0.5) l | |
| burning sensation (v) | -- | 1 (0.5) x | |
| neutropenia (c, t) | -- | 1 (0.5) l | |
| absolute arrhythmia | -- | 1 (0.5) д | |
| congenital, familial and genetic disorders | hypersensitivity (c, t, v) | 1 (0.8) л | 1 (0.5) u |
| endocrine disorders | exsiccosis | 1 (0.8) г | -- |
| gastrointestinal disorders | dysphagia | 1 (0.8) г | -- |
| nausea (c,t,v) | 3 (2.4) h, б, и | -- | |
| vomiting (c,t,v) | 3 (2.4) r, б, и | -- | |
| diarrhea (c, t, v) | -- | 1 (0.5) b | |
| stomatitis (c, t) | -- | 1 (0.5) l | |
| general disorders and administration site conditions | discomfort | 1 (0.8) c | -- |
| loss of appetite (c, t) | 1 (0.8) h | -- | |
| chills (c, t, v) | 2 (1.6) d, j | 1 (0.5) н | |
| pain (c, t, v) | 3 (2.4) h, I, y | 1 (0.5) f | |
| pyrexia (c, t, v) | 1 (0.8) v | 4 (2.2) s, u, f, н | |
| cachexia | -- | 1 (0.5) l | |
| edema (v) | -- | 2 (1.1) l, o | |
| induration (v) | -- | 1 (0.5) x | |
| local reaction (c, t, v) | -- | 1 (0.5) f | |
| swelling (c, t, v) | -- | 1 (0.5) o | |
| hepatobiliary disorders | ascites | -- | 1 (0.5) g |
| immune system disorder | polyneuropathy (c) | -- | 1 (0.5) o |
| infections and infestations | sepsis | 1 (0.8) q | 1 (0.5) l |
| metabolism and nutrition disorders | hypocalcemia | 1 (0.8) h | 1 (0.5) l |
| hypokalemia | -- | 1 (0.5) l | |
| musculoskeletal and connective tissue disorders | Dupuyrtren’s contracture | 1 (0.8) q | -- |
| hypotonia | 1 (0.8) c | -- | |
| nervous system disorders | Vertigo (c, t) | 1 (0.8) h | -- |
| psychiatric disorders | reduced general condition | 1 (0.8) y | 4 (2.2) g, m, n, w |
| renal and urinary disorders | renal insufficiency | -- | 1 (0.5) m |
| urinary retention | -- | 1 (0.5) i | |
| respiratory, thoracic and mediastinal disorders | cough | 1 (0.8) r | -- |
| atelectasis | -- | 1 (0.5) e | |
| pleural effusion | -- | 1 (0.5) l | |
| skin and subcutaneous tissue disorder | erythema (c, t, v) | 1 (0.8) з | 2 (1.1) t, x |
| vascular disorder | hemorrhoids (com) | 1 (0.8) k | -- |
| thrombosis (t) | -- | 1 (0.5) m | |
| Not specified | AE, unspecified | 1 (0.8) ж | -- |
| AE frequency, n (%) | 38 (30.2) 1) | 37 (20.1) 1) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thronicke, A.; Oei, S.L.; Merkle, A.; Matthes, H.; Schad, F. Clinical Safety of Combined Targeted and Viscum album L. Therapy in Oncological Patients. Medicines 2018, 5, 100. https://doi.org/10.3390/medicines5030100
Thronicke A, Oei SL, Merkle A, Matthes H, Schad F. Clinical Safety of Combined Targeted and Viscum album L. Therapy in Oncological Patients. Medicines. 2018; 5(3):100. https://doi.org/10.3390/medicines5030100
Chicago/Turabian StyleThronicke, Anja, Shiao Li Oei, Antje Merkle, Harald Matthes, and Friedemann Schad. 2018. "Clinical Safety of Combined Targeted and Viscum album L. Therapy in Oncological Patients" Medicines 5, no. 3: 100. https://doi.org/10.3390/medicines5030100
APA StyleThronicke, A., Oei, S. L., Merkle, A., Matthes, H., & Schad, F. (2018). Clinical Safety of Combined Targeted and Viscum album L. Therapy in Oncological Patients. Medicines, 5(3), 100. https://doi.org/10.3390/medicines5030100





