Study Protocol for a Randomized Double Blind, Placebo Controlled Trial Exploring the Effectiveness of a Micronutrient Formula in Improving Symptoms of Anxiety and Depression
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Study Aims
2.3. Participant Eligibility
2.4. Sample Recruitment
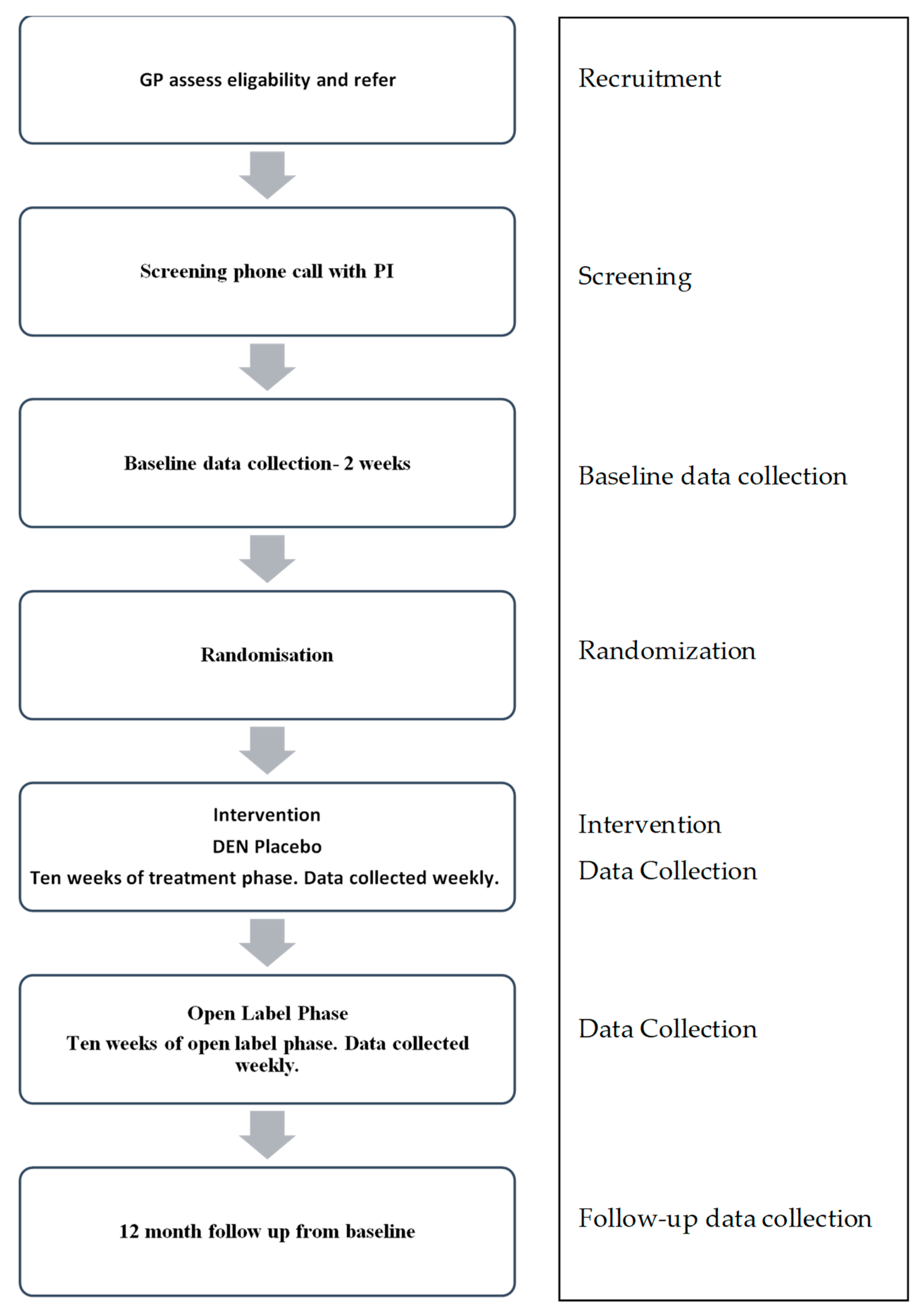
2.5. Study Procedure
2.5.1. Screening Assessment
2.5.2. Randomization and Allocation
2.5.3. Blinding
2.5.4. Intervention
2.6. Data Collection and Outcome Measures
2.6.1. Primary Outcome Measure
2.6.2. Secondary Outcome Measure
2.6.3. Other Measures
2.7. Data Management
2.8. Study Integrity
2.9. Sample Size
2.10. Data Analyses
3. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Mental Health Action Plan 2013–2020; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Hegel, M.T.; Oxman, T.E.; Hull, J.G.; Swain, K.; Swick, H. Watchful waiting for minor depression in primary care: Remission rates and predictors of improvement. Gen. Hosp. Psychiatry 2006, 28, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Skapinakis, P.; Lewis, G.; Davies, S.; Brugha, T.; Prince, M.; Singleton, N. Panic disorder and subthreshold panic in the UK general population: Epidemiology, comorbidity and functional limitation. Eur. Psychiatry 2011, 26, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.E.; Oakley Browne, M.A.; Scott, K.M.; Mcgee, M.A.; Baxter, J.; Kokaua, J. Te Rau Hinengaro: The New Zealand mental health survey: Overview of methods and findings. Aust. N. Z. J. Psychiatry 2006, 40, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Konnopka, A.; Leichsenring, F.; Leibing, E.; König, H.-H. Cost-of-illness studies and cost-effectiveness analyses in anxiety disorders: A systematic review. J. Affect. Disorders 2009, 114, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Ophuis, R.H.; Lokkerbol, J.; Heemskerk, S.C.M.; van Balkom, A.J.L.M.; Hiligsmann, M.; Evers, S.M.A.A. Cost-effectiveness of interventions for treating anxiety disorders: A systematic review. J. Affect. Disord. 2017, 210, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.; Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines Team for Depression. Australian and New Zealand clinical practice guidelines for the treatment of depression. Aust. N. Z. J. Psychiatry 2004, 38, 389–407. [Google Scholar] [PubMed]
- Olfson, M.; Marcus, S.C. National patterns in antidepressant medication treatment. Arch. Gen. Psychiatry 2009, 66, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Kohn, R.; Saxena, S.; Levav, I.; Saraceno, B. The treatment gap in mental health care. Bull. World Health Organ. 2004, 82, 858–866. [Google Scholar] [PubMed]
- Hieronymus, F.; Emilsson, J.F.; Nilsson, S.; Eriksson, E. Consistent superiority of selective serotonin reuptake inhibitors over placebo in reducing depressed mood in patients with major depression. Mol. Psychiatry 2016, 21, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, D.; Woods, R.; Lawson, R.; Taylor, D. Efficacy of drug treatments for generalised anxiety disorder: Systematic review and meta-analysis. Br. Med. J. 2011, 342. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.G.; Sawyer, A.T.; Korte, K.J.; Smits, J.A. Is it beneficial to add pharmacotherapy to cognitive-behavioral therapy when treating anxiety disorders? A meta-analytic review. Int. J. Cogn. Ther. 2009, 2, 160–175. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Amminger, G.P.; Balanzá-Martínez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry 2015, 2, 271–274. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef]
- Ferguson, J.M. SSRI Antidepressant Medications: Adverse Effects and Tolerability. Prim. Care Companion J. Clin. Psychiatry 2001, 3, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, I.; Maguire, K.; Ng, C. Sexual side-effects of contemporary antidepressants: Review. Aust. N. Z. J. Psychiatry 2009, 43, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Serretti, A.; Mandelli, L. Antidepressants and body weight: A comprehensive review and meta-analysis. J. Clin. Psychiatry 2010, 71, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, P.; Cristea, I.A.; Weitz, E.; Gentili, C.; Berking, M. The effects of cognitive and behavioural therapies for anxiety disorders on depression: A meta-analysis. Psychol. Med. 2016, 46, 3451–3462. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, P.; Cristea, I.A.; Karyotaki, E.; Reijnders, M.; Huibers, M.J.H. How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta-analytic update of the evidence. World Psychiatry 2016, 15, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Kolovos, S.; van Tulder, M.W.; Cuijpers, P.; Prigent, A.; Chevreul, K.; Riper, H.; Bosmans, J.E. The effect of treatment as usual on major depressive disorder: A meta-analysis. J. Affect. Disord. 2017, 210, 72–81. [Google Scholar] [CrossRef] [PubMed]
- McMain, S.; Newman, M.G.; Segal, Z.V.; DeRubeis, R.J. Cognitive behavioral therapy: Current status and future research directions. Psychother. Res. 2015, 25, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.; Ogles, B. The efficacy and effectiveness of psychotherapy. In Bergin and Garfields Handbook of Psychotherapy and Behavior Change; Lambert, M.J., Ed.; Wiley: New York, NY, USA, 2004; pp. 139–193. [Google Scholar]
- Patel, V.; Belkin, G.S.; Chockalingam, A.; Cooper, J.; Saxena, S.; Unützer, J. Grand Challenges: Integrating Mental Health Services into Priority Health Care Platforms. PLoS Med. 2013, 10, e1001448. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, A.; Quirk, S.E.; Housden, S.; Brennan, S.L.; Williams, L.J.; Pasco, J.A.; Berk, M.; Jacka, F.N. Relationship between diet and mental health in children and adolescents: A systematic review. Am. J. Public Health 2014, 104, e31–e42. [Google Scholar] [CrossRef] [PubMed]
- Robson, D.; Gray, R. Serious mental illness and physical health problems: A discussion paper. Int. J. Nurs. Stud. 2007, 44, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Happell, B. The high prevalence of poor physical health and unhealthy lifestyle behaviours in individuals with severe mental illness. Issues Ment. Health Nurs. 2011, 32, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Davison, K.M.; Kaplan, B.J. Nutrient intakes are correlated with overall psychiatric functioning in adults with mood disorders. Can. J. Psychiatry 2012, 57, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Opie, R.S.; O’Neil, A.; Itsiopoulos, C.; Jacka, F.N. The impact of whole-of-diet interventions on depression and anxiety: A systematic review of randomised controlled trials. Public Health Nutr. 2015, 18, 2074–2093. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Davison, K.M.; Kaplan, B.J. Nutrient- and non-nutrient-based natural health product (NHP) use in adults with mood disorders: Prevalence, characteristics and potential for exposure to adverse events. BMC Complement. Altern. Med. 2013, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.L. Emotional influences on food choice: Sensory, physiological and psychological pathways. Physiol. Behav. 2006, 89, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Rothman, A.J.; Sheeran, P.; Wood, W. Reflective and automatic processes in the initiation and maintenance of dietary change. Ann. Behav. Med. 2009, 38, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.; Alexander, N.; Almeida, N.; Black, R.; Burns, R.; Bush, L.; Crawford, P.; Keim, N.; Kris-Etherton, P.; Weaver, C. Food science challenge: Translating the Dietary Guidelines for Americans to bring about real behavior change. J. Food Sci. 2011, 76, R29–R37. [Google Scholar] [CrossRef] [PubMed]
- Stunkard, A.J.; Faith, M.S.; Allison, K.C. Depression and obesity. Biol. Psychiatry 2003, 54, 330–337. [Google Scholar] [CrossRef]
- Lewis, J.E.; Tiozzo, E.; Melillo, A.B.; Leonard, S.; Chen, L.; Mendez, A.; Woolger, J.M.; Konefal, J. The effect of methylated vitamin B complex on depressive and anxiety symptoms and quality of life in adults with depression. ISRN Psychiatry 2013, 2013, 621453. [Google Scholar] [CrossRef] [PubMed]
- Long, S.-J.; Benton, D. Effects of vitamin and mineral supplementation on stress, mild psychiatric symptoms, and mood in nonclinical samples: A meta-analysis. Psychosom. Med. 2013, 75, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Papakostas, G.I.; Shelton, R.C.; Zajecka, J.M.; Etemad, B.; Rickels, K.; Clain, A.; Baer, L.; Dalton, E.D.; Sacco, G.R.; Schoenfeld, D.; et al. l-methylfolate as adjunctive therapy for SSRI-resistant major depression: Results of two randomized, double-blind,,parallel-sequential trials. Am. J. Psychiatry 2012, 169, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.R. Depression, Suicide and Deficiencies of Omega–3 Essential Fatty Acids in Modern Diets. In Omega-3 Fatty Acids, the Brain and Retina; Karger Publishers: Basel, Switzerland, 2009; Volume 99, pp. 17–30. [Google Scholar]
- De Souza, M.C.; Walker, A.F.; Robinson, P.A.; Bolland, K. A synergistic effect of a daily supplement for 1 month of 200 mg magnesium plus 50 mg vitamin B6 for the relief of anxiety-related premenstrual symptoms: A randomized, double-blind, crossover study. J. Womens Health Gend. Based Med. 2000, 9, 131–139. [Google Scholar] [CrossRef] [PubMed]
- DiGirolamo, A.M.; Ramirez-Zea, M.; Wang, M.; Flores-Ayala, R.; Martorell, R.; Neufeld, L.M.; Ramakrishnan, U.; Sellen, D.; Black, M.M.; Stein, A.D. Randomized trial of the effect of zinc supplementation on the mental health of school-age children in Guatemala. Am. J. Clin. Nutr. 2010, 92, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Popper, C.W. Single-Micronutrient and Broad-Spectrum Micronutrient Approaches for Treating Mood Disorders in Youth and Adults. Child Adolesc. Psychiatr. Clin. N. Am. 2014, 23, 591–672. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.J.; Crawford, S.G.; Field, C.J.; Simpson, J.S.A. Vitamins, Minerals, and Mood. Psychol. Bull. 2007, 133, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Gariballa, S.; Forster, S. Effects of dietary supplements on depressive symptoms in older patients: A randomised double-blind placebo-controlled trial. Clin. Nutr. 2007, 26, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.J.; Rucklidge, J.J.; Romijn, A.R.; Dolph, M. A randomised trial of nutrient supplements to minimise psychological stress after a natural disaster. Psychiatry Res. 2015, 228, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Rucklidge, J.J.; Andridge, R.; Gorman, B.; Blampied, N.; Gordon, H.; Boggis, A. Shaken but unstirred? Effects of micronutrients on stress and trauma after an earthquake: RCT evidence comparing formulas and doses. Hum. Psychopharmacol. Clin. Exp. 2012, 27, 440–454. [Google Scholar] [CrossRef] [PubMed]
- Rucklidge, J.J.; Blampied, N.M. Post-earthquake psychological functioning in adults with attention-deficit/hyperactivity disorder: Positive effects of micronutrients on resilience. N. Z. J. Psychol. 2011, 40, 51–57. [Google Scholar]
- Lothian, J.; Blampied, N.M.; Rucklidge, J.J. Effect of micronutrients on insomnia in adults: A multiple-baseline study. Clin. Psychol. Sci. 2016, 4, 1112–1124. [Google Scholar] [CrossRef]
- Rucklidge, J.J.; Eggleston, M.; Johnston, J.M.; Darling, K.; Frampton, C.M. Vitamin-mineral treatment improves aggression and emotional regulation in children with ADHD: A fully-blinded, randomized, placebo-controlled trial. J. Child Psychol. Psychiatry 2018, 59, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D.; Ring, C.; Suter, M.; Willemsen, G. The effects of an oral multivitamin combination with calcium, magnesium, and zinc on psychological well-being in healthy young male volunteers: A double-blind placebo-controlled trial. Psychopharmacology 2000, 150, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Schlebusch, L.; Bosch, B.; Polglase, G.; Kleinschmidt, I.; Pillay, B.; Cassimjee, M. A double-blind, placebo-controlled, double-centre study of the effects of an oral multivitamin-mineral combination on stress. S. Afr. Med. J. 2000, 90, 1216–1223. [Google Scholar] [PubMed]
- Leung, B.M.Y.; Kaplan, B.J.; Field, C.J.; Tough, S.; Eliasziw, M.; Gomez, M.F.; McCargar, L.J.; Gagnon, L.; Dewey, D.; Bell, R.C.; et al. Prenatal micronutrient supplementation and postpartum depressive symptoms in a pregnancy cohort. BMC Pregnancy Childbirth 2013, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Popper, C.W. Do vitamins or minerals (apart from lithium) have mood-stabilizing effects? J. Clin. Psychiatry 2001, 62, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Lowe, B.; Decker, O.; Muller, S.; Brahler, E.; Schellberg, D.; Herzog, W.; Herzberg, P.Y. Validation and Standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the General Population. Med. Care 2008, 46, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.A.; Zamorano, E.; García-Campayo, J.; Pardo, A.; Freire, O.; Rejas, J. Validity of the GAD-7 scale as an outcome measure of disability in patients with generalized anxiety disorders in primary care. J. Affect. Disord. 2011, 128, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Cameron, I.M.; Crawford, J.R.; Lawton, K.; Reid, I.C. Psychometric comparison of PHQ-9 and HADS for measuring depression severity in primary care. Br. J. Gen. Pract. 2008, 58, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Titov, N.; Dear, B.F.; McMillan, D.; Anderson, T.; Zou, J.; Sunderland, M. Psychometric Comparison of the PHQ-9 and BDI-II for Measuring Response during Treatment of Depression. Cogn. Behav. Ther. 2011, 40, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Manea, L.; Gilbody, S.; McMillan, D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): A meta-analysis. Can. Med. Assoc. J. 2012, 184, E191–E196. [Google Scholar] [CrossRef] [PubMed]
- Fervaha, G.; Takeuchi, H.; Agid, O.; Lee, J.; Foussias, G.; Remington, G. Determinants of patient-rated and clinician-rated illness severity in schizophrenia. J. Clin. Psychiatry 2015, 76, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Spearing, M.K.; Post, R.M.; Leverich, G.S.; Brandt, D.; Nolen, W. Modification of the Clinical Global Impressions (CGI) scale for use in bipolar illness (BP): The CGI-BP. Psychiatry Res. 1997, 73, 159–171. [Google Scholar] [CrossRef]
- Gloster, A.T.; Rhoades, H.M.; Novy, D.; Klotsche, J.; Senior, A.; Kunik, M.; Wilson, N.; Stanley, M.A. Psychometric properties of the Depression Anxiety and Stress Scale-21 in older primary care patients. J. Affect. Disord. 2008, 110, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Connor, K.M.; Davidson, J.R.; Churchill, L.E.; Sherwood, A.; Weisler, R.H.; FOA, E. Psychometric properties of the social phobia inventory (SPIN). Br. J. Psychiatry 2000, 176, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Antony, M.M.; Coons, M.J.; McCabe, R.E.; Ashbaugh, A.; Swinson, R.P. Psychometric properties of the social phobia inventory: Further evaluation. Behav. Res. Ther. 2006, 44, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Gori, A.; Giannini, M.; Socci, S.; Luca, M.; Dewey, D.E.; Schuldberg, D.; Craparo, G. Assessing social anxiety disorder: Psychometric properties of the Italian Social Phobia Inventory (I-SPIN). Clin. Neuropsychiatry 2013, 10, 37–42. [Google Scholar]
- Osório, F.d.L.; Crippa, J.A.S.; Loureiro, S.R. Cross-cultural validation of the Brazilian Portuguese version of the Social Phobia Inventory (SPIN): Study of the items and internal consistency. Rev. Bras. Psiquiatr. 2009, 31, 25–29. [Google Scholar] [CrossRef]
- Ranta, K.; Kaltiala-Heino, R.; Koivisto, A.-M.; Tuomisto, M.T.; Pelkonen, M.; Marttunen, M. Age and gender differences in social anxiety symptoms during adolescence: The Social Phobia Inventory (SPIN) as a measure. Psychiatry Res. 2007, 153, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Rosario-Campos, M.C.; Miguel, E.C.; Quatrano, S.; Chacon, P.; Ferrao, Y.; Findley, D.; Katsovich, L.; Scahill, L.; King, R.A.; Woody, S.R.; et al. The Dimensional Yale-Brown Obsessive-Compulsive Scale (DY-BOCS): An instrument for assessing obsessive-compulsive symptom dimensions. Mol. Psychiatry 2006, 11, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Elkins, R.M.; Pincus, D.B.; Comer, J.S. A psychometric evaluation of the panic disorder severity scale for children and adolescents. Psychol. Assess. 2014, 26, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Wuyek, L.A.; Antony, M.M.; McCabe, R.E. Psychometric properties of the panic disorder severity scale: Clinician-administered and self-report versions. Clin. Psychol. Psychother. 2011, 18, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Sundin, E.C.; Horowitz, M.J. Impact of Event Scale: Psychometric properties. Br. J. Psychiatry 2002, 180, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Creamer, M.; Bell, R.; Failla, S. Psychometric properties of the Impact of Event Scale—Revised. Behav. Res. Ther. 2003, 41, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Salkovskis, P.M.; Rimes, K.A.; Warwick, H.M.C.; Clark, D.M. The Health Anxiety Inventory: Development and validation of scales for the measurement of health anxiety and hypochondriasis. Psychol. Med. 2002, 32, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Hedman, E.; Ljótsson, B.; Andersson, E.; Andersson, G.; Lindefors, N.; Rück, C.; Axelsson, E.; Lekander, M. Psychometric properties of Internet-administered measures of health anxiety: An investigation of the Health Anxiety Inventory, the Illness Attitude Scales, and the Whiteley Index. J. Anxiety Disord. 2015, 31, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Raistrick, D.; Bradshaw, J.; Tober, G.; Weiner, J.; Allison, J.; Healey, C. Development of the Leeds Dependence Questionnaire (LDQ): A questionnaire to measure alcohol and opiate dependence in the context of a treatment evaluation package. Addiction 1994, 89, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Heather, N.; Raistrick, D.; Tober, G.; Godfrey, C.; Parrott, S. Leeds Dependence Questionnaire: New Data from a Large Sample of Clinic Attenders. Addict. Res. Theory 2001, 9, 253–269. [Google Scholar] [CrossRef]
- Kelly, J.F.; Magill, M.; Slaymaker, V.; Kahler, C. Psychometric validation of the Leeds Dependence Questionnaire (LDQ) in a young adult clinical sample. Addict. Behav. 2010, 35, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, C.S.; Anderson, K.L. The Quality of Life Scale (QOLS): Reliability, validity, and utilization. Health Qual. Life Outcomes 2003, 1, 60. [Google Scholar] [CrossRef] [PubMed]
- Uher, R.; Pavlova, B. Long-term effects of depression treatment. Lancet Psychiatry 2016, 3, 95–96. [Google Scholar] [CrossRef]
- Bailey, R.L.; Miller, P.E.; Mitchell, D.C.; Hartman, T.J.; Lawrence, F.R.; Sempos, C.T.; Smiciklas-Wright, H. Dietary screening tool identifies nutritional risk in older adults. Am. J. Clin. Nutr. 2009, 90, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Rammstedt, B.; John, O.P. Measuring personality in one minute or less: A 10-item short version of the Big Five Inventory in English and German. J. Res. Personal. 2007, 41, 203–212. [Google Scholar] [CrossRef]
- Rucklidge, J.J.; Blampied, N.; Gorman, B.; Gordon, H.A.; Sole, E. Psychological functioning 1 year after a brief intervention using micronutrients to treat stress and anxiety related to the 2011 Christchurch earthquakes: A naturalistic follow-up. Hum. Psychopharmacol. 2014, 29, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Rucklidge, J.J.; Johnstone, J.; Gorman, B.; Boggis, A.; Frampton, C.M. Moderators of treatment response in adults with ADHD treated with a vitamin-mineral supplement. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 50, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Mech, A.W.; Farah, A. Correlation of clinical response with homocysteine reduction during therapy with reduced B vitamins in patients with MDD who are positive for MTHFR C677T or A1298C polymorphism: A randomized, double-blind, placebo-controlled study. J. Clin. Psychiatry 2016, 77, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Dumville, J.C.; Torgerson, D.J.; Hewitt, C.E. Reporting attrition in randomised controlled trials. BMJ Br. Med. J. 2006, 332, 969–971. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, C.E.; Kumaravel, B.; Dumville, J.C.; Torgerson, D.J. Assessing the impact of attrition in randomized controlled trials. J. Clin. Epidemiol. 2010, 63, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.; Lemenager, T.; Hoffmann, S.; Reinhard, I.; Hermann, D.; Batra, A.; Berner, M.; Wodarz, N.; Heinz, A.; Smolka, M.N.; et al. Results of a double-blind, placebo-controlled pharmacotherapy trial in alcoholism conducted in Germany and comparison with the US COMBINE study. Addict. Biol. 2013, 18, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Blampied, N.M. Analyzing therapeutic change using modified Brinley plots: History, construction, and interpretation. Behav. Ther. 2017, 48, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, N.S.; Truax, P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J. Consult. Clin. Psychol. 1991, 59, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, P.; Gentili, C.; Banos, R.M.; Garcia-Campayo, J.; Botella, C.; Cristea, I.A. Relative effects of cognitive and behavioral therapies on generalized anxiety disorder, social anxiety disorder and panic disorder: A meta-analysis. J. Anxiety Disord. 2016, 43, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Warner, H.A.; Brown, W.A. Symptom reduction and suicide risk in patients treated with placebo in antidepressant clinical trials: An analysis of the Food and Drug Administration database. Arch. Gen. Psychiatry 2000, 57, 311–317. [Google Scholar] [CrossRef] [PubMed]
| Daily Essential Nutrients (DEN) Advanced Supplement Facts | |||
| Amount Per Full Dose (12 capsules) | |||
| Vitamin A (as retinyl palmitate) | 5760 IU | Calcium (as NutraTek™ chelation complex) | 1320 mg |
| Vitamin C (as ascorbic acid) | 600 mg | Iron (as NutraTek™ chelation complex) | 13.8 mg |
| Vitamin D (as cholecalciferol) | 3000 IU | Phosphorus (as NutraTek™ chelation complex) | 840 mg |
| Vitamin E (as d-alpha tocopheryl succinate) | 360 IU | Iodine (as NutraTek™ chelation complex) | 204 mcg |
| Thiamin (as thiamin mononitrate) | 60 mg | Magnesium (as NutraTek™ chelation complex) | 600 mg |
| Riboflavin | 18 mg | Zinc (as NutraTek™ chelation complex) | 48 mg |
| Niacin (as niacinamide) | 90 mg | Selenium (as NutraTek™ chelation complex) | 204 mcg |
| Vitamin B6 (as pyridoxine hydrochloride) | 69.9 mg | Copper (as NutraTek™ chelation complex) | 7.2 mg |
| Folate (as calcium l-5 methyletrahydrofolate) | 828 mcg | Manganese (as NutraTek™ chelation complex) | 9.6 mg |
| Vitamin B12 (as methylcobalamin & adenosylcobalamin) | 900 mcg | Chromium (as NutraTek™ chelation complex) | 624 mcg |
| Biotin | 1080 mcg | Molybdenum (as NutraTek™ chelation complex) | 144 mcg |
| Pantothenic acid (as d-calcium pantothenate) | 30 mg | Potassium (as NutraTek™ chelation complex) | 240 mg |
| Propriety blend: Choline bitartrate, alpha-lipoic acid, mineral wax, inositol, acetyl-l carnitine, grape seed extract, gingko biloba leaf extract, N-acetyl-l-cysteine, l-methionine, trace minerals as NutraTek™ chelation complex, lithium orotate, boron, vanadium, nickel.Other ingredients: Vegetarian capsule (hypromellose), microcrystalline cellulose, magnesium stearate, silicon dioxide, titanium dioxide. | |||
| Placebo Facts | |||
| Amount Per Full Dose (12 capsules) | |||
| Fiber Acacia Gum | 3600 mg | Cocoa Powder | 48 mg |
| Maltodextrin | 4750.8 mg | Riboflavin Powder | 1.2 mg |
| Variable | Instrument | Time Point |
|---|---|---|
| Self-report | ||
| Primary outcome measures (anxiety and depressive symptoms) | Generalised Anxiety Disorder-7 Question Scale (GAD-7) Patient Health Questionnaire-9 (PHQ-9) | Baseline, weekly during treatment, end of treatment, weekly during open label, end of open label, one-year follow up. |
| Anxiety symptoms | Social Phobia Inventory (SPIN) Yale-Brown Obsessive Compulsive Scale (Y-BOCS) Panic Disorder Severity Scale (PDSS) Impact of Events Scale-Revised (IES-R) Health Anxiety Inventory (HAI) | Baseline, end of treatment, end of open label, one year follow up. |
| Psychiatric status | Web Screening Questionnaire (WSQ) Depression, Anxiety and Stress Scale (DASS-21) | Baseline, end of treatment, end of open label, one-year follow up. |
| Personality | Big Five Inventory (BFI-10) | Baseline |
| Quality of life | Quality of Life Scale (QOLS) | Baseline, end of treatment, end of open label, one-year follow up. |
| Diet quality, alcohol and drug intake | Dietary Screening Tool (DST), Leeds Dependency Questionnaire (LDS) | Baseline, end of treatment, end of open label, one-year follow up. |
| Treatment side effects | Modified Antidepressant Side-Effects Checklist (ASEC) | Baseline, weekly during treatment, end of treatment, weekly during open label, end of open label. |
| Treatment effectiveness | Modified Clinical Global Impression-Improvements (MCGI-I) | End of treatment, end of open label, one year follow up. |
| Observer report | ||
| Treatment effectiveness | Clinical Global Impression-Improvement (CGI-I) * | End of treatment, end of open label, one year follow up. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blampied, M.; Bell, C.; Gilbert, C.; Boden, J.; Nicholls, R.; Rucklidge, J.J. Study Protocol for a Randomized Double Blind, Placebo Controlled Trial Exploring the Effectiveness of a Micronutrient Formula in Improving Symptoms of Anxiety and Depression. Medicines 2018, 5, 56. https://doi.org/10.3390/medicines5020056
Blampied M, Bell C, Gilbert C, Boden J, Nicholls R, Rucklidge JJ. Study Protocol for a Randomized Double Blind, Placebo Controlled Trial Exploring the Effectiveness of a Micronutrient Formula in Improving Symptoms of Anxiety and Depression. Medicines. 2018; 5(2):56. https://doi.org/10.3390/medicines5020056
Chicago/Turabian StyleBlampied, Meredith, Caroline Bell, Claire Gilbert, Joseph Boden, Rebecca Nicholls, and Julia J. Rucklidge. 2018. "Study Protocol for a Randomized Double Blind, Placebo Controlled Trial Exploring the Effectiveness of a Micronutrient Formula in Improving Symptoms of Anxiety and Depression" Medicines 5, no. 2: 56. https://doi.org/10.3390/medicines5020056
APA StyleBlampied, M., Bell, C., Gilbert, C., Boden, J., Nicholls, R., & Rucklidge, J. J. (2018). Study Protocol for a Randomized Double Blind, Placebo Controlled Trial Exploring the Effectiveness of a Micronutrient Formula in Improving Symptoms of Anxiety and Depression. Medicines, 5(2), 56. https://doi.org/10.3390/medicines5020056





