Synthesis, Antimicrobial, and Antioxidant Activities of Chalcogen-Containing Nitrone Derivatives from (R)-citronellal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Oil Material
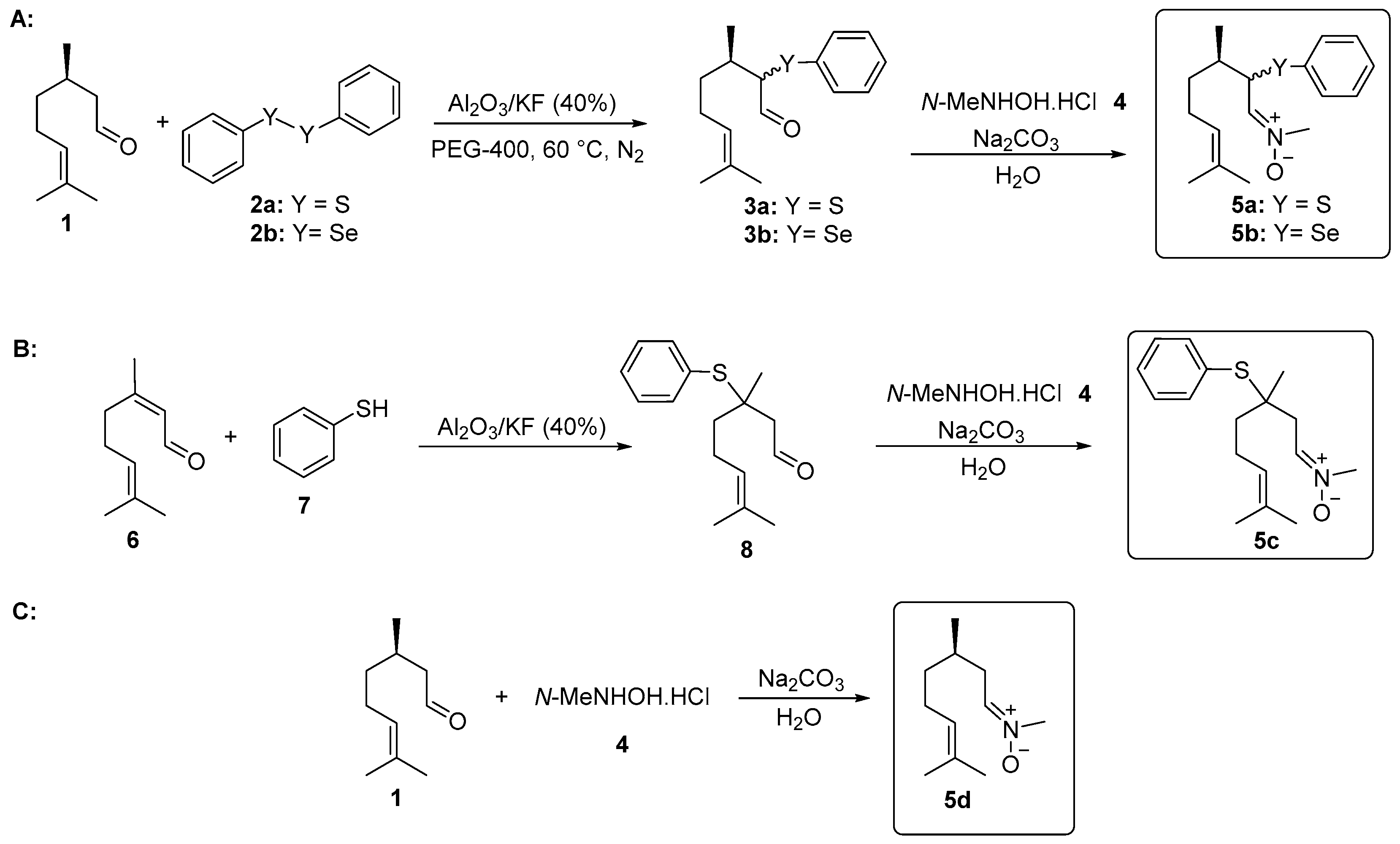
2.2. Synthesis of α-phenylselanyl citronellal 3a, α-phenylthio citronellal 3b, and β-phenylthio citronellal 8
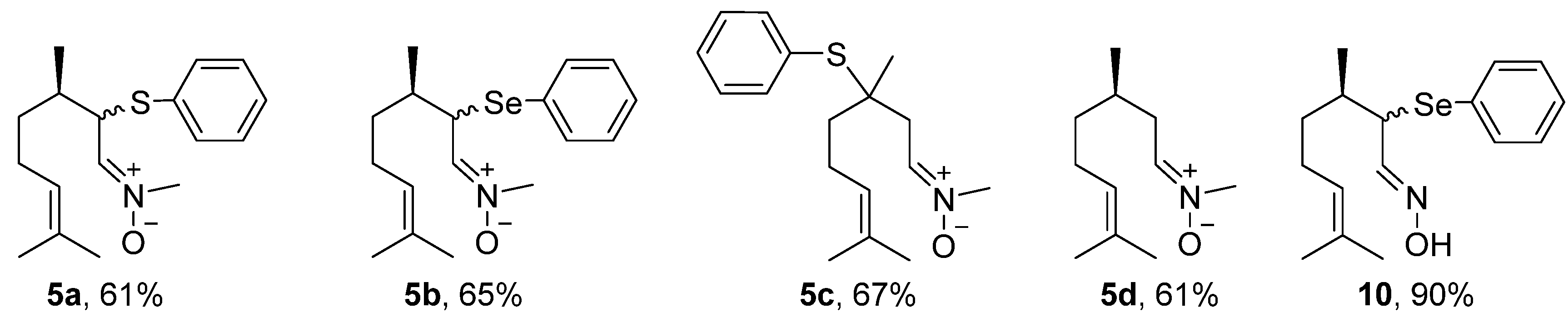
2.3. General Procedure for the Synthesis of Nitrones 5a–d Derived from Citronellal
2.4. Synthesis of the Selenium-Containing Oxime 10
2.5. Bacterial Strains
2.6. Antioxidant Activity Assays
2.7. In Vitro Toxicity
3. Results and Discussion
3.1. Chemistry
3.2. Antimicrobial Activity
3.3. Antioxidant Activity
3.4. In Vitro Toxicity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Damodaran, S.; Parkin, K.; Fennema, O.R. Fennema’s Food Chemistry, 4th ed.; Taylor & Francis: Boca Raton, FL, USA, 2008. [Google Scholar]
- Hoeden, B.P.; Davies, J.K.; Johnson, P.D.R.; Stinear, T.P.; Grayson, M.L. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin intermediate and heterogeneous vancomycin-intermediate strains: Resistance mechanisms, laboratory detection, and clinical implications. Clin. Microbiol. 2010, 23, 99–139. [Google Scholar] [CrossRef] [PubMed]
- Pybus, D.; Sell, C. The Chemistry of Fragrances: From Perfumer to Consumer; The Royal Society of Chemistry: Cambridge, UK, 1999. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.M.; Barcellos, A.; Casaril, A.M.; Perin, G.; Schiesser, C.H.; Callaghan, K.L.; Lenardão, E.J.; Savegnago, L. Twice acting antioxidants: Synthesis and antioxidant properties of selenium and sulfur-containing zingerone derivatives. Tetrahedron Lett. 2015, 56, 2243–2246. [Google Scholar] [CrossRef]
- Neukirch, H.; D’Ambrosio, M.; Sosa, S.; Altinier, G.; Loggia, R.D.; Guerriero, A. Improved anti-inflammatory activity of three new terpenoids derived, by systematic chemical modifications, from the abundant triterpenes of the flower plant Calendula officinalis. Chem. Biodivers. 2005, 2, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Junkai, L.; Shengtao, X.; Zheying, Z.; Jinyi, X. The structural modification of natural products for novel drug discovery. Exp. Opin. Drug Disc. 2017, 12, 121–140. [Google Scholar] [CrossRef]
- Lenardão, E.J.; Silva, W.P.; Jacob, R.G.; Volcan, D.S.M.; Goldbeck, J.C.S.; Fonseca, S.F. Semi-Synthetic Compounds as Antimicrobial Agents in Food Preservation. In The Battle against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2015; Volume 1, pp. 576–583. [Google Scholar]
- Victória, F.N.; Radatz, C.S.; Sachini, M.; Jacob, R.G.; Alves, D.; Savegnago, L.; Perin, G.; Motta, A.S.; Silva, W.P.; Lenardão, E.J. Further analysis of the antimicrobial activity of α-phenylseleno citronellal and α-phenylseleno citronellol. Food Control 2012, 23, 95–99. [Google Scholar] [CrossRef]
- Savegnago, L.; Borges, V.C.; Alves, D.; Jesse, C.R.; Rocha, J.B.T.; Nogueira, C.W. Evaluation of antioxidant activity and potential toxicity of 1-buthyltelurenyl-2-methylthioheptene. Life Sci. 2006, 79, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Victória, F.N.; Martinez, D.M.; Castro, M.; Casaril, A.M.; Alves, D.; Lenardão, E.J.; Salles, H.D.; Schneider, P.H.; Savegnago, L. Antioxidant properties of (R)-Se-aryl thiazolidine-4-carboselenoate. Chem. Biol. Interact. 2013, 205, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, H. Asymmetric 1,3-dipolar cycloadditions. Tetrahedron 2007, 63, 3235–3285. [Google Scholar] [CrossRef]
- Hamer, J.; Macaluso, A. Nitrones. Chem. Rev. 1964, 64, 473–795. [Google Scholar] [CrossRef]
- McQueen, D.M. U.S. Patent 2,426,894. Available online: https://www.google.com/patents/US2426894#forward-citations (accessed on 23 May 2017).
- Petkesa, H.I.; Gál, E.; Gãinã, L.; Sabou, M.; Majdik, C.; Silaghi-Dumitrescu, L. Synthesis and antibacterial properties of new phenothiazinyl- and phenyl-nitrones. C. R. Chim. 2014, 17, 1050–1056. [Google Scholar] [CrossRef]
- Shijum, C.; Kang, Z.; Gang, C. Synthesis and application of phenyl nitrone derivatives as acidic and microbial corrosion inhibitors. J. Chem. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Franco, S.; Merchan, F.L.; Merino, P.; Tejero, T. An Improved Synthesis of Ketonitrones. Synth. Commun. 1995, 25, 2275–2284. [Google Scholar] [CrossRef]
- Flick, A.C.; Padwa, A. A conjugate addition-dipolar cycloaddition approach towards the synthesis of various alkaloids. ARKIVOC 2011, vi, 137–161. [Google Scholar]
- Nazari, M.; Movassagh, B. α-Phenylselenenylation of aldehydes and ketones with diphenyl diselenide mediated by KF/Al2O3. Tetrahedron Lett. 2009, 50, 1453–1455. [Google Scholar] [CrossRef]
- Lenardão, E.J.; Trecha, D.O.; Ferreira, P.C.; Jacob, R.G.; Perin, G. Green Michael addition of thiols to electron deficient alkenes using KF/alumina and recyclable solvent or solvent-free conditions. J. Braz. Chem. Soc. 2009, 20, 93–99. [Google Scholar] [CrossRef]
- Isager, P.; Thomsen, I.; Torssell, K.B.G. Reactions with α,β-unsaturated nitrile oxides. Synthetic studies in the terpene field. Synthesis of tagetones, ocimenones, deodarone and atlantone. Acta Chem. Scand. 1990, 44, 806–813. [Google Scholar] [CrossRef]
- Clinical Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement (M100-S25); CLSI: Wayne, PA, USA, 2015. [Google Scholar]
- Rota, C.; Carramiñana, J.J.; Burillo, J.; Herrera, A. In vitro antimicrobial activity of essential oils from aromatic plants against selected foodborne pathogens. J. Food Prot. 2004, 67, 1252–1256. [Google Scholar] [CrossRef]
- Sassa, S. Delta-aminolevulinic acid dehydratase assay. Enzyme 1982, 28, 133–145. [Google Scholar] [PubMed]
- Costa, J.R.M.A.; Mela, M.; Assis, H.C.S.; Pelletier, E.; Randi, M.A.F.; Ribeiro, C.A.O. Enzymatic inhibition and morphological changes in Hopliasmalabaricus from dietary exposure to lead(II) or methylmercury. Ecotoxicol. Environ. Saf. 2007, 67, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Smith-Palmer, A.; Stewart, J.; Fyfe, L. Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett. Appl. Microbiol. 1998, 26, 118–124. [Google Scholar] [CrossRef]
- Goldbeck, J.C.; Victória, F.N.; Motta, A.; Savegnago, L.; Jacob, R.G.; Perin, G.; Lenardão, E.J.; da Silva, W.P. Bioactivity and morphological changes of bacterial cells after exposure to 3-(p-chlorophenyl)thio citronellal. LWT Food Sci. Technol. 2014, 59, 813–819. [Google Scholar] [CrossRef]
- Wiedander, E.; Engman, L.; Suensjö, E.; Erlansson, M.; Johansson, U.; Linden, M.; Andersson, C.M.; Brattsand, R. Antioxidative properties of organotellurium compounds in cell systems. Biochem. Pharmacol. 1998, 55, 573–584. [Google Scholar] [CrossRef]
- Gunlçin, I. Antioxidant activity of L-adrenaline: A structure–activity insight. Chem. Biol. Interact. 2009, 179, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Soong, Y.; Barlow, P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4303. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; Bukman, L.; Vargas, A.M.M.; Barizão, E.O.; Moraes, J.C.G.; Visentainer, J.V.; Almeida, V.C. The antioxidant activity of teas measured by the FRAP method adapted to the FIA system: Optimizing the conditions using the response surface methodology. Food Chem. 2013, 138, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Berker, K.I.; Güçlü, K.; Tor, I.; Apak, R. Comparative evaluation of Fe(III) reducing power- based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP), and ferricyanide reagents. Talanta 2007, 72, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
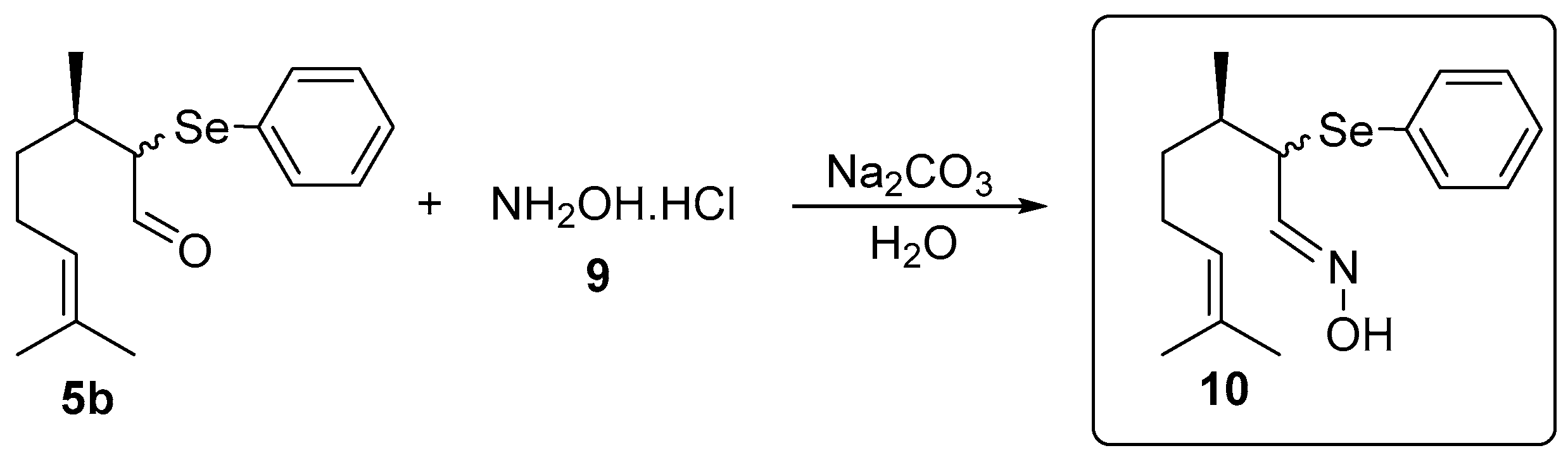
| Bacteria ** | Inhibition Zone * (mm) Compounds | ||||||
|---|---|---|---|---|---|---|---|
| Streptomycin (10 µg) | 5a | 5b | 5c | 5d | 10 | 9e | |
| L. monocytogenes | 21 | 18 | 19.5 | 26 | 31 | 21 | 21 |
| S. aureus | 21 | 15 | 19.5 | 24 | 24.5 | 22 | 22 |
| B. cereus | 26 | 21.5 | 18.5 | 24.5 | 29 | 19 | 19 |
| S. Typhimurium | 18 | 8.5 | 8.5 | 21.5 | 23 | 10.5 | 10.5 |
| P. aeruginosa | 13 | 8.5 | 8.5 | 24 | 23 | 9 | 9 |
| E. coli O157:H7 | 24 | 11 | 9 | 21.5 | 22.5 | 9 | 9 |
| Compound | Bacteria ** | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L. monocytogenes | S. mureus | B. mereus | S. Typhimurium | P. aeruginosa | E. coli O157:H7 | |||||||
| MIC a,* | MBC a,* | MIC a,* | MBC a,* | MIC a,* | MBC a,* | MIC a,* | MBC a,* | MIC a,* | MBC a,* | MIC a,* | MBC a,* | |
| 5a | >0.65 | >0.65 | >0.65 | >0.65 | 0.48 | 0.59 | >0.65 | >0.65 | >0.65 | >0.65 | >0.65 | >0.65 |
| 5b | >0.64 | >0.64 | 0.64 | >0.64 | 0.64 | >0.64 | >0.64 | >0.64 | >0.64 | >0.64 | >0.64 | >0.64 |
| 5c | 0.69 | >0.69 | 0.61 | 0.69 | 0.69 | 0.69 | >0.69 | >0.69 | 0.61 | 0.61 | 0.52 | 0.52 |
| 5d | 1.26 | 1.26 | 1.26 | 1.26 | >1.26 | >1.26 | 1.13 | 1.26 | 1.13 | 1.26 | 1.13 | 1.26 |
| 10 | >0.70 | >0.70 | >0.70 | >0.70 | >0.70 | >0.70 | >0.70 | >0.70 | >0.70 | >0.70 | >0.70 | >0.70 |
| Concentration (μM) | Compounds | |
|---|---|---|
| 5a | 5c | |
| 100 | 2.43 ± 1.08 | 2.43 ± 0.61 |
| 500 | 20.31 ± 1.26 *** | 29.34 ± 1.00 *** |
| Concentration (μM) | Compounds | ||||
|---|---|---|---|---|---|
| 5a | 5b | 5c | 5d | 10 | |
| 5 | nt | nt | nt | nt | 2.39 ± 0.57 |
| 10 | - | - | - | - | 27.81 ± 2.12 *** |
| 50 | 11.74 ± 0.47 *** | 9.69 ± 0.57 ** | 4.18 ± 1.23 | 0.30 ± 0.33 | 87.59 ± 4.81 *** |
| 100 | 22.84 ± 1.02 *** | 22.32 ± 1.65 *** | 11.60 ± 1.50 *** | 3.10 ± 0.48 *** | 94.03 ± 1.05 *** |
| 500 | 77.61 ± 0.81 *** | 73.92 ± 4.96 ** | 59.51 ± 4.16 *** | 17.05 ± 0.47 *** | x |
| IC50 | 297.00 ± 1.00 | 315.30 ± 30.02 | 419.30 ± 35.80 | - | 25.00 ± 2.00 |
| Concentration (μM) | Compounds | ||||
|---|---|---|---|---|---|
| 5a | 5b | 5c | 5d | 10 | |
| Control | 0.118 ± 0.009 | 0.094 ± 0.026 | 0.071 ± 0.020 | 0.117 ± 0.009 | 0.071 ± 0.020 |
| 1 | - | nt | nt | 0.166 ± 0.008 | nt |
| 5 | 0.165 ± 0.007 | - | - | 0.280 ± 0.022 ** | - |
| 10 | 0.207 ± 0.019 * | 0.133 ± 0.097 | 0.153 ± 0.047 | 0.412 ± 0.055 *** | 0.084 ± 0.010 |
| 50 | 0.572 ± 0.023 *** | 0.256 ± 0.181 | 0.543 ± 0.047 *** | 1.198 ± 0.024 *** | 0.180 ± 0.008 *** |
| 100 | 1.039 ± 0.067 *** | 0.416 ± 0.234 | 1.121 ± 0.160 *** | x | 0.257 ± 0.007 *** |
| 500 | x | 1.602 ± 0.408 *** | x | x | 1.291 ± 0.045 *** |
| Concentration (μM) | Compounds | ||||
|---|---|---|---|---|---|
| 5a | 5b | 5c | 5d | 10 | |
| Liver | |||||
| control | 4,79 ± 0.87 | 4.79 ± 0.87 | 4.79 ± 0.87 | 4.30 ± 0.74 | 4.30 ± 0.74 |
| 10 | 4.99 ± 0.84 | 2.59 ± 0.29 *** | 4.63 ± 0.82 | 4.50 ± 0.60 | 4.90 ± 0.58 |
| 50 | 4.87 ± 0.67 | 0.42 ± 0.13 *** | 4.31 ± 0.81 | 4.22 ± 0.78 | 4.71 ± 0.53 |
| 100 | 4.71 ± 0.89 | 0.07 ± 0.02 *** | 4.12 ± 0.90 | 3.54 ± 0.84 | 4.57 ± 0.62 |
| 500 | 4.46 ± 0.80 | 0.03 ± 0.01 *** | 3.88 ± 0.87 | 2.84 ± 0.57 | 3.97 ± 0.82 |
| Kidney | |||||
| control | 1.01 ± 0.12 | 1.01 ± 0.12 | 1.01 ± 0.12 | 0.98 ± 0.11 | 0.98 ± 0.11 |
| 10 | 1.17 ± 0.05 | 1.08 ± 0.05 | 1.10 ± 0.12 | 1.02 ± 0.11 | 0.96 ± 0.12 |
| 50 | 1.17 ± 0.07 | 0.99 ± 0.07 | 1.02 ± 0.12 | 0.96 ± 0.12 | 0.95 ± 0.10 |
| 100 | 1.09 ± 0.08 | 0.68 ± 0.12 ** | 0.99 ± 0.10 | 0.89 ± 0.12 | 0.84 ± 0.10 |
| 500 | 0.90 ± 0.08 | 0.24 ± 0.03 *** | 0.90 ± 0.07 | 0.72 ± 0.07 | 0.61 ± 0.12 * |
| Brain | |||||
| control | 0.30 ± 0.05 | 0.26 ± 0.05 | 0.30 ± 0.05 | 0.30 ± 0.05 | 0.30 ± 0.05 |
| 10 | 0.44 ± 0.09 | 0.30 ± 0.11 | 0.30 ± 0.10 | 0.29 ± 0.09 | 0.30 ± 0.10 |
| 50 | 0.42 ± 0.10 | 0.25 ± 0.05 | 0.28 ± 0.09 | 0.27 ± 0.10 | 0.28 ± 0.09 |
| 100 | 0.40 ± 0.11 | 0.18 ± 0.03 | 0.26 ± 0.08 | 0.26 ± 0.09 | 0.26 ± 0.08 |
| 500 | 0.36 ± 0.10 | 0.14 ± 0.04 | 0.26 ± 0.07 | 0.24 ± 0.08 | 0.26 ± 0.07 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz, M.C.; Mano, R.A.; Oliveira, D.H.; Maia, D.S.V.; Silva, W.P.; Savegnago, L.; Lenardão, E.J.; Jacob, R.G. Synthesis, Antimicrobial, and Antioxidant Activities of Chalcogen-Containing Nitrone Derivatives from (R)-citronellal. Medicines 2017, 4, 39. https://doi.org/10.3390/medicines4020039
Ferraz MC, Mano RA, Oliveira DH, Maia DSV, Silva WP, Savegnago L, Lenardão EJ, Jacob RG. Synthesis, Antimicrobial, and Antioxidant Activities of Chalcogen-Containing Nitrone Derivatives from (R)-citronellal. Medicines. 2017; 4(2):39. https://doi.org/10.3390/medicines4020039
Chicago/Turabian StyleFerraz, Mariana C., Renata A. Mano, Daniela H. Oliveira, Darla S. V. Maia, Wladimir P. Silva, Lucielli Savegnago, Eder J. Lenardão, and Raquel G. Jacob. 2017. "Synthesis, Antimicrobial, and Antioxidant Activities of Chalcogen-Containing Nitrone Derivatives from (R)-citronellal" Medicines 4, no. 2: 39. https://doi.org/10.3390/medicines4020039






