Isolation and Cytotoxic Investigation of Flacourtin from Oncoba spinosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection and Purification
2.2. Cytotoxicity Assay
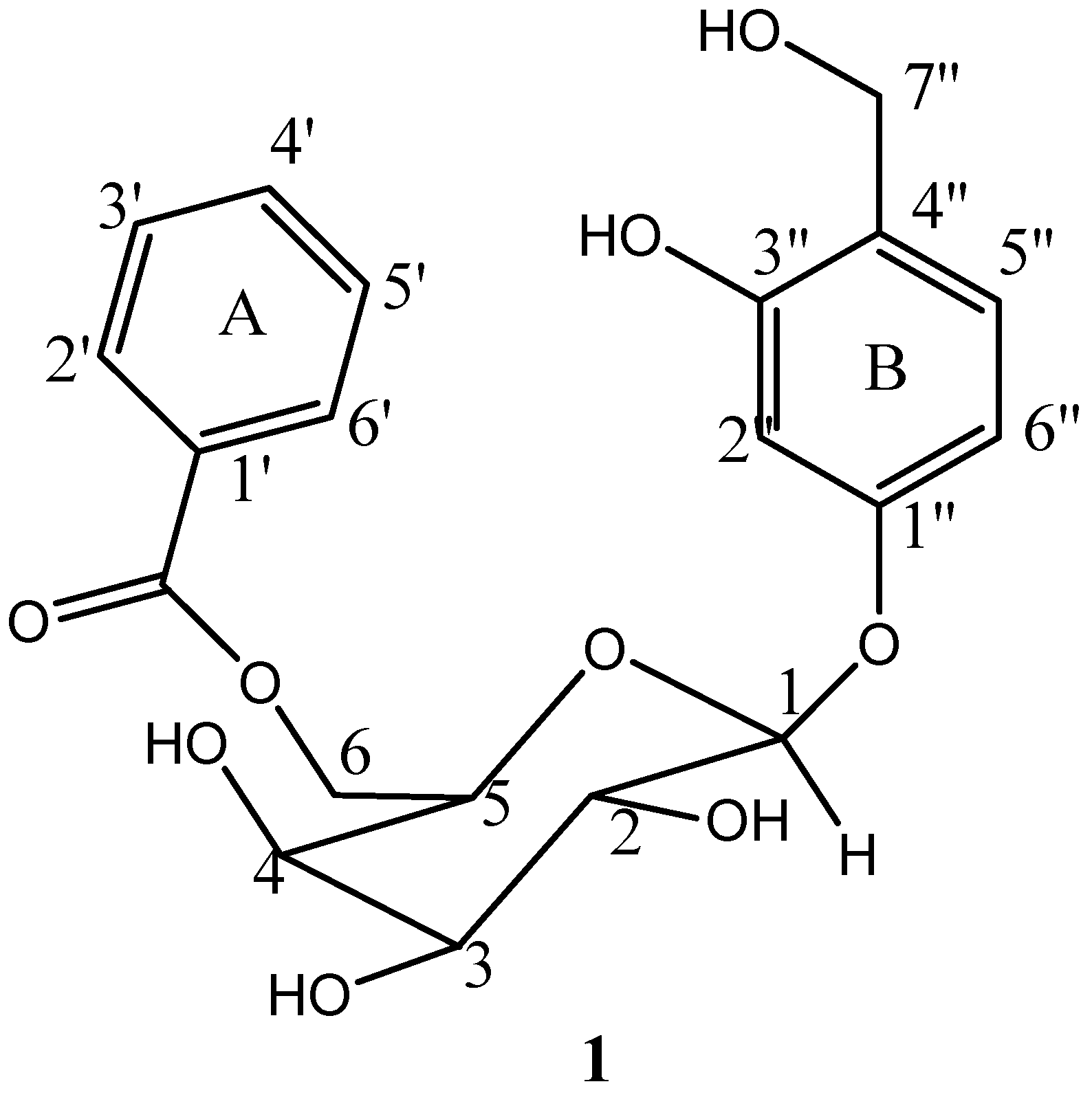
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Gbadamosi, I.T.; Oloyede, A.A. Mineral, proximate and phytochemical components of ten Nigeria medicinal plants used in the management of arthritis. Afr. J. Pharm. Pharmacol. 2014, 8, 638–643. [Google Scholar] [CrossRef]
- Ramzi, A.; Mothana, A.; Kriegisch, S.; Harms, M.; Kristian, W.; Landequist, U. Assessment of Yemeni medinal plant for their in vitro antimicrobial, anticancer, and antioxidant activities. J. Pharm. Biol. 2011, 49, 200–210. [Google Scholar]
- Djoussi, M.G.; Jean, D.M.; David, N.; Jules, R.K.; Leon, A.T.; Dominique, H.; Laurence, V.N. Antimicrobial and antioxidant flavonoids from the leaves of Oncoba spinosa. BMC Complement. Altern. Med. 2005, 15, 134. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, P.K.; Guha, K.P.; Biswas, G.K.; Mukherjee, B. (−)Flacourtin, a phenolic glucoside ester from Flacourtia indica. Phytochemistry 2005, 26, 3090–3091. [Google Scholar] [CrossRef]
- Nazneen, M.; Mazid, M.A.; Kundu, J.K.; Bachar, S.C.; Begum, F.; Datta, B.K. Protective effects of Flacourtia indica aerial parts extracts against paracetamol-induced hepatotoxiciy in rats. J. Taibah Univ. Sci. 2009, 2, 1–6. [Google Scholar] [CrossRef]
- Saxena, A.; Patel, B.D. In vitro antioxidant activity of methanolic and aqueous extract of Flacourtia indica Merr. Am.-Eurasian J. Sci. Res. 2010, 5, 201–206. [Google Scholar]
- Singh, T.S.; Singh, M.; Yadav, D.; Singh, I.; Mansoori, M.H. Antiasthmatic potential of Flacourtia indica Merr. Afr. J. Basic Appl. Sci. 2011, 3, 201–204. [Google Scholar]
- Eramma, N.; Gayathri, D. Antibacterial potential and phytochemical analysis of Flacourtia indica (Burm.f.) Merr. root extract against human pathogens. Indo Am. J. Pharm. Res. 2013, 3, 3832–3846. [Google Scholar]
- Patro, S.K.; Behera, P.C.; Kumar, P.M.; Sasmal, D.; Padhy, R.K.; Dash, S.K. Pharmacological Review of Flacourtia sepiaria (Ruxb.). Sch. Acad. J. Pharm. 2013, 2, 89–93. [Google Scholar]
- Amarasinghe, N.R.; Jayasinghe, L.; Hara, N.; Fujimoto, Y. Flacourside, a new 4-oxo-2-cyclopentenylmethyl glucoside from the fruit juice of Flacourtia indica. Food Chem. 2007, 102, 95–97. [Google Scholar] [CrossRef]
| Sample | Crude | n-Hexane Fraction | Defatted Fraction | Flacourtin |
|---|---|---|---|---|
| LD50 (µg/mL) | 1203 | >10,000 | >10,000 | >10,000 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balogun, O.S.; Ajayi, O.S.; Lawal, O.S. Isolation and Cytotoxic Investigation of Flacourtin from Oncoba spinosa. Medicines 2016, 3, 31. https://doi.org/10.3390/medicines3040031
Balogun OS, Ajayi OS, Lawal OS. Isolation and Cytotoxic Investigation of Flacourtin from Oncoba spinosa. Medicines. 2016; 3(4):31. https://doi.org/10.3390/medicines3040031
Chicago/Turabian StyleBalogun, Olaoye S., Olukayode S. Ajayi, and Olayinka S. Lawal. 2016. "Isolation and Cytotoxic Investigation of Flacourtin from Oncoba spinosa" Medicines 3, no. 4: 31. https://doi.org/10.3390/medicines3040031






