Development of a Biomarker for Penconazole: A Human Oral Dosing Study and a Survey of UK Residents’ Exposure
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Sample Preparation
2.3. Sample Analysis
2.4. Method Characteristics
2.5. Creatinine Analysis
2.6. Volunteer Study
2.7. Residents’ Study
3. Results
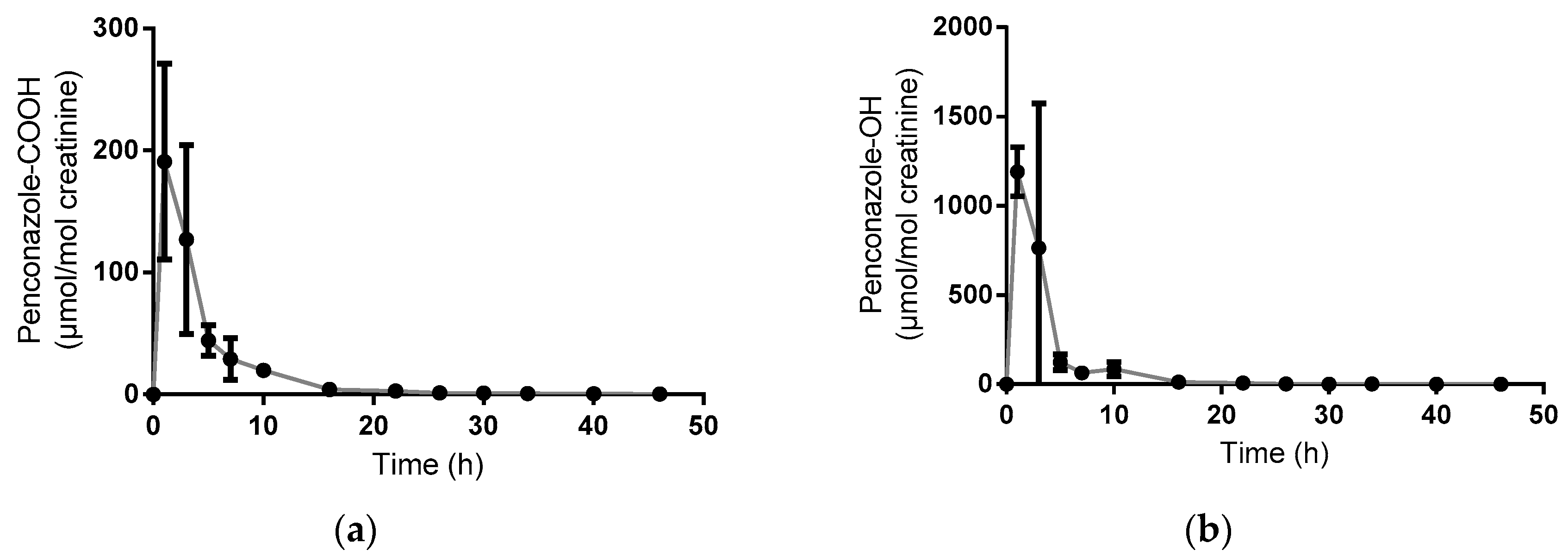
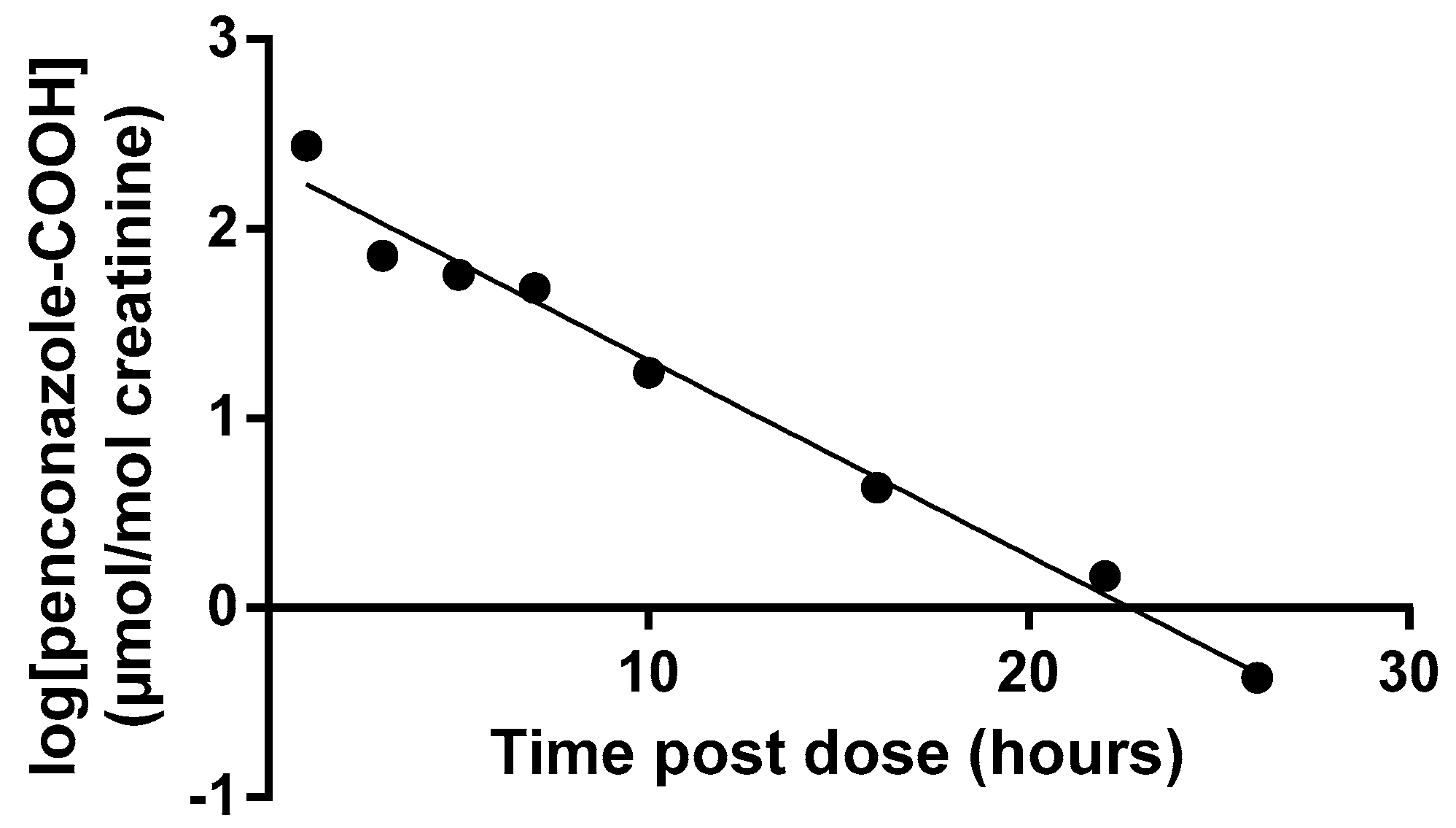
3.1. Volunteer Study
3.2. Residents’ Study
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- JMPR. 848. Penconazole. 1992 19/11/15. Available online: http://www.inchem.org/documents/jmpr/jmpmono/v92pr14.htm (accessed on 6 May 2016).
- HSE. Pesticides Register of UK Authorised Products. 2015 19/11/15. Available online: https://secure.pesticides.gov.uk/pestreg/ (accessed on 6 May 2016).
- EFSA. Conclusion Regarding the Peer Review of the Pesticide Risk Assessment of the Active Substance Penconazole. 2008. Available online: http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/175r.pdf (accessed on 6 May 2016).
- Perdichizzi, S.; Mascolom, M.G.; Silingardi, P.; Morandi, E.; Rotondo, F.; Guerrini, A.; Prete, L.; Vaccari, M.; Colacci, A. Cancer-related genes transcriptionally induced by the fungicide penconazole. Toxicol. in Vitro 2014, 28, 125–130. [Google Scholar] [PubMed]
- PubChem. Penconazole: Compound Summary for CID 91693. 2016 14/04/2016. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Penconazole (accessed on 6 May 2016).
- Galea, K.S.; MacCalman, L.; Jones, K.; Cocker, J.; Teedon, P.; Cherrie, J.W.; van Tongeren, M. Comparison of residents' pesticide exposure with predictions obtained using the UK regulatory exposure assessment approach. Regul. Toxicol. Pharmacol. 2015, 73, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Galea, K.S.; MacCalman, L.; Jones, K.; Cocker, J.; Teedon, P.; Cherrie, J.W.; van Tongeren, M. Urinary biomarker concentrations of captan, chlormequat, chlorpyrifos and cypermethrin in UK adults and children living near agricultural land. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Galea, K.S.; MacCalman, L.; Jones, K.; Cocker, J.; Teedon, P.; Sleeuwenhoek, A.J.; Cherrie, J.W.; van Tongeren, M. Biological monitoring of pesticide exposures in residents living near agricultural land. BMC Public Health 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Garthwaite, D.; Barker, I.; Laybourn, R.; Huntly, A.; Parrish, G.; Hudson, S.; Thygesen, H.; Macarthur, R. Pesticide usage survey report 265. Orchards in the United Kingdom 2014 2015 30/11/15. Available online: https://secure.fera.defra.gov.uk/pusstats/surveys/documents/orchards2014.pdf (accessed on 6 May 2016).
- Gough, K.C.; Patel, S.; Baker, C.A.; Maddison, B.C. Development of Immunoassays for the Detection of the Fungicide Penconazole and Its Urinary Metabolite. J. Agric. Food Chem. 2009, 57, 9393–9399. [Google Scholar] [PubMed]
- Garner, F.; Jones, K. Biological monitoring for exposure to methamidophos: A human oral dosing study. Toxicol. Lett. 2014, 231, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Griffin, P.; Mason, H.; Heywood, K.; Cocker, J. Oral and dermal absorption of chlorpyrifos: A human volunteer study. Occup. Environ. Med. 1999, 56, 10–13. [Google Scholar] [CrossRef]
- Sams, C.; Jones, K. Biological monitoring for exposure to deltamethrin: A human oral dosing study and background levels in the UK general population. Toxicol. Lett. 2011. [Google Scholar] [CrossRef] [PubMed]
- Cocker, J.; Mason, H.J.; Warren, N.D.; Cotton, R.J. Creatinine adjustment of biological monitoring results. Occup. Med.-Oxf. 2011, 61, 349–353. [Google Scholar] [CrossRef]
- Biological monitoring of pesticide exposure in residents–PS2620. 2015 30/11/15. Available online: http://randd.defra.gov.uk/Default.aspx?Menu=Menu&Module=More&Location=None&ProjectID=17319&FromSearch=Y&Publisher=1&SearchText=PS2620&SortString=ProjectCode&SortOrder=Asc&Paging=10#Description (accessed on 6 May 2016).
- Fustinoni, S. Biomonitoring of exposure to penconazole in agriculture. In Proceedings of the 31st International Congress on Occupational Health, Seoul, Korea, 31 May–5 June 2015.
- Fustinoni, S. Urine and hair specimens for biomonitoring short and long term penconazole exposure. In Proceedings of the International Congress on Rural Health, Lodi, Italy, 8–11 September 2015.
- Fustinoni, S.; Mercadante, R.; Polledri, E.; Rubino, F.M.; Mandic-Rajcevic, S.; Vianello, G.; Colosio, C.; Moretto, A. Biological monitoring of exposure to tebuconazole in winegrowers. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Fustinoni, S.; Polledri, E.; Mercadante, R.; Rubino, F.; Colosio, C.; Moretto, A. Time course of excretion of tebuconazole and its metabolites in vineyard workers. G. Ital. Med. Lav. Ergon. 2012, 34, 423–424. [Google Scholar] [PubMed]
- Flack, S.; Goktepe, I.; Ball, L.M.; Nylander-French, L.A. Development and application of quantitative methods for monitoring dermal and inhalation exposure to propiconazole. J. Environ. Monit. 2008, 10, 336–344. [Google Scholar] [CrossRef] [PubMed]



| Code | Sex | Height (m) | Weight (kg) | Age (Years) | BMI* |
|---|---|---|---|---|---|
| A | M | 1.78 | 72 | 59 | 22.7 |
| B | F | 1.58 | 62 | 50 | 24.8 |
| C | M | 1.88 | 83 | 62 | 23.5 |
| Mean Half-Life (h) | Range (h) | |
|---|---|---|
| Penconazole-OH | 3.1 | 2.6–3.5 |
| Penconazole-COOH | 3.7 | 2.9–4.0 |
| Code | % Administered Dose Recovered in Urine | Metabolite Fraction of Total | ||
|---|---|---|---|---|
| Pencon-OH | Pencon-COOH | Penconazole | ||
| A | 53 | 0.85 | 0.15 | 0.001 |
| B | 54 | 0.87 | 0.13 | 0.001 |
| C | 33 | 0.77 | 0.23 | 0.002 |
| Sample Type | N | N < LOD | %<LOD | Maximum | 95th Percentile * |
|---|---|---|---|---|---|
| Background sample—Outwith spray season | 483 | 427 | 88 | 1.73 | 0.22 |
| Background sample—Within spray season | 556 | 500 | 90 | 1.19 | 0.29 |
| After spray event | 89 | 72 | 81 | 1.84 | 0.32 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sams, C.; Jones, K.; Galea, K.S.; MacCalman, L.; Cocker, J.; Teedon, P.; Cherrie, J.W.; Van Tongeren, M. Development of a Biomarker for Penconazole: A Human Oral Dosing Study and a Survey of UK Residents’ Exposure. Toxics 2016, 4, 10. https://doi.org/10.3390/toxics4020010
Sams C, Jones K, Galea KS, MacCalman L, Cocker J, Teedon P, Cherrie JW, Van Tongeren M. Development of a Biomarker for Penconazole: A Human Oral Dosing Study and a Survey of UK Residents’ Exposure. Toxics. 2016; 4(2):10. https://doi.org/10.3390/toxics4020010
Chicago/Turabian StyleSams, Craig, Kate Jones, Karen S. Galea, Laura MacCalman, John Cocker, Paul Teedon, John W. Cherrie, and Martie Van Tongeren. 2016. "Development of a Biomarker for Penconazole: A Human Oral Dosing Study and a Survey of UK Residents’ Exposure" Toxics 4, no. 2: 10. https://doi.org/10.3390/toxics4020010








