Mercury, Lead, Cadmium, Arsenic, Chromium and Selenium in Feathers of Shorebirds during Migrating through Delaware Bay, New Jersey: Comparing the 1990s and 2011/2012
Abstract
:1. Introduction
2. Methods
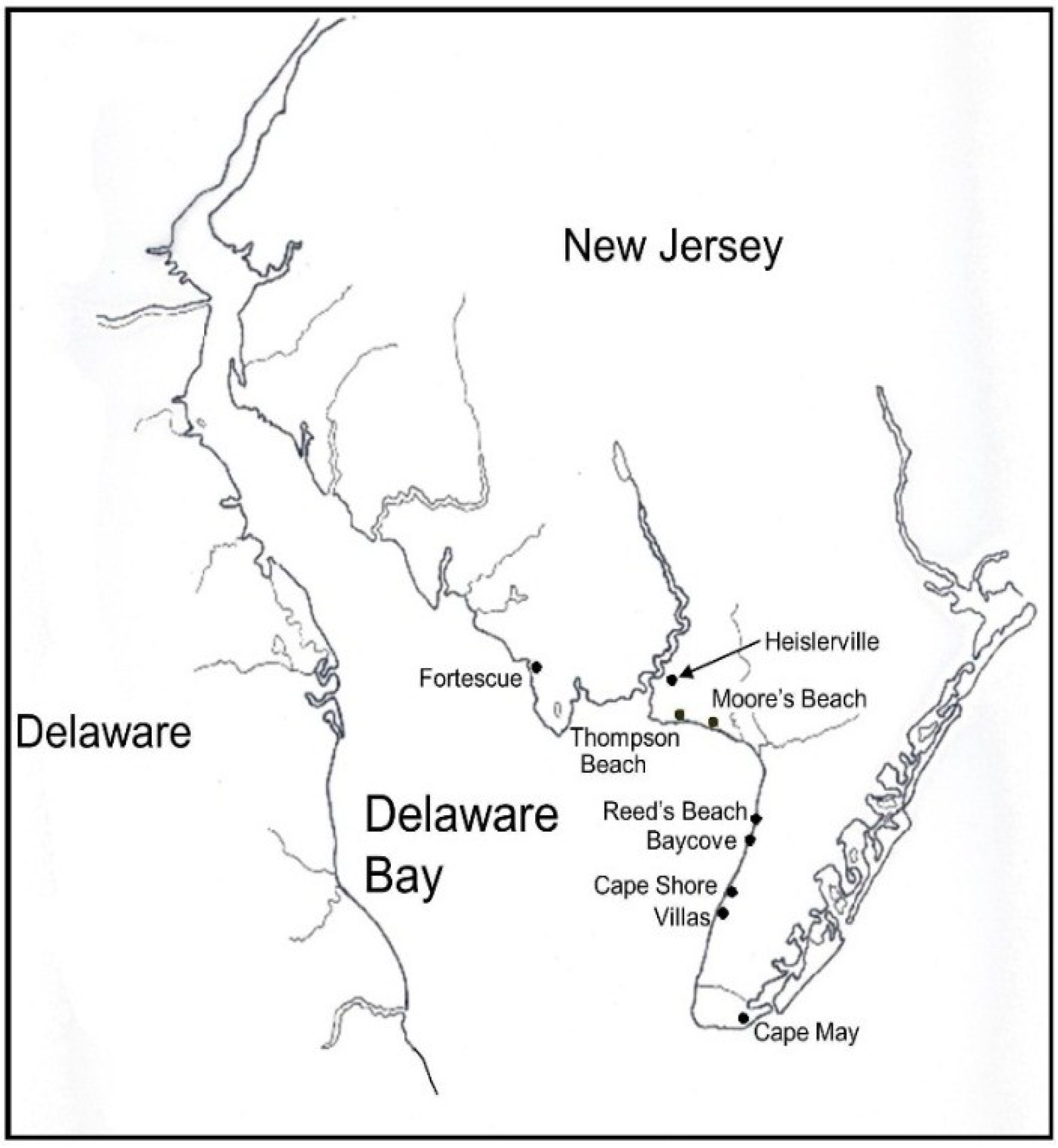
2.1. Collecting Methods
2.2. Chemical Analysis
2.3. Data Analysis
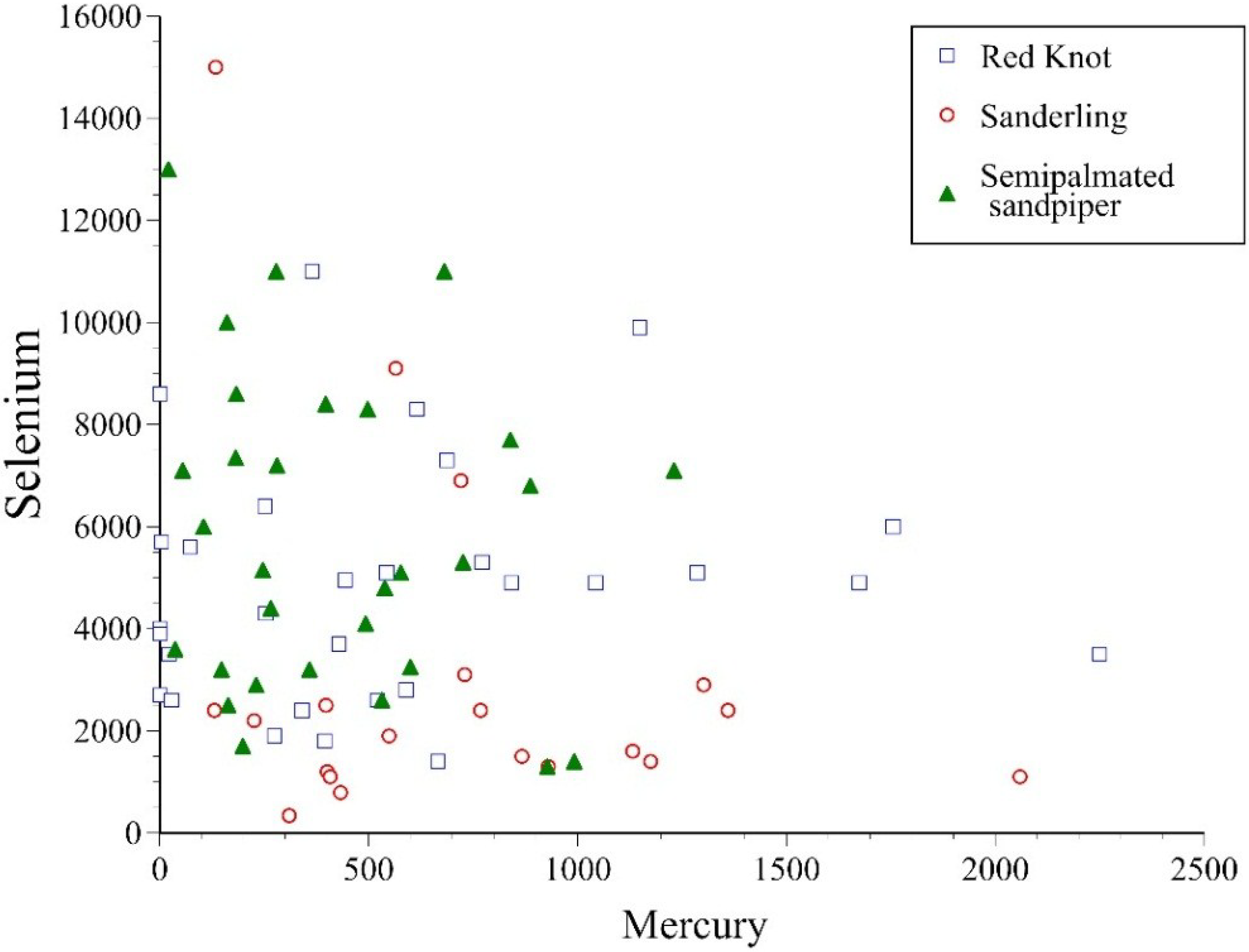
3. Results
| 1991–1992 | 1995 | 2011–2012 | Temporal Kruskal-Wallis Chi-Square (p) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyzed in 2013 | Analyzed in 2013 | ||||||||||
| Mean ± STD Error | Mean ± STD Error | Mean ± STD Error | |||||||||
| Red Knot | n | 16 | 30 | ||||||||
| Arsenic | 209 | ± | 22 | 446 | ± | 42 | 15.4 (<0.0001) | ||||
| Cadmium | 19 | ± | 4 | 17 | ± | 2 | NS | ||||
| Chromium | 291 | ± | 42 | 578 | ± | 83 | 7.6 (0.006) | ||||
| Lead | 139 | ± | 13 | 484 | ± | 67 | 24.3 (<0.0001) | ||||
| Mercury | 791 | ± | 77 | 576 | ± | 105 | 5.0 (0.03) | ||||
| Selenium | 7550 | ± | 536 | 4835 | ± | 432 | 13.0 (0.0003) | ||||
| Sanderling | n | 12 | 20 | ||||||||
| Arsenic | 238 | ± | 39 | 311 | ± | 64 | NS | ||||
| Cadmium | 14 | ± | 3 | 11 | ± | 3 | NS | ||||
| Chromium | 764 | ± | 260 | 463 | ± | 62 | NS | ||||
| Lead | 268 | ± | 52 | 367 | ± | 52 | NS | ||||
| Mercury | 2007 | ± | 352 | 730 | ± | 109 | 8.5 (0.004) | ||||
| Selenium | 2063 | ± | 266 | 3057 | ± | 781 | NS | ||||
| Semipalmated Sandpiper | n | 12 | 28 | 30 | |||||||
| Arsenic | 1140 | ± | 217 | 563 | ± | 52 | 842 | ± | 101 | 7.7 (0.02) | |
| Cadmium | 48 | ± | 9 | 25 | ± | 7 | 14 | ± | 3 | 18.1 (0.0001) | |
| Chromium | 1149 | ± | 294 | 450 | ± | 66 | 523 | ± | 64 | 11.0 (0.004) | |
| Lead | 849 | ± | 165 | 553 | ± | 70 | 411 | ± | 46 | 9.2 (0.01) | |
| Mercury | 420 | ± | 82 | 509 | ± | 31 | 428 | ± | 58 | NS | |
| Selenium | 3462 | ± | 604 | 4165 | ± | 434 | 5802 | ± | 562 | 7.0 (0.03) | |
| Species Comparisons Chi-Square (p) | |||||||||||
| Arsenic | 15.0 (0.0005) | 25.4 (<0.0001) | |||||||||
| Cadmium | 15.3 (0.0005) | 2.9 (NS) | |||||||||
| Chromium | 12.1 (0.002) | 0.7 (NS) | |||||||||
| Lead | 21.1 (<0.0001) | 0.8 (NS) | |||||||||
| Mercury | 15.3 (0.0005) | 5.1 (NS) | |||||||||
| Selenium | 24.8 (<0.0001) | 17.6 (0.0001) | |||||||||
4. Discussion
4.1. Temporal Patterns
4.2. Interspecific Comparisons
4.3. Effects Levels
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clark, K.; Niles, L.; Burger, J. Abundance and distribution of shorebirds migrating on Delaware Bay, 1986–1992. Condor 1993, 95, 694–705. [Google Scholar] [CrossRef]
- Niles, L.J.; Sitters, H.P.; Dey, A.D.; Arce, N.; Atkinson, P.W.; Baker, A.J.; Bennett, K.A.; Buchanan, J.; Carmona, R.; Harrington, B.A.; et al. Status of the Red Knot, Calidris canutus rufa, in the Western Hemisphere. Stud. Avian Biol. 2008, 36, 1–185. [Google Scholar]
- Tsipoura, N.; Burger, J. Shorebird diet during spring migration stopover on Delaware Bay. Condor 1999, 101, 633–644. [Google Scholar] [CrossRef]
- Morrison, R.I.G.; Davidson, N.C.; Wilson, J.R. Survival of the fattest: Body stores on migration and survival in red knots Calidris canutus islandica. J. Avian Biol. 2007, 38, 479–487. [Google Scholar] [CrossRef]
- Niles, L.J.; Bart, J.; Sitters, H.P.; Dey, A.D.; Clark, K.E.; Atkinson, P.W.; Baker, A.J.; Bennett, K.A.; Kalasz, K.S.; Clark, J.; et al. Effects of horseshoe crab harvest in Delaware Bay on Red Knots: Are harvest restrictions working? BioScience 2009, 59, 153–164. [Google Scholar] [CrossRef]
- Burger, J. A Naturalist along the Jersey Shore; Rutgers University Press: New Brunswick, NJ, USA, 1996; p. 305. [Google Scholar]
- Mizrahi, D.; Peters, K. Relationships between Sandpipers and Horse Shoe Crabs in Delaware Bay: A synthesis. In Biology and Conservation of Horseshoe Crabs; Tanacredi, J.T., Botton, M.L., Smith, D.R., Eds.; Springer: New York, NY, USA, 2009; pp. 65–88. [Google Scholar]
- Kraemer, G.; Michels, S. History of Horseshoe Crab Harvest in Delaware Bay. In Biology and Conservation of Horseshoe Crabs; Tanacredi, J.T., Botton, M.L., Smith, D.R., Eds.; Springer: New York, NY, USA, 2009; pp. 299–313. [Google Scholar]
- McGowan, C.P.; Hines, J.E.; Nichols, J.D.; Lyons, J.E.; Smith, D.R.; Kalasz, K.S.; Niles, L.J.; Dey, A.D.; Clark, N.A.; Atkinson, P.W.; et al. Demographic consequences of migratory stopover: Linking red knot survival to horseshoe crab spawning abundance. Ecosphere 2011, 2, 11–22. [Google Scholar] [CrossRef]
- Bart, J.; Brown, B.; Harrington, B.A.; Morrison, R.I.G. Survey trends of North American shorebirds: Population declines or shifting distributions? J. Avian Biol. 2007, 38, 73–82. [Google Scholar] [CrossRef]
- Andres, B.A.; Smith, P.A.; Morrison, R.G.; Gratto-Trevor, C.L.; Brown, S.C.; Friis, C.A. Population estimates of North American shorebirds, 2012. Int. Wader Stud. Group 2013, 119, 178–194. [Google Scholar]
- Burger, J.; Clark, K.L.; Niles, L. Importance of beach, mudflat and marsh for migrant shorebirds on Delaware Bay. Biol. Conserv. 1997, 7, 283–292. [Google Scholar] [CrossRef]
- Burger, J.; Jeitner, C.; Clark, K.L.; Niles, L. The effect of human activities on migrant shorebirds: Successful adaptive management. Environ. Conserv. 2004, 31, 283–288. [Google Scholar] [CrossRef]
- Burger, J.; Carlucci, C.; Jeitner, C.; Niles, L. Habitat choice, disturbance, and management of foraging shorebirds and gulls at a migratory stopover. J. Coast. Res. 2007, 23, 1159–1166. [Google Scholar] [CrossRef]
- Galbraith, H.; Jones, R.; Park, R.; Clough, J.; Herod-Julius, S.; Harrington, B.; Page, G. Global climate change and sea level rise: Potential losses of intertidal habitat for shorebirds. Waterbirds 2002, 25, 173–183. [Google Scholar] [CrossRef]
- Goss-Custard, J.D.; Triplet, P.; Sueur, F.; West, A.D. Critical thresholds of disturbance by people and raptors in foraging wading birds. Biol. Conserv. 2005, 127, 88–97. [Google Scholar] [CrossRef]
- Hargreaves, A.L.; Douglas, P.; Whiteside, R.; Gilchrist, G. Concentrations of 17 elements, including mercury, in the tissues, food and abiotic environment of Arctic shorebirds. Sci. Total Environ. 2011, 409, 3757–3770. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.W. Critical review of selected heavy metal and chlorinated hydrocarbon concentrations in the marine environment. Mar. Environ. Res. 1990, 29, 1–64. [Google Scholar] [CrossRef]
- Spahn, S.A.; Sherry, T.W. Cadmium and lead in exposure associated with reduced growth rates, poorer fledging success of Little Blue heron chicks (Egretta caerulea) in South Louisiana wetlands. Arch. Environ. Contam. Toxicol. 1999, 37, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.; Gochfeld, M. Metal levels in eggs of common terns (Sterna hirundo) in New Jersey: Temporal trends from 1971 to 2002. Environ. Res. 2004, 94, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Neff, J.M. Ecotoxicology of arsenic in the marine environment. Environ. Toxicol. Chem. 1997, 16, 917–927. [Google Scholar]
- Burke, T.; Fagliano, J.; Goldoft, M.; Hazen, R.E.; Iglewicz, R.; McKee, T. Chromite ore processing residue in Hudson County, New Jersey. Environ. Health Perspect. 1991, 92, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Burger, J. Food chain differences affect heavy metals in bird eggs in Barnegat Bay, New Jersey. Environ. Res. 2002, 90, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Weis, J.S. Diet and food web support of the white perch, Morone americana in the Hackensack Meadowlands. Environ. Biol. Fishes 2005, 74, 109–113. [Google Scholar] [CrossRef]
- Eisler, R. Handbook of Chemical Risk Assessment: Health Hazards to Humans, Plants and Animals; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Burger, J.; Seyboldt, S.; Morganstein, N.; Clark, K. Heavy metals and selenium in feathers of three shorebird species from Delaware Bay. Environ. Monit. Assess. 1993, 28, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Ornithological Council. Guidelines to the Use of Wild Birds in Research. Available online: http://www.nmnh.si.edu/BIRDNET/guide/guidelines.html (accessed on 30 January 2015).
- Burger, J. Temporal trends (1989–2011) in levels of mercury and other heavy metals in feathers of fledgling great egrets nesting in Barnegat Bay, New Jersey. Environ. Res. 2013, 122, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, M.; Norman, D. Effects of waterborne mercury on terrestrial wildlife at clear lake: Evaluation and testing of a predictive model. Environ. Toxicol. Chem. 1998, 17, 214–227. [Google Scholar] [CrossRef]
- SAS. Statistical Analysis System; SAS Institute: Cary, NC, USA, 2005. [Google Scholar]
- Ralston, N.V.C.; Raymond, L.J. Dietary selenium’s protective effects against methylmercury toxicity. Toxicology 2010, 278, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, C.R.; Fitzgerald, W.F.; Lamborg, C.H.; Balcom, P.H.; Tseng, C.M. Biogeochemical cycling of methylmercury in lakes and tundra watersheds of Arctic Alaska. Environ. Sci. Technol. 2006, 40, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Evers, D.C.; Burgess, N.M.; Champous, L.; Hoskins, B.; Major, A.; Goodale, W.M.; Taylor, R.J.; Poppenga, R.; Daigle, T. Patterns and interpretation of mercury exposure in freshwater avian communities in northeastern North America. Ecotoxicology 2005, 14, 191–221. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Lead. Agency for Toxic Substances and Disease Registry; US Public Health Service: Atlanta, GA, USA, 1997. [Google Scholar]
- Burger, J. Metals in avian feathers: Bioindicators of environmental pollution. Rev. Environ. Toxicol. 1993, 5, 203–311. [Google Scholar]
- Lucia, M.; Bocher, P.; Chambosse, M.; Delaporte, P.; Bustamante, P. Trace element accumulation in relation to trophic niches of shorebirds using intertidal mudflats. J. Sea Res. 2014, 92, 134–143. [Google Scholar] [CrossRef]
- Lucia, M.; Bocher, P.; Cosson, R.P.; Churlaud, C.; Bustamante, P. Evidence of species-specific detoxification processes for trace elements in shorebirds. Ecotoxicology 2012, 21, 2349–2362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinz, G.H. Effects of low dietary levels of methyl mercury on mallard reproduction. Bull. Environ. Contam. Toxicol. 1974, 11, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Ohlendorf, H.M.; Hothem, R.L.; Welsh, D. Nest success, cause-specific nest failure, and hatchability of aquatic birds at selenium-contaminated Kesterson Reservoir and a reference site. Condor 1989, 91, 787–796. [Google Scholar] [CrossRef]
- Wolfe, M.; Schwarzbach, S.; Sulaiman, R.S. Effects of mercury on wildlife: A comprehensive review. Environ. Toxicol. Chem. 1998, 17, 146–160. [Google Scholar] [CrossRef]
- Custer, T.W.; Custer, C.M.; Hines, R.K.; Gutreuter, S.; Stromborg, K.L. Organochlorine contaminants and reproductive success of double-crested cormorants from Green Bay, Wisconsin, USA. Environ. Toxicol. Chem. 1999, 18, 1209–1217. [Google Scholar]
- Burger, J.; Gochfeld, M. Metals in albatross feathers from midway atoll: Influence of species, age, and nest location. Environ. Res. 2000, 82, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Bouton, S.N.; Frederick, P.C.; Spalding, M.G.; McGill, H. Effects of chronic low concentrations of dietary methylmercury on the behavior of juvenile Great Egrets. Environ. Toxicol. Chem. 1999, 18, 1934–1939. [Google Scholar] [CrossRef]
- Eisler, R. Mercury Hazards to Fish, Wildlife and Invertebrates: A Synoptic Review; U.S. Fish & Wildlife Service: Washington, DC, USA, 1987. [Google Scholar]
- Elliott, J.E.; Scheuhammer, A.M.; Leighton, F.A.; Pearce, P.A. Heavy metal and metallothionein concentrations in Atlantic Canadian seabirds. Arch. Environ. Contam. Toxicol. 1992, 22, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Spalding, M.G.; Frederick, P.C.; McGill, H.C.; Bouton, S.N.; McDowell, L.R. Methylmercury accumulation in tissues and its effects on growth and appetite in captive great egrets. J. Wildl. Dis. 2000, 36, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.; Gochfeld, M. Effects of lead on birds (Laridae): A review of laboratory and field studies. J. Toxicol. Environ. Health 2000, 3, 59–78. [Google Scholar] [CrossRef]
- Wiener, J.G.; Spry, D.J. Toxicological significance of mercury in freshwater fish. In Environmental Contaminants in Wildlife: Interpreting Tissue Concentrations; Beyer, W.N., Heinz, G.H., Redmon-Norwood, A.W., Eds.; Special Publications, Lewis Publishers: Boca Raton, FL, USA, 1996; pp. 297–339. [Google Scholar]
- Eisler, R. Cadmium Hazards to Fish, Wildlife and Invertebrates: A Synoptic Review; U.S. Fish & Wildlife Service: Washington, DC, USA, 1985. [Google Scholar]
- Jackson, A.K.; Evers, D.C.; Etterson, M.A.; Condon, A.M.; Folsom, S.B.; Detweiler, J.; Schmerfeld, J.; Cristol, D.A. Mercury exposure affects the reproductive success of a free-living terrestrial songbird, the Carolina Wren (Thryothorus ludovicianus). The Auk 2011, 128, 759–769. [Google Scholar] [CrossRef]
- Heinz, G.H. Selenium in birds. In Environmental Contaminants in Wildlife: Interpreting Tissue Concentrations; Beyer, W.M., Heinz, W.M., Eds.; CRC Press: Boca Raton, FL, USA, 1996; pp. 447–458. [Google Scholar]
- Ackerman, J.T.; Eagles-Smith, C.A. Selenium bioaccumulation and body condition in shorebirds and terns breeding in San Francisco Bay, California, USA. Environ. Toxicol. Chem. 2009, 28, 2134–2141. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burger, J.; Tsipoura, N.; Niles, L.J.; Gochfeld, M.; Dey, A.; Mizrahi, D. Mercury, Lead, Cadmium, Arsenic, Chromium and Selenium in Feathers of Shorebirds during Migrating through Delaware Bay, New Jersey: Comparing the 1990s and 2011/2012. Toxics 2015, 3, 63-74. https://doi.org/10.3390/toxics3010063
Burger J, Tsipoura N, Niles LJ, Gochfeld M, Dey A, Mizrahi D. Mercury, Lead, Cadmium, Arsenic, Chromium and Selenium in Feathers of Shorebirds during Migrating through Delaware Bay, New Jersey: Comparing the 1990s and 2011/2012. Toxics. 2015; 3(1):63-74. https://doi.org/10.3390/toxics3010063
Chicago/Turabian StyleBurger, Joanna, Nellie Tsipoura, Lawrence J. Niles, Michael Gochfeld, Amanda Dey, and David Mizrahi. 2015. "Mercury, Lead, Cadmium, Arsenic, Chromium and Selenium in Feathers of Shorebirds during Migrating through Delaware Bay, New Jersey: Comparing the 1990s and 2011/2012" Toxics 3, no. 1: 63-74. https://doi.org/10.3390/toxics3010063







