Nanostructure and Luminescent Properties of Bimetallic Lanthanide Eu/Gd, Tb/Gd and Eu/Tb Coordination Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of the LnMOFs
2.3. Characterization of the LnMOFs
3. Results and Discussion
3.1. Structural Characterization of the LnMOFs
3.2. XPS Characterization on the Surface of the LnMOF
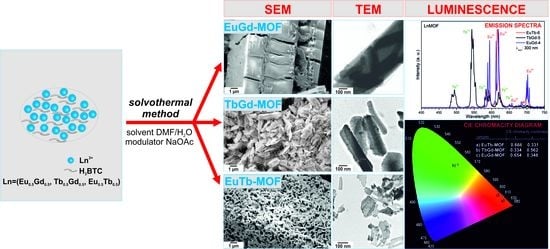
3.3. Surface Morphologies of LnMOFs
3.4. Luminescence of the LnMOFs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Batten, S.R.; Champness, N.R.; Chen, X.M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Terminology of metal-organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef] [Green Version]
- Peedikakkal, A.M.P.; Adarsh, N.N. Coordination Polymers. In Porous Coordination Polymers; Mazumder, M.J., Sheardown, H., Al-Ahmed, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 2–6. [Google Scholar] [CrossRef]
- Lee, J.S.M.; Otake, K.; Kitagawa, S. Transport properties in porous coordination polymers. Coord. Chem. Rev. 2020, 421, 213447. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Matveevskaya, V.; Pavlov, D.; Yakunenkov, A.; Potapov, A. Coordination Polymers Based on Highly Emissive Ligands: Synthesis and Functional Properties. Materials 2020, 13, 2699. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, K.A.; Potapov, A.S.; Fedin, V.P. Micro- and mesoporous metal-organic coordination polymers for separation of hydrocarbons. Rus. Chem. Rev. 2021, 90. [Google Scholar] [CrossRef]
- Xu, B.; Guo, H.; Wang, S.; Li, Y.; Zhang, H.; Liu, C. Solvothermal synthesis of luminescent Eu(BTC)(H2O)DMF hierarchical architectures. CrystEngComm 2012, 14, 2914–2919. [Google Scholar] [CrossRef]
- Neufeld, M.J.; Winter, H.; Landry, M.R.; Goforth, A.M.; Khan, S.; Pratx, G.; Sun, C. Lanthanide Metal-Organic Frameworks for Multispectral Radioluminescent Imaging. ACS Appl. Mater. Interfaces 2020, 12, 26943–26954. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, F.; Fan, L.; Zhao, W.; Chen, B.; Chen, X.; Zhou, S.F.; Xiao, J.; Zhan, G. Improved hydrolytic robustness and catalytic performance of flexible lanthanide-based metal-organic frameworks: A mater of coordination environments. Mater. Des. 2020, 194, 108881. [Google Scholar] [CrossRef]
- Dang, S.; Song, S.; Feng, J.; Zhang, H. Microwave-assisted synthesis of nanoscale Eu(BTC)(H2O)·DMF with tunable luminescence. Sci. China Chem. 2015, 58, 973–978. [Google Scholar] [CrossRef]
- Alammar, T.; Hlova, I.Z.; Gupta, S.; Balema, V.; Pecharskya, V.K.; Mudring, A.V. Luminescent Properties of Mechanochemically Synthesized Lanthanide Containing MIL-78 MOF. Dalton Trans. 2018, 47, 7594–7601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramya, A.R.; Varughese, S.; Reddy, M.L.P. Tunable white-light emission from mixed lanthanide (Eu3+, Gd3+, Tb3+) coordination polymers derived from 4-(dipyridin-2-yl)-aminobenzoate. Dalton Trans. 2014, 43, 10940–10946. [Google Scholar] [CrossRef]
- Gonzalez, A.; Chu, X.M.; Pitton, K.A.; Crichton, R.; Einkauf, J.D. Developing Luminescent Lanthanide Coordination Polymers and Metal-Organic Frameworks for Bioimaging Applications. Springs 2017, 6, 37–43. [Google Scholar]
- Zhao, Y.; Li, D. Lanthanide-functionalized metal-organic frameworks as ratiometric luminescent sensors. J. Mater. Chem. C 2020, 8, 12739–12754. [Google Scholar] [CrossRef]
- Wang, M.; Hu, M.; Li, Z.; He, L.; Song, Y.; Jia, Q.; Zhang, Z.; Du, M. Construction of Tb-MOF-on-Fe-MOF conjugate as a novel platform for ultrasensitive detection of carbohydrate antigen 125 and living cancer cells. Biosens. Bioelectron. 2019, 142, 111536. [Google Scholar] [CrossRef]
- Nasruddin; Zulys, A.; Yulia, F.; Buhori, A.; Muhadzib, N.; Ghiyats, M.; Saha, B.B. Synthesis and characterization of a novel microporous lanthanide based metal-organic framework (MOF) using napthalenedicarboxylate acid. J. Mater. Res. Technol. 2020, 9, 7409–7417. [Google Scholar] [CrossRef]
- Lian, X.; Yan, B. A lanthanide metal–organic framework (MOF-76) for adsorbing dyes and fluorescence detecting aromatic pollutants. RSC Adv. 2016, 6, 11570–11576. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Ma, H.; Zhang, L.; Jiang, H.; Song, H.; Zhang, L.; Luo, Y. A nanoscaled lanthanide metal-organic framework as a colorimetric fluorescent sensor for dipicolinic acid based on modulating energy transfer. J. Mater. Chem. C 2016, 4, 7294–7301. [Google Scholar] [CrossRef]
- Ren, K.; Guo, X.F.; Tang, Y.J.; Huang, B.H.; Wang, H. Size-controlled synthesis of metal-organic frameworks and their performance as fluorescence sensors. Analyst 2020, 145, 7349–7356. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, J.F.S.D.; Barros, B.S.; Kulesza, J.; de Oliveira, J.B.L.; Leite, A.K.P.; de Oliveira, R.S. Influence of synthesis time on the microstructure and photophysical properties of Gd-MOFs doped with Eu3+. Mater. Chem. Phys. 2017, 190, 166–174. [Google Scholar] [CrossRef]
- Gomez, G.E.; Kaczmarek, A.M.; Van Deun, R.; Brusau, E.V.; Narda, G.E.; Vega, D.; Iglesias, M.; Gutierrez-Puebla, E.; Monge, M.A. Photoluminescence, Unconventional-Range Temperature Sensing, and Efficient Catalytic Activities of Lanthanide Metal-Organic Frameworks. Eur. J. Inorg. Chem. 2016, 10, 1577–1588. [Google Scholar] [CrossRef]
- Chen, D.M.; Sun, C.X.; Peng, Y.; Zhang, N.N.; Si, H.H.; Liu, C.S.; Du, M. Ratiometric fluorescence sensing and colorimetric decoding methanol by a bimetallic lanthanide-organic framework. Sens. Actuators B 2018, 265, 104–109. [Google Scholar] [CrossRef]
- Zhou, J.; Li, H.; Zhang, H.; Li, H.; Shi, W.; Cheng, P. A Bimetallic Lanthanide Metal–Organic Material as a Self-Calibrating Color-Gradient Luminescent Sensor. Adv. Mater. 2015, 27, 7072–7077. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Hu, J.; Li, J.; Zhang, M. Tunable emission and selective luminescence sensing for nitro-pollutants and metal ions based on bifunctional lanthanide metal-organic frameworks. J. Lumin. 2020, 221, 117100. [Google Scholar] [CrossRef]
- Xia, T.; Wang, J.; Jiang, K.; Cui, Y.; Yang, Y.; Qian, G. A Eu/Gd-mixed metal-organic framework for ultrasensitive physiological temperature sensing. Chin. Chem. Lett. 2018, 29, 861–864. [Google Scholar] [CrossRef]
- Brunckova, H.; Mudra, E.; Rocha, L.; Nassar, E.; Nascimento, W.; Kolev, H.; Kovalcikova, A.; Molcanova, Z.; Podobova, M.; Medvecky, L. Preparation and characterization of isostructural lanthanide Eu/Gd/Tb metal-organic framework thin films for luminescent applications. Appl. Surf. Sci. 2021, 542, 148731. [Google Scholar] [CrossRef]
- Che, H.; Li, Y.; Zhang, S.; Chen, W.; Tian, X.; Yang, C.; Lu, L.; Zhou, Z.; Nie, Y. A portable logic detector based on Eu-MOF for multi-target, on-site, visual detection of Eu3+ and fluoride in groundwater. Sens. Actuators B Chem. 2020, 324, 128641. [Google Scholar] [CrossRef]
- Binh, N.T.; Tien, D.M.; Giang, L.T.K.; Khuyen, H.T.; Huong, N.T.; Huong, T.T.; Lam, T.D. Study on preparation and characterization of MOF based lanthanide doped luminescent coordination polymers. Mater. Chem. Phys. 2014, 143, 946–951. [Google Scholar] [CrossRef]
- Song, K.; Yu, H.; Zhang, J.; Bai, Y.; Guan, Y.; Yu, J.; Guo, L. Rosebengal-Loaded Nanoporous Structure Based on Rare Earth Metal-Organic-Framework: Synthesis, Characterization and Photophysical Performance. Crystals 2020, 10, 185. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Tong, C. Dual-functional lanthanide metal organic frameworks for visual and ultrasensitive ratiometric fluorescent detection of phosphate based on aggregation-induced energy transfer. Anal. Chimica Acta 2020, 1133, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Colomer, M.T.; Rodríguez, E.; Moran-Pedroso, M.; Vattier, F.; de Andres, A. Impact of Tb4+ and morphology on the thermal evolution of Tb-doped TiO2 nanostructured hollow spheres and nanoparticles. J. Alloys Compd. 2020, 853, 156973. [Google Scholar] [CrossRef]
- Balaguer, M.; Yoo, C.Y.; Bouwmeester, H.J.M.; Serra, J.M. Bulk transport and oxygen surface exchange of the mixed ionic–electronic conductor Ce1−xTbxO2−δ (x = 0.1, 0.2, 0.5). J. Mater. Chem. A 2013, 1, 10234–10242. [Google Scholar] [CrossRef]
- Cheng, J.; Liang, J.; Dong, L.; Chai, J.; Zhao, N.; Ullah, S.; Wang, H.; Zhang, D.; Imtiaz, S.; Shan, G.; et al. Self-assembly of 2D-metal–organic framework/graphene oxide membranes as highly efficient adsorbents for the removal of Cs+ from aqueous solutions. RSC Adv. 2018, 8, 40813–40822. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, J.; Han, Y.; Zhu, M.; Shang, S.; Li, W. MOF-derived various morphologies of N-doped carbon composites for acetylene hydrochlorination. J. Mater. Sci. 2018, 53, 4913–4926. [Google Scholar] [CrossRef]
- Jiang, T.; Sun, X.; Wei, L.; Li, M. Electrochemical determination of artemisinin based on signal inhibition for the reduction of hemin. Anal. Bioanal. Chem. 2021, 413, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Fechler, N.; Fellinger, T.P.; Antonietti, M. One-pot synthesis of nitrogen–sulfur-co-doped carbons with tunable composition using a simple isothiocyanate ionic liquid. J. Mater. Chem. A 2013, 1, 14097–14102. [Google Scholar] [CrossRef] [Green Version]
- Sheta, S.M.; El-Sheikh, S.M.; Abd-Elzaher, M.M.; Wassel, A.R. A novel nano-size lanthanum metal–organic framework based on 5-amino-isophthalic acid and phenylenediamine: Photoluminescence study and sensing applications. Appl. Organometal Chem. 2019, 33, 4777. [Google Scholar] [CrossRef]
- Du, S.Z.; Sun, Z.; Han, L.; Qing, M.; Luo, H.Q.; Li, N.B. Two 3d-4f metal-organic frameworks as fluorescent sensor array for the discrimination of phosphates based on different response patterns. Sens. Actuators B. Chem. 2020, 324, 128757. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, K.; Sun, X.; Guan, R.; Su, H.; You, H.; Qi, C. A series of nano/micro-sized metal-organic frameworks with tunable photoluminescence properties. CrystEngComm 2015, 17, 2321–2326. [Google Scholar] [CrossRef]
- Medina-Velazquez, D.Y.; Alejandre-Zuniga, B.Y.; Loera-Serna, S.; Ortiz, E.M.; Morales-Ramirez, J.; Garfias-Garcia, E.; Garcia-Murillo, A.; Falcony, C. An alkaline one-pot reaction to synthesize luminescent Eu-BTC MOF nanorods, highly pure and water-insoluble, under room conditions. J. Nanoparticle Res. 2016, 18, 352–362. [Google Scholar] [CrossRef]
- Moscardini, S.B.; Sverzut, L.; Massarotto, W.L.; Nassar, E.J.; Rocha, L.A. Multi-color emission from lanthanide ions doped into niobium oxide. J. Mater. Sci. Mater Electron. 2020, 31, 5241–5252. [Google Scholar] [CrossRef]
- Miura, B.A.; Ferreira, N.H.; Oliveira, P.F.; Faria, E.H.; Tavares, D.C.; Rocha, L.A.; Ciuffi, K.J.; Nassar, E.J. Functionalization of luminescent YVO4:Eu3+ nanoparticles by sol-gel. J. Lumin. 2015, 159, 93–99. [Google Scholar] [CrossRef]
- Rocha, L.A.; Ciuffi, K.J.; Sacco, H.C.; Nassar, E.J. Influence on deposition speed and stirring type in the obtantion of titania films. Mater. Chem. Phys. 2004, 85, 245–250. [Google Scholar] [CrossRef]
- Haugland, R.P. Handbook of Fluorescent Probes and Research Products, 9th ed.; Molecular Probes: Eugene, OR, USA, 2002. [Google Scholar]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Matias, C.R.; Nassar, E.J.; Verelst, M.; Rocha, L.A. Synthesis and Characterization of Nb2O5:La3+,Eu3+ Phosphors Obtained by the Non-Hydrolytic Sol-Gel Process. J. Braz. Chem. Soc. 2015, 26, 2558–2570. [Google Scholar] [CrossRef]
- Pereira, P.F.S.; Matos, M.G.; Ferreira, C.M.A.; De Faria, E.H.; Calefi, P.S.; Rocha, L.A.; Ciuffi, K.J.; Nassar, E.J. Aluminate matrix doped with Tm3+/Tb3+/Eu3+ obtained by non-hydrolytic sol-gel route: White light emission. J. Lumin. 2014, 146, 394–397. [Google Scholar] [CrossRef]
- Santa-Cruz, P.A.; Teles, F.S. Spectra Lux Software v.1.0, Ponto Quântico Nanodispositivos/Renami; SciELO: São Paulo, Brazil, 2003. [Google Scholar]
- Zhou, L.; Huang, J.; Gong, F.; Lan, Y.; Tong, Z.; Sun, J. A new red phosphor LaNb0.70V0.30O4:Eu3+ for white light-emitting diodes. J. Alloys Compd. 2010, 495, 268–271. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, L.; Liang, Z.; Gong, F.; Han, J.; Wang, R. Promising red phosphors LaNbO4:Eu3+, Bi3+ for LED solid-state lighting application. J. Rare Earths 2010, 28, 356–360. [Google Scholar] [CrossRef]








| LnMOF | Size of Nanorod (nm) | Size of Included Particles (nm) | ||
|---|---|---|---|---|
| Length | Width | Length | Width | |
| Eu-1 | 820 | 280 | 350 | 100 |
| 600 | 150 | 200 | 50 | |
| Gd-2 | 730 | 150 | 150 | 50 |
| 600 | 150 | 100 | 40 | |
| Tb-3 | 400 | 50 | 150 | 60 |
| 250 | 50 | 100 | 50 | |
| EuGd-4 | 850 | 400 | 450 | 100 |
| 300 | 50 | |||
| TbGd-5 | 400 | 150 | 150 | 30 |
| 300 | 30 | 130 | 20 | |
| EuTb-6 | 140 | 20 | 40 | 20 |
| 110 | 20 | 30 | 20 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunckova, H.; Mudra, E.; Rocha, L.; Nassar, E.; Nascimento, W.; Kolev, H.; Lisnichuk, M.; Kovalcikova, A.; Molcanova, Z.; Strečkova, M.; et al. Nanostructure and Luminescent Properties of Bimetallic Lanthanide Eu/Gd, Tb/Gd and Eu/Tb Coordination Polymers. Inorganics 2021, 9, 77. https://doi.org/10.3390/inorganics9100077
Brunckova H, Mudra E, Rocha L, Nassar E, Nascimento W, Kolev H, Lisnichuk M, Kovalcikova A, Molcanova Z, Strečkova M, et al. Nanostructure and Luminescent Properties of Bimetallic Lanthanide Eu/Gd, Tb/Gd and Eu/Tb Coordination Polymers. Inorganics. 2021; 9(10):77. https://doi.org/10.3390/inorganics9100077
Chicago/Turabian StyleBrunckova, Helena, Erika Mudra, Lucas Rocha, Eduardo Nassar, Willian Nascimento, Hristo Kolev, Maksym Lisnichuk, Alexandra Kovalcikova, Zuzana Molcanova, Magdalena Strečkova, and et al. 2021. "Nanostructure and Luminescent Properties of Bimetallic Lanthanide Eu/Gd, Tb/Gd and Eu/Tb Coordination Polymers" Inorganics 9, no. 10: 77. https://doi.org/10.3390/inorganics9100077