Single Crystal Growth of Sillén–Aurivillius Perovskite Oxyhalides Bi4NbO8X (X = Cl, Br)
Abstract
:1. Introduction
2. Results and Discussion
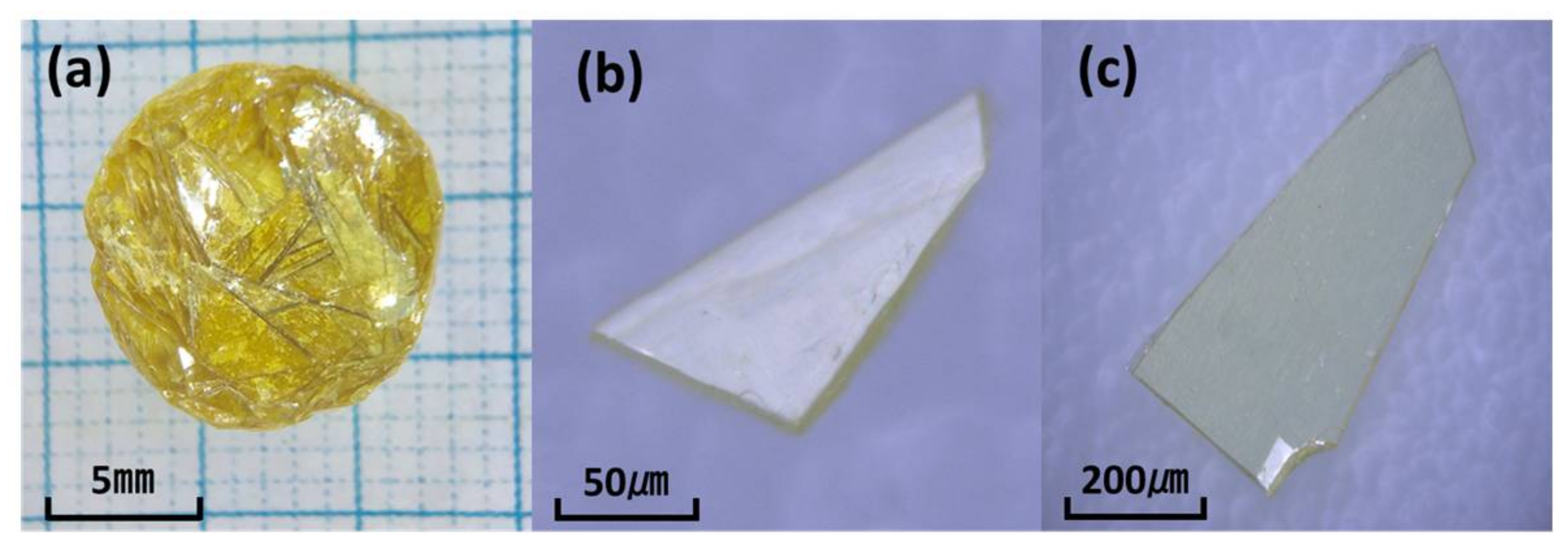
2.1. Growth Condition of Bi4NbO8Cl
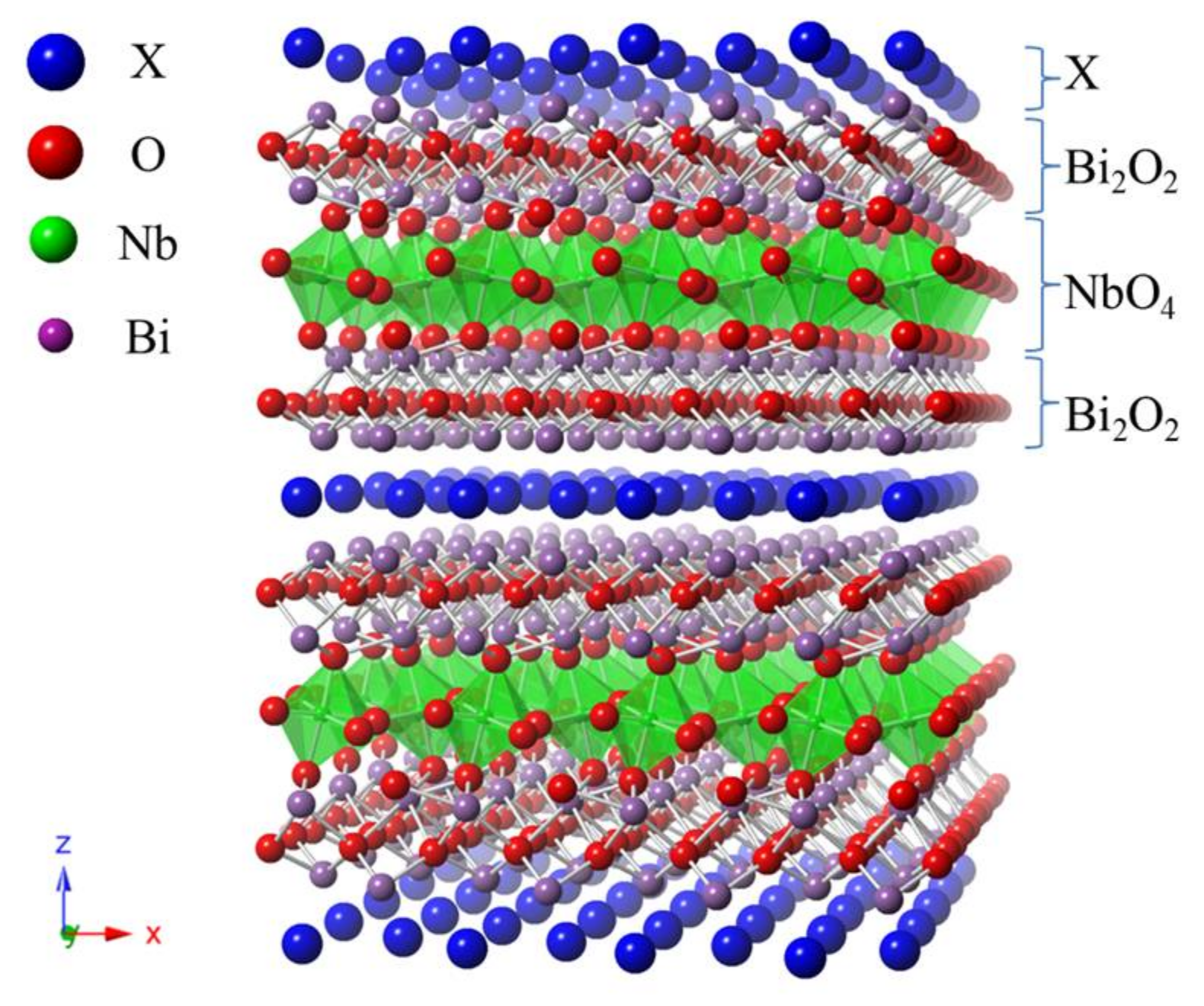
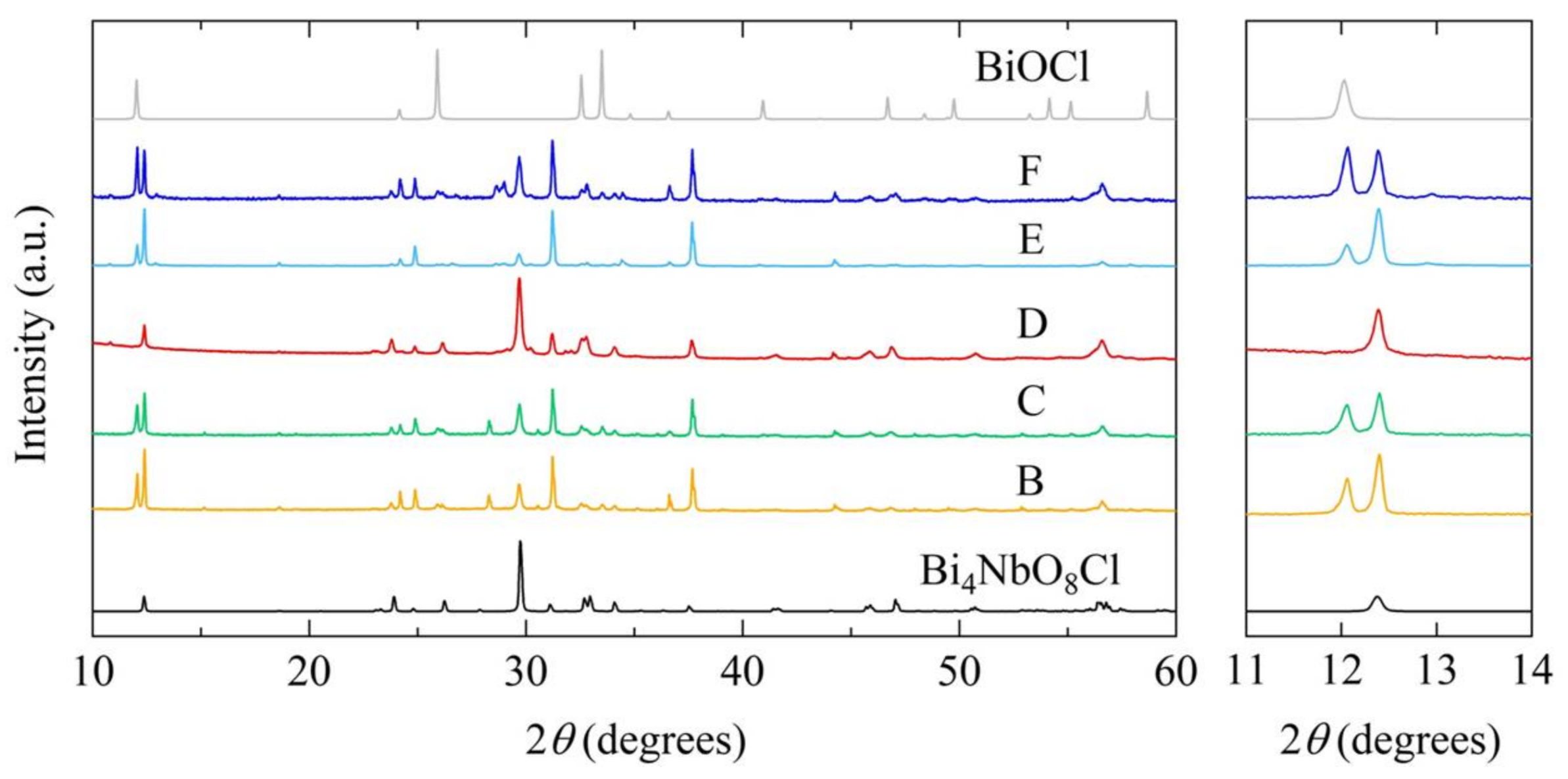
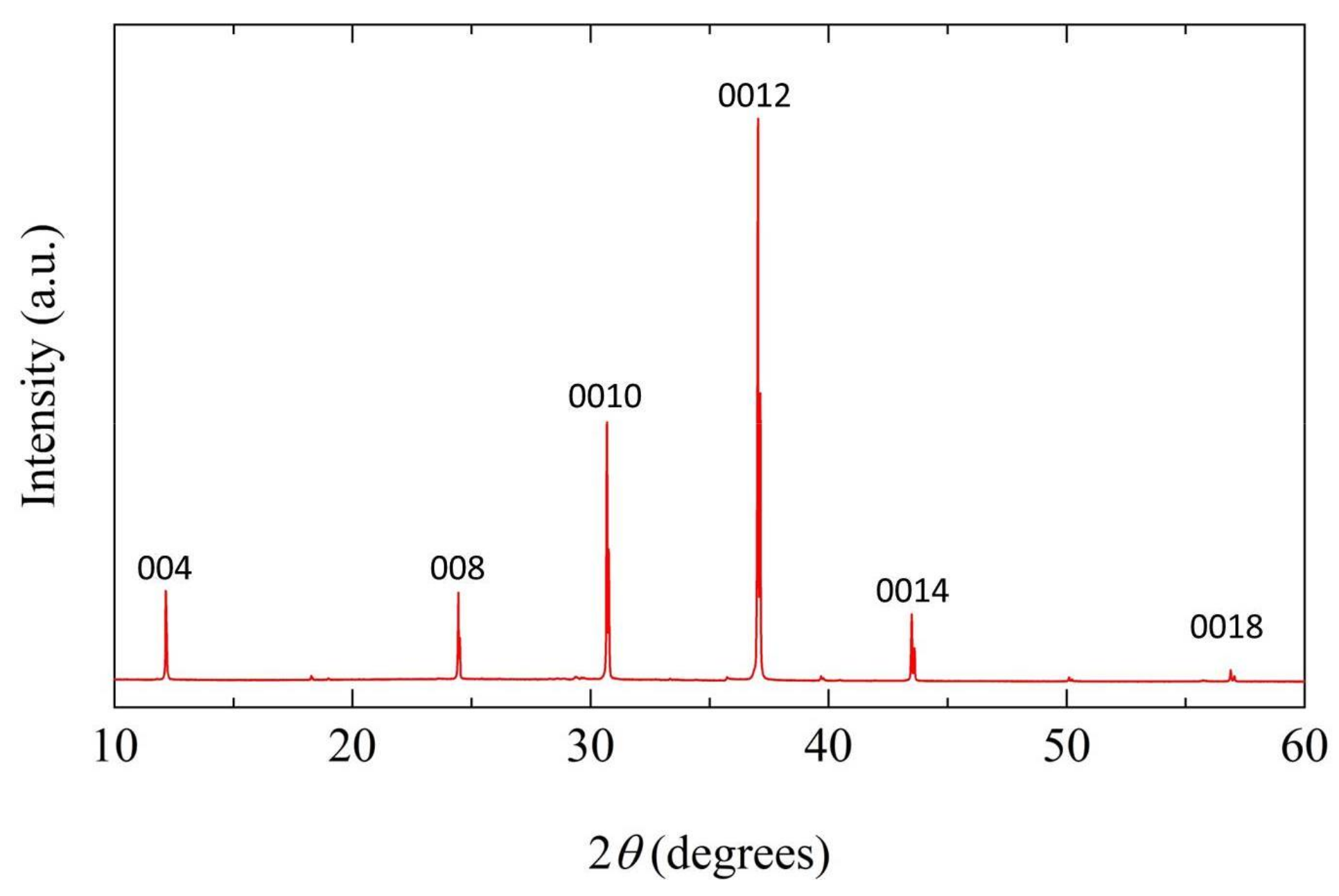
2.2. Characterization of Bi4NbO8Cl
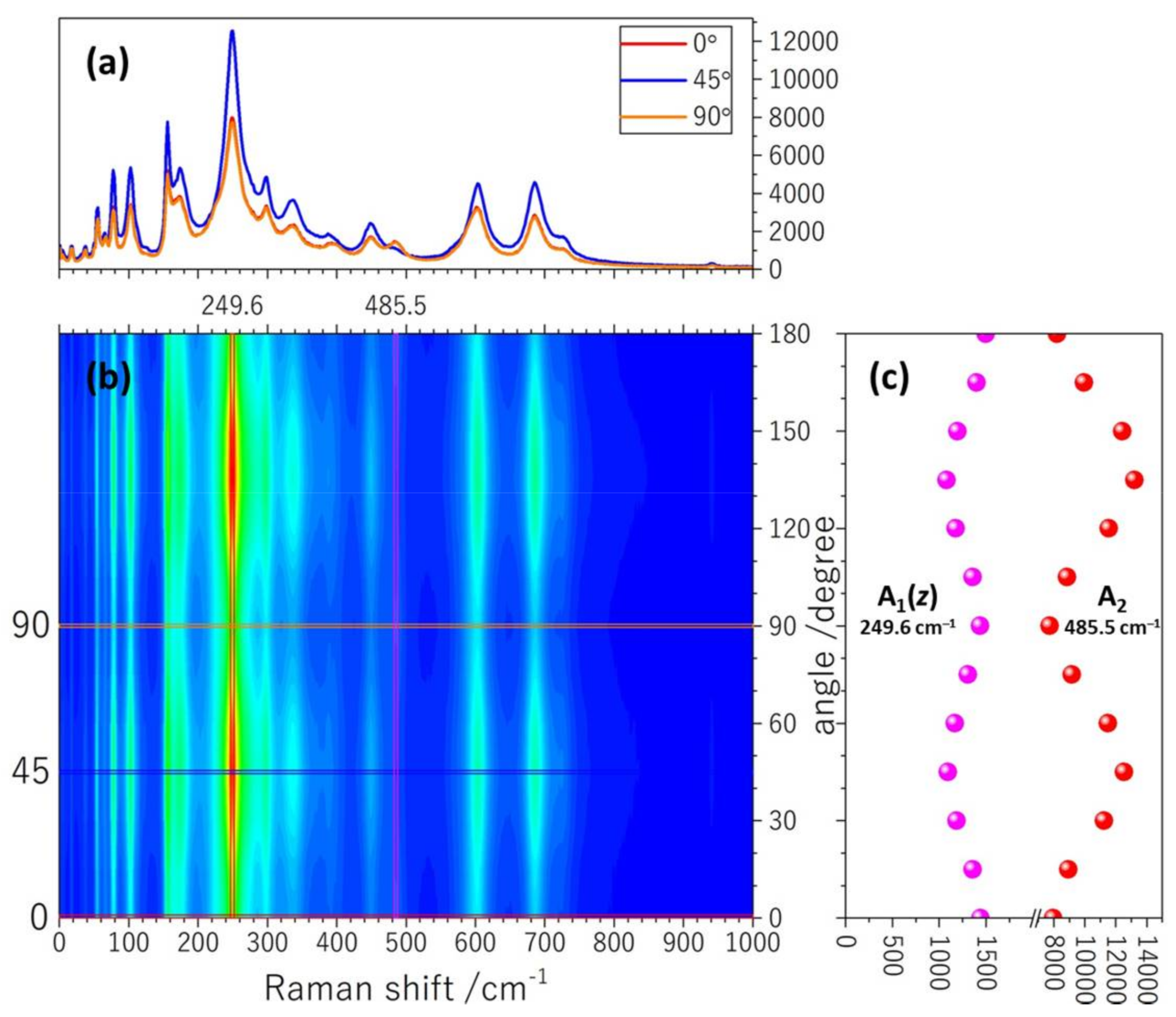
2.3. Polarized Raman Measurement of Bi4NbO8Cl
2.4. Single Crystal Growth of Bi4NbO8Br
3. Materials and Methods
3.1. Crystal Growth
3.2. Characterizations
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kageyama, H.; Hayashi, K.; Maeda, K.; Attfield, J.P.; Hiroi, Z.; Rondinelli, J.M.; Poeppelmeier, K.R. Expanding frontiers in materials chemistry and physics with multiple anions. Nat. Commun. 2018, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Atuchin, V.V.; Isaenko, L.I.; Kesler, V.G.; Lin, Z.S.; Molokeev, M.S.; Yelisseyev, A.P.; Zhurkov, S.A. Exploration on anion ordering, optical properties and electronic structure in K3WO3F3 elpasolite. J. Solid State Chem. 2012, 187, 159–164. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Molokeev, M.S.; Yurkin, G.Y.; Gavrilova, T.A.; Kesler, V.G.; Laptash, N.M.; Flerov, I.N.; Patrin, G.S. Synthesis, structural, magnetic, and electronic properties of cubic CsMnMoO3F3 oxyfluoride. J. Phys. Chem. C 2012, 116, 10162–10170. [Google Scholar] [CrossRef]
- Maeda, K.; Takata, T.; Hara, M.; Saito, N.; Inoue, Y.; Kobayashi, H.; Domen, K. GaN: ZnO solid solution as a photocatalyst for visible-light-driven overall water splitting. J. Am. Chem. Soc. 2005, 127, 8286–8287. [Google Scholar] [CrossRef] [PubMed]
- Yashima, M.; Yamada, H.; Maeda, K.; Domen, K. Experimental visualization of covalent bonds and structural disorder in a gallium zinc oxynitride photocatalyst (Ga1−xZnx)(N1−xOx): Origin of visible light absorption. Chem. Commun. 2010, 46, 2379–2381. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Takata, T.; Kondo, J.N.; Hara, M.; Kobayashi, H.; Domen, K. Oxysulfide Sm2Ti2S2O5 as a stable photocatalyst for water oxidation and reduction under visible light irradiation (λ ≤ 650 nm). J. Am. Chem. Soc. 2002, 124, 13547–13553. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Takata, T.; Matsumura, T.; Kondo, J.N.; Hara, M.; Kobayashi, H.; Domen, K. Oxysulfides Ln2Ti2S2O5 as stable photocatalysts for water oxidation and reduction under visible-light irradiation. J. Phys. Chem. B 2004, 108, 2637–2642. [Google Scholar] [CrossRef]
- Fujito, H.; Kunioku, H.; Kato, D.; Suzuki, H.; Higashi, M.; Kageyama, H.; Abe, R. Layered perovskite oxychloride Bi4NbO8Cl: A stable visible light responsive photocatalyst for water splitting. J. Am. Chem. Soc. 2016, 138, 2082–2085. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, J.F. The structures of Bi3PbWO8Cl and Bi4NbO8Cl and the evolution of the bipox structure series. J. Solid State Chem. 1986, 62, 92–104. [Google Scholar] [CrossRef]
- Kunioku, H.; Higashi, M.; Tomita, O.; Yabuuchi, M.; Kato, D.; Fujito, H.; Kageyama, H.; Abe, R. Strong hybridization between Bi-6s and O-2p orbitals in Sillén–Aurivillius perovskite Bi4MO8X (M = Nb, Ta; X = Cl, Br), visible light photocatalysts enabling stable water oxidation. J. Mater. Chem. A 2018, 6, 3100–3107. [Google Scholar] [CrossRef]
- Kato, D.; Hongo, K.; Maezono, R.; Higashi, M.; Kunioku, H.; Yabuuchi, M.; Suzuki, H.; Okajima, H.; Zhong, C.; Nakano, K.; et al. Valence band engineering of layered bismuth oxyhalides toward stable visible-light water splitting: Madelung site potential analysis. J. Am. Chem. Soc. 2017, 139, 18725–18731. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, V.J.; Sedgwick, T.O.; Landermann, J.B. Vapor growth of Bi12GeO20, γ-Bi2O3 and BiOCl. J. Cryst. Growth 1973, 20, 165–168. [Google Scholar] [CrossRef]
- Peng, H.; Chan, C.K.; Meister, S.; Zhang, X.F.; Cui, Y. Shape evolution of layer-structured bismuth oxychloride nanostructures via low-temperature chemical vapor transport. Chem. Mater. 2008, 21, 247–252. [Google Scholar] [CrossRef]
- Binnewies, M.; Glaum, R.; Schmidt, M.; Schmidt, P. Chemical Vapor Transport Reactions; Walter de Gruyter: Berlin, Germany, 2012. [Google Scholar]
- Zhao, L.; Zhang, X.; Fan, C.; Liang, Z.; Han, P. First-principles study on the structural, electronic and optical properties of BiOX (X = Cl, Br, I) crystals. Physica B 2012, 407, 3364–3370. [Google Scholar] [CrossRef]
- Kusainova, A.M.; Stefanovich, S.Y.; Dolgikh, V.A.; Mo-sunov, A.V.; Hervoches, C.H.; Lightfoot, P. Dielectric properties and structure of Bi4NbO8Cl and Bi4TaO8Cl. J. Mater. Chem. 2001, 11, 1141–1145. [Google Scholar] [CrossRef]
- Schneemeyer, L.F.; Waszczak, J.V.; Siegrist, T.; Van Dover, R.B.; Rupp, L.W.; Batlogg, B.; Cava, R.J.; Murphy, D.W. Superconductivity in YBa2Cu3O7 single crystals. Nature 1987, 328, 601. [Google Scholar] [CrossRef]
- Ishiguro, T.; Kitazawa, A.; Mizutani, N.; Kato, M. Single-crystal growth and crystal structure refinement of CuAlO2. J. Solid State Chem. 1981, 40, 170–174. [Google Scholar] [CrossRef]
- Kusainova, A.M.; Zhou, W.; Irvine, J.T.; Lightfoot, P. Layered Intergrowth Phases Bi4MO8X (X = Cl, M = Ta and X = Br, M = Ta or Nb): Structural and Electrophysical Characterization. J. Solid State Chem. 2002, 166, 148–157. [Google Scholar] [CrossRef]
- He, Y.; Zhang, Y.; Huang, H.; Tian, N.; Guo, Y.; Luo, Y. A novel Bi-based oxybromide Bi4NbO8Br: Synthesis, characterization and visible-light-active photocatalytic activity. Colloid Surf. A Physicochem. Eng. Asp. 2014, 462, 131–136. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Katayama, D.; Koreeda, A. Broadband light scattering spectroscopy utilizing an ultra-narrowband holographic notch filter. Jpn. J. Appl. Phys. 2016, 55, 10TC03. [Google Scholar] [CrossRef]
- Fujii, Y.; Noju, M.; Shimizu, T.; Taniguchi, H.; Itoh, M.; Nishio, I. Raman tensor analysis of crystalline lead titanate by quantitative polarized spectroscopy. Ferroelectrics 2014, 462, 8–13. [Google Scholar] [CrossRef]
- Kusainova, A.M.; Lightfoot, P.; Zhou, W.; Stefanovich, S.Y.; Mosunov, A.V.; Dolgikh, V.A. Ferroelectric properties and crystal structure of the layered intergrowth phase Bi3Pb2Nb2O11Cl. Chem. Mater. 2001, 13, 4731–4737. [Google Scholar] [CrossRef]
- Liu, S.; Miiller, W.; Liu, Y.; Avdeev, M.; Ling, C.D. Sillen–Aurivillius intergrowth phases as templates for naturally layered multiferroics. Chem. Mater. 2012, 24, 3932–3942. [Google Scholar] [CrossRef]

| Samples | Soak Temperature (°C) | Soak Time (h) | Cooling Rate (°C/h) | Slow Cooling Temperature (°C) |
|---|---|---|---|---|
| A | 1150 | 5 | 10 | 25 |
| B | 1075 | 5 | 2 | 875 |
| C | 1075 | 5 | 5 | 875 |
| D | 1075 | 5 | 10 | 875 |
| E | 1075 | 10 | 10 | 875 |
| F | 1075 | 20 | 10 | 875 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, C.; Kato, D.; Takeiri, F.; Fujii, K.; Yashima, M.; Nishiwaki, E.; Fujii, Y.; Koreeda, A.; Tassel, C.; Abe, R.; et al. Single Crystal Growth of Sillén–Aurivillius Perovskite Oxyhalides Bi4NbO8X (X = Cl, Br). Inorganics 2018, 6, 41. https://doi.org/10.3390/inorganics6020041
Zhong C, Kato D, Takeiri F, Fujii K, Yashima M, Nishiwaki E, Fujii Y, Koreeda A, Tassel C, Abe R, et al. Single Crystal Growth of Sillén–Aurivillius Perovskite Oxyhalides Bi4NbO8X (X = Cl, Br). Inorganics. 2018; 6(2):41. https://doi.org/10.3390/inorganics6020041
Chicago/Turabian StyleZhong, Chengchao, Daichi Kato, Fumitaka Takeiri, Kotaro Fujii, Masatomo Yashima, Emily Nishiwaki, Yasuhiro Fujii, Akitoshi Koreeda, Cédric Tassel, Ryu Abe, and et al. 2018. "Single Crystal Growth of Sillén–Aurivillius Perovskite Oxyhalides Bi4NbO8X (X = Cl, Br)" Inorganics 6, no. 2: 41. https://doi.org/10.3390/inorganics6020041
APA StyleZhong, C., Kato, D., Takeiri, F., Fujii, K., Yashima, M., Nishiwaki, E., Fujii, Y., Koreeda, A., Tassel, C., Abe, R., & Kageyama, H. (2018). Single Crystal Growth of Sillén–Aurivillius Perovskite Oxyhalides Bi4NbO8X (X = Cl, Br). Inorganics, 6(2), 41. https://doi.org/10.3390/inorganics6020041






