Activity and Stability of the Tetramanganese Polyanion [Mn4(H2O)2(PW9O34)2]10— during Electrocatalytic Water Oxidation
Abstract
:1. Introduction
2. Results and Discussion
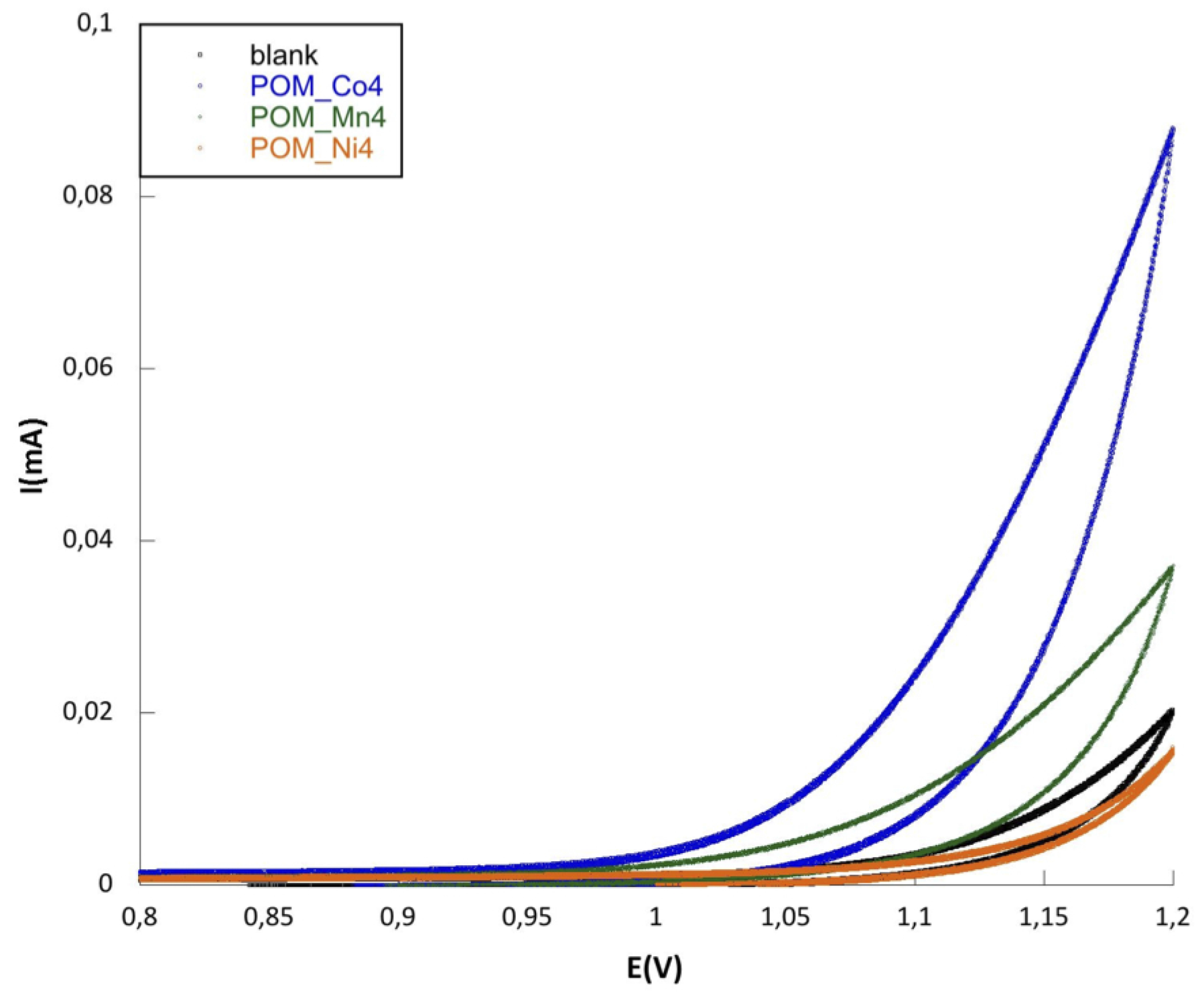
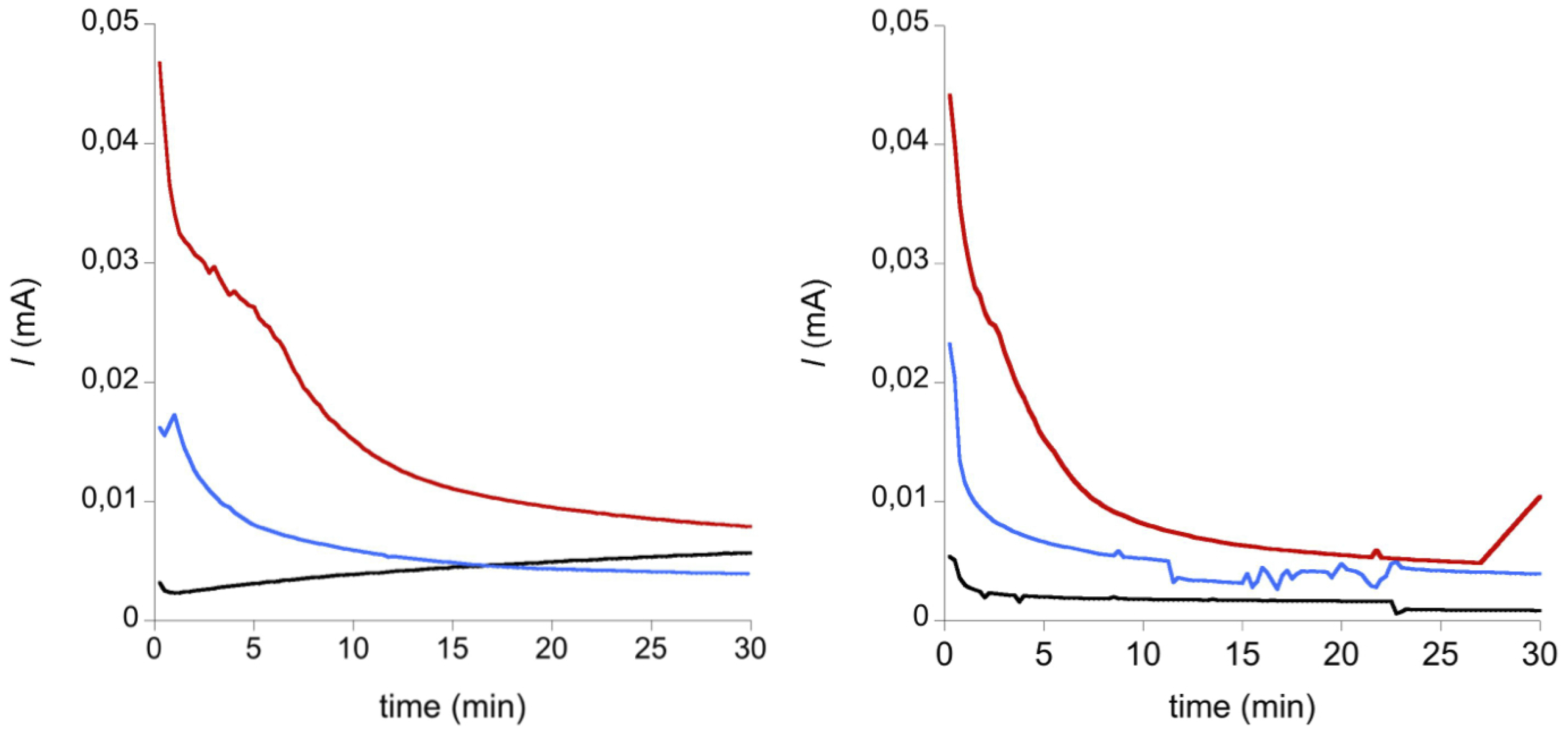
3. Experimental Section
3.1. Materials and Instrumentation
3.2. Synthesis and Characterization

3.3. Electrochemistry
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Spiccia, L.; Singh, A. Water oxidation catalysts based on abundant 1st row transition metals. Coord. Chem. Rev. 2013, 257, 2607–2622. [Google Scholar]
- Dau, H.; Limberg, C.; Reier, T.; Risch, M.; Roggan, S.; Strasser, P. The mechanism of water oxidation: From electrolysis via homogeneous to biological catalysis. ChemCatChem 2010, 2, 724–761. [Google Scholar] [CrossRef]
- Galan-Mascaros, J.R. Water oxidation at electrodes modified with earth-abundant transition-metal catalysts. ChemElectroChem 2015, 2, 37–50. [Google Scholar] [CrossRef]
- McKone, J.R.; Lewis, N.S.; Gray, H.B. Will solar-driven water-splitting devices see the light of day? Chem. Mater. 2014, 26, 407–414. [Google Scholar] [CrossRef]
- Barber, J. Photosynthetic energy conversion: Natural and artificial. Chem. Soc. Rev. 2009, 38, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Geletii, Y.V.; Zhao, C.; Vickers, J.W.; Zhu, G.; Luo, Z.; Song, J.; Lian, T.; Musaev, D.G.; Hill, C.L. Polyoxometalate water oxidation catalysts and the production of green fuel. Chem. Soc. Rev. 2012, 41, 7572–7589. [Google Scholar] [CrossRef] [PubMed]
- Streb, C. New trends in polyoxometalate photoredox chemistry: From photosensitisation to water oxidation catalysis. Dalton Trans. 2012, 41, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.L. Progress and challenges in polyoxometalate-based catalysis and catalytic materials chemistry. J. Mol. Catal. 2007, 262, 2–6. [Google Scholar] [CrossRef]
- Geletii, Y.V.; Botar, B.; Kögerler, P.; Hillesheim, D.A.; Musaev, D.G.; Hill, C.L. An all-inorganic, stable, and highly active tetraruthenium homogeneous catalyst for water oxidation. Angew. Chem. Int. Ed. 2008, 47, 3896–3899. [Google Scholar] [CrossRef] [PubMed]
- Sartorel, A.; Miro, P.; Salvadori, E.; Romain, S.; Carraro, M.; Scorrano, G.; di Valentin, M.; Llobet, A.; Bo, C.; Bonchio, M. Water oxidation at a tetraruthenate core stabilized by polyoxometalate ligands: experimental and computational evidence to trace the competent intermediates. J. Am. Chem. Soc. 2009, 131, 16051–16052. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.X.; Liu, Y.; Lee, C.Y.; Bond, A.M.; Zhang, J.; Geletii, Y.V.; Hill, C.L. Graphene-supported [Ru4O4(OH)2(H2O)4(γ-SiW10O36)2]10– for highly efficient electrocatalytic water oxidation. Energy Environ. Sci. 2013, 6, 2654–2663. [Google Scholar] [CrossRef]
- Orlandi, M.; Argazzi, R.; Sartorel, A.; Carraro, M.; Scorrano, G.; Bonchio, M.; Scandola, F. Ruthenium polyoxometalate water splitting catalyst: Very fast hole scavenging from photogenerated oxidants. Chem. Commun. 2010, 46, 3152–3154. [Google Scholar] [CrossRef] [PubMed]
- Toma, F.M.; Sartorel, A.; Iurlo, M.; Carraro, M.; Parisse, P.; Maccato, C.; Rapino, S.; Rodriguez Gonzalez, B.; Amenitsch, H.; da Ros, T.; et al. Efficient water oxidation at carbon nanotube-polyoxometalate electrocatalytic interfaces. Nat. Chem. 2010, 2, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Besson, C.; Huang, Z.; Geletii, Y.V.; Lense, S.; Hardcastle, K.I.; Musaev, D.G.; Lian, T.; Proust, A.; Hill, C.L. Cs9)[(γ-PW10O36)2Ru4O5(OH)(H2O)4)], a new all-inorganic, soluble catalyst for the efficient visible-light-driven oxidation of water. Chem. Commun. 2010, 46, 2784–2786. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Tan, J.M.; Besson, C.; Geletii, Y.V.; Musaev, D.G.; Kuznetsov, A.E.; Luo, Z.; Hardcastle, K.I.; Hill, C.L. A fast soluble carbon-free molecular water oxidation catalyst based on abundant metals. Science 2010, 328, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Luo, Z.; Geletii, Y.V.; Vickers, J.W.; Yin, Q.; Wu, D.; Hou, Y.; Ding, Y.; Song, J.; Musaev, D.G.; et al. Efficient light-driven carbon-free cobalt-based molecular catalyst for water oxidation. J. Am. Chem. Soc. 2011, 133, 2068–2071. [Google Scholar] [CrossRef] [PubMed]
- Stracke, J.J.; Finke, R.G. Electrocatalytic water oxidation beginning with the cobalt polyoxometalate [Co4)(H2O)2(PW9O34)2]10–: Identification of Heterogeneous CoOx as the dominant catalyst. J. Am. Chem. Soc. 2011, 133, 14872–14875. [Google Scholar] [CrossRef] [PubMed]
- Stracke, J.J.; Finke, R.G. Water oxidation catalysis beginning with 2.5 μM [Co4(H2O)2(PW9O34)2]10–: Investigation of the true electrochemically driven catalyst at ≥ 600 mV overpotential at a glassy carbon electrode. ACS Catal. 2013, 3, 1209–1219. [Google Scholar] [CrossRef]
- Natali, M.; Berardi, S.; Sartorel, A.; Bonchio, M.; Campagna, S.; Scandola, F. Is [Co4(H2O)2(α-PW9O34)2]10– a genuine molecular catalyst in photochemical water oxidation? Answers from time-resolved hole scavenging experiments. Chem. Commun. 2012, 48, 8808–8810. [Google Scholar] [CrossRef] [PubMed]
- Vickers, J.W.; Lv, H.; Sumliner, J.M.; Zhu, G.; Luo, Z.; Musaev, D.G.; Geletii, Y.V.; Hill, C.L. Differentiating homogeneous and heterogeneous water oxidation catalysis: Confirmation that [Co4(H2O)2(α-PW9O34)2]10– is a molecular water oxidation catalyst. J. Am. Chem. Soc. 2013, 135, 14110–14118. [Google Scholar] [CrossRef] [PubMed]
- Schiwon, R.; Klingan, K.; Dau, H.; Limberg, C. Shining light on integrity of a tetracobalt-polyoxometalate water oxidation catalyst by X-ray spectroscopy before and after catalysis. Chem. Commun. 2013, 50. [Google Scholar] [CrossRef] [PubMed]
- Goberna-Ferron, S.; Vigara, L.; Soriano-López, J.; Galán-Mascarós, J.R. Identification of a nonanuclear polyoxometalate cluster as a homogeneous catalyst for water oxidation. Inorg. Chem. 2012, 51, 11707–11715. [Google Scholar] [CrossRef] [PubMed]
- Stracke, J.J.; Finke, R.G. Water oxidation catalysis beginning with Co4(H2O)2(PW9O34 when driven by the chemical oxidant ruthenium(III)tris(2,2-bipyridine): Stoichiometry, kinetic, and mechanistic studies en route to identifying the true catalyst. ACS Catal. 2014, 4, 79–89. [Google Scholar] [CrossRef]
- Song, F.; Ding, Y.; Ma, B.; Wang, C.; Wang, Q.; Du, X.; Fua, S.; Song, J. K7[(CoCoII)CoIII(H2O)W11O39]: a molecular mixed-valence Keggin polyoxometalate catalyst of high stability and efficiency for visible light-driven water oxidation. Energy Environ. Sci. 2013, 6, 1170–1184. [Google Scholar] [CrossRef]
- Zhu, G.; Glass, E.N.; Zhao, C.; Lv, H.; Vickers, J.W.; Geletii, Y.V.; Musaev, D.G.; Song, J.; Hill, C.L. A nickel containing polyoxometalate water oxidation catalyst. Dalton Trans. 2012, 41, 13043–13049. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Ma, H.; Geletii, Y.V.; Hardcastle, K.I.; Hill, C.L. Structurally characterized iridium(III)-containing polytungstate and catalytic water oxidation activity. Inorg. Chem. 2009, 48, 5596–5598. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Geletii, Y.V.; P., K.; Schilder, H.; Song, J.; Lense, S.; Zhao, C.; I., H.K.; Musaev, D.G.; Hill, C.L. Water oxidation catalyzed by a new tetracobalt-substituted polyoxometalate complex: [{Co4(μ-OH)(H2O)3}(Si2W19O70)]11–. Dalton Trans. 2012, 41, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Song, J.; Geletii, Y.V.; Vickers, J.W.; Sumliner, J.M.; Musaev, D.G.; Kögerler, P.; Zhuk, P.F.; Bacsa, J.; Zhu, G.; et al. An exceptionally fast homogeneous carbon-free cobalt-based water oxidation catalyst. J. Am. Chem. Soc. 2014, 136, 9268–9271. [Google Scholar] [CrossRef] [PubMed]
- Suga, M.; Akita, F.; Hirata, K.; Ueno, G.; Murakami, H.; Nakajima, Y.; Shimizu, T.; Yamashita, K.; Yamamoto, M.; Ago, H.; et al. Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses. Nature 2015, 517, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.; Alonso-Mori, R.; Hellmich, J.; Tran, R.; Hattne, J.; Laksmono, H.; Glöckner, C.; Echols, N.; Sierra, R.G.; Sellberg, J.; et al. Room temperature femtosecond X-ray diffraction of photosystem II microcrystals. Proc. Natl. Acad. Sci. USA 2012, 109, 9721–9726. [Google Scholar] [CrossRef] [PubMed]
- Umena, Y.; Kawakami, K.; Shen, J.R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, K.N.; Iverson, T.M.; Maghlaoui, K.; Barber, J.; Iwata, S. Architecture of the photosynthetic oxygen-evolving center. Science 2004, 303, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Al-Oweini, R.; Sartorel, A.; Bassil, B.S.; Natali, M.; Berardi, S.; Scandola, F.; Kortz, U.; Bonchio, M. Photocatalytic water oxidation by a mixed-valent MnIVO3 manganese oxo core that mimics the natural oxygen-evolving center. Angew. Chem. Int. Ed. 2014, 53, 11182–11185. [Google Scholar] [CrossRef] [PubMed]
- Zaharieva, I.; Chernev, P.; Risch, M.; Klingan, K.; Kohlhoff, M.; Fischer, A.; Dau, H. Electrosynthesis, functional, and structural characterization of a water-oxidizing manganese oxide. Energy Environ. Sci. 2012, 5, 7081–7089. [Google Scholar] [CrossRef]
- Wiechen, M.; Najafpour, M.M.; Allakhverdiev, S.I.; Spiccia, L. Water oxidation catalysis by manganese oxides: Learning from evolution. Energy Environ. Sci. 2014, 7, 2203–2212. [Google Scholar] [CrossRef]
- Kuo, C.H.; Mosa, I.M.; Poyraz, A.S.; Biswas, S.; El-Sawy, A.M.; Song, W.; Luo, Z.; Chen, S.Y.; Rusling, J.F.; He, J.; et al. Robust mesoporous manganese oxide catalysts for water oxidation. ACS Catal. 2015, 5, 1693–1699. [Google Scholar] [CrossRef]
- Najafpour, M.M.; Moghaddam, A.N.; Dau, H.; Zaharieva, I. Fragments of layered manganese oxide are the real water oxidation catalyst after transformation of molecular precursor on clay. J. Am. Chem. Soc. 2014, 136, 7245–7248. [Google Scholar] [CrossRef] [PubMed]
- Zaharieva, I.; Chernev, P.; Risch, M.; Klingan, K.; Kohlhoff, M.; Fischer, A.; Dau, H. Electrosynthesis, functional, and structural characterization of a water-oxidizing manganese oxide. Energy Environ. Sci. 2012, 5, 7081–7089. [Google Scholar] [CrossRef]
- Soriano-López, J.; Goberna-Ferron, S.; Vigara, L.; Carbó, J.J.; Poblet, J.M.; Galan-Mascaros, J.R. Cobalt polyoxometalates as heterogeneous water oxidation catalysts. Inorg. Chem. 2013, 52, 4753–4755. [Google Scholar] [CrossRef] [PubMed]
- Weakley, T.J.R.; Evans, H.T.; Showell, J.S.; Tourne, G.F.; Tourne, C.M. 18-Tungstotetracobaltato(II)diphosphate and related anions. A novel structural class of heteropolyanions. Chem. Commun. 1973, 139–140. [Google Scholar] [CrossRef]
- Gómez Garcia, C.; Coronado, E.; Gomez-Romero, P.; Casan-Pastor, N. A tetranuclear rhomblike cluster of manganese(II). Crystal structure and magnetic properties of the heteropoly complex K10[Mn4(H2O)2(PW9O34)2] 20H2O. Inorg. Chem. 1993, 32, 3378–3381. [Google Scholar] [CrossRef]
- Hocking, R.K.; Brimblecombe, R.; Chang, L.Y.; Singh, A.; Cheah, M.H.; Glover, C.; Casey, W.H.; Spiccia, L. Water-oxidation catalysis by manganese in a geochemical-like cycle. Nat. Chem. 2011, 3, 461–466. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goberna-Ferrón, S.; Soriano-López, J.; Galán-Mascarós, J.R. Activity and Stability of the Tetramanganese Polyanion [Mn4(H2O)2(PW9O34)2]10— during Electrocatalytic Water Oxidation. Inorganics 2015, 3, 332-340. https://doi.org/10.3390/inorganics3030332
Goberna-Ferrón S, Soriano-López J, Galán-Mascarós JR. Activity and Stability of the Tetramanganese Polyanion [Mn4(H2O)2(PW9O34)2]10— during Electrocatalytic Water Oxidation. Inorganics. 2015; 3(3):332-340. https://doi.org/10.3390/inorganics3030332
Chicago/Turabian StyleGoberna-Ferrón, Sara, Joaquín Soriano-López, and José Ramón Galán-Mascarós. 2015. "Activity and Stability of the Tetramanganese Polyanion [Mn4(H2O)2(PW9O34)2]10— during Electrocatalytic Water Oxidation" Inorganics 3, no. 3: 332-340. https://doi.org/10.3390/inorganics3030332






