Fully Oxidized and Mixed-Valent Polyoxomolybdates Structured by Bisphosphonates with Pendant Pyridine Groups: Synthesis, Structure and Photochromic Properties
Abstract
:1. Introduction
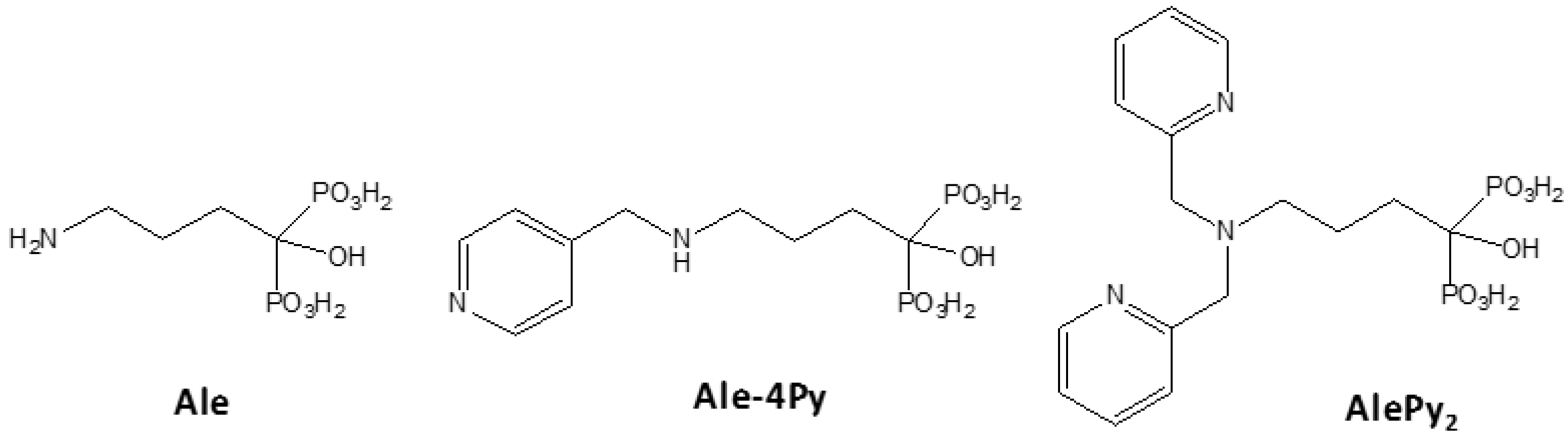
2. Results and Discussion
2.1. Synthesis and Characterization
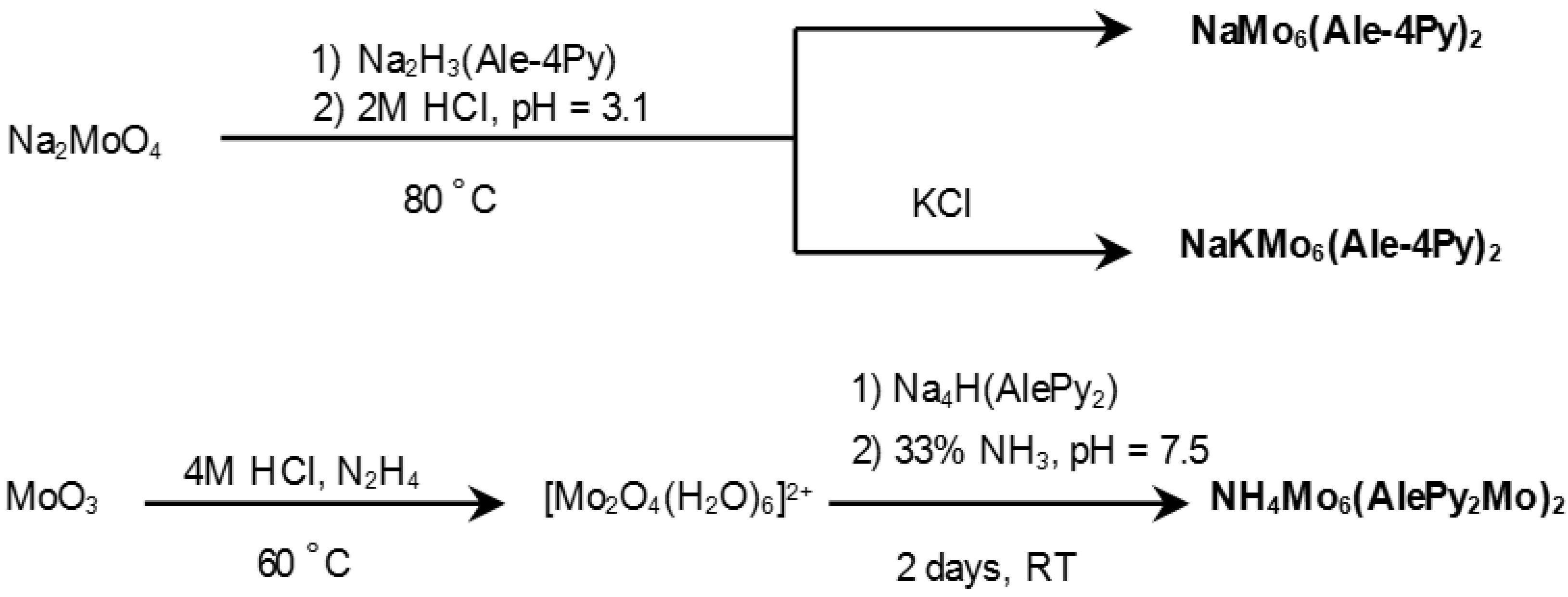

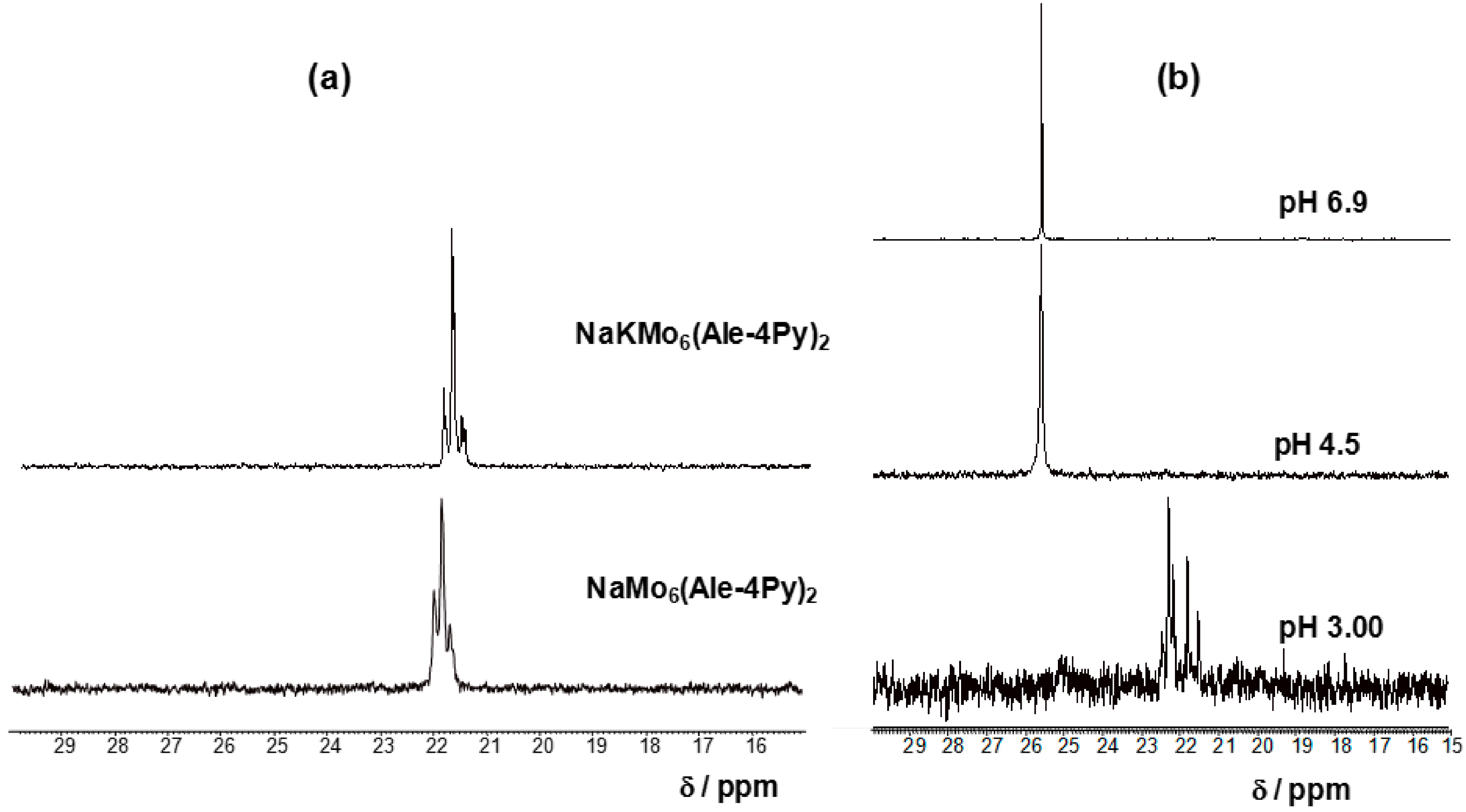
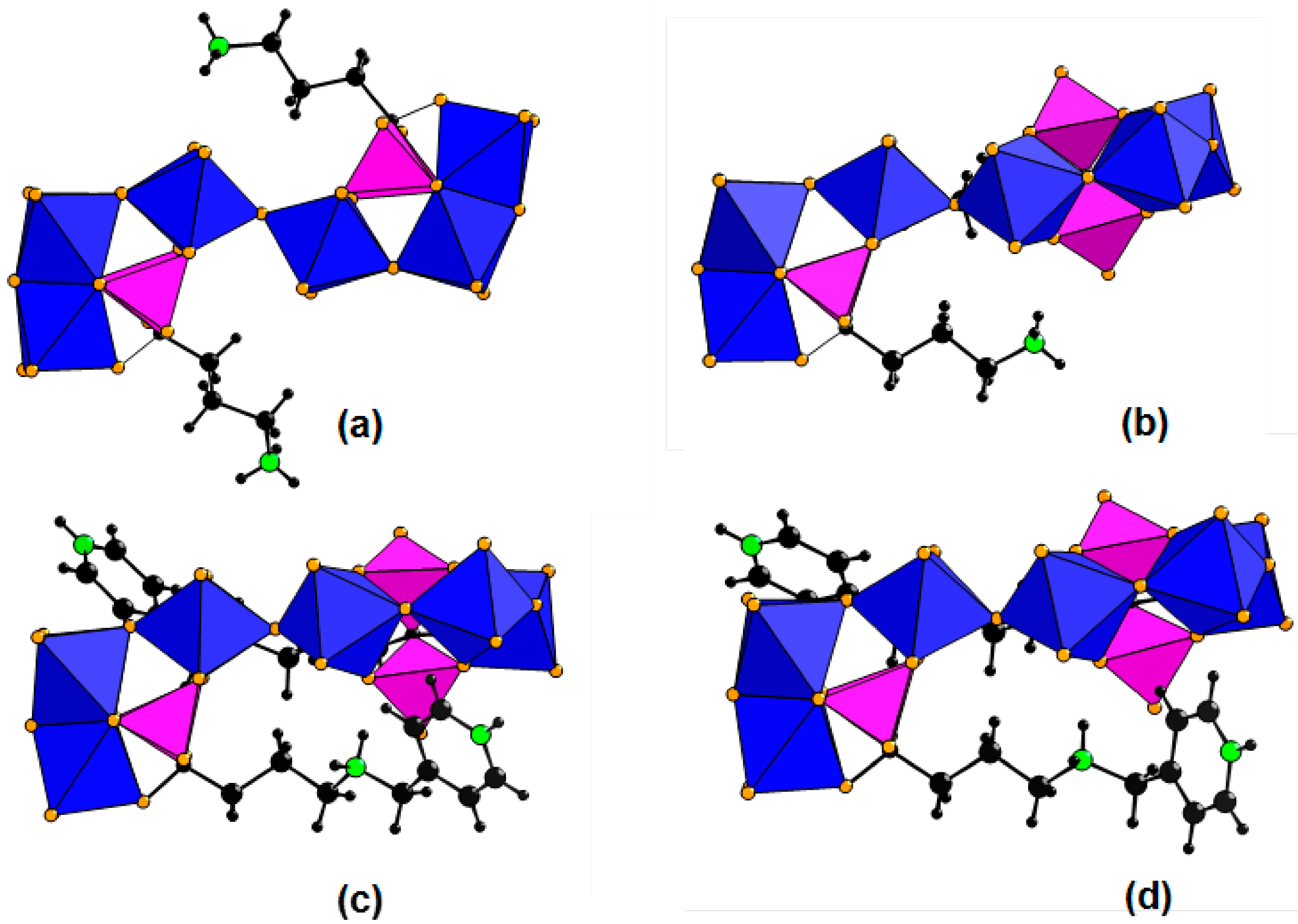
2.2. Structures


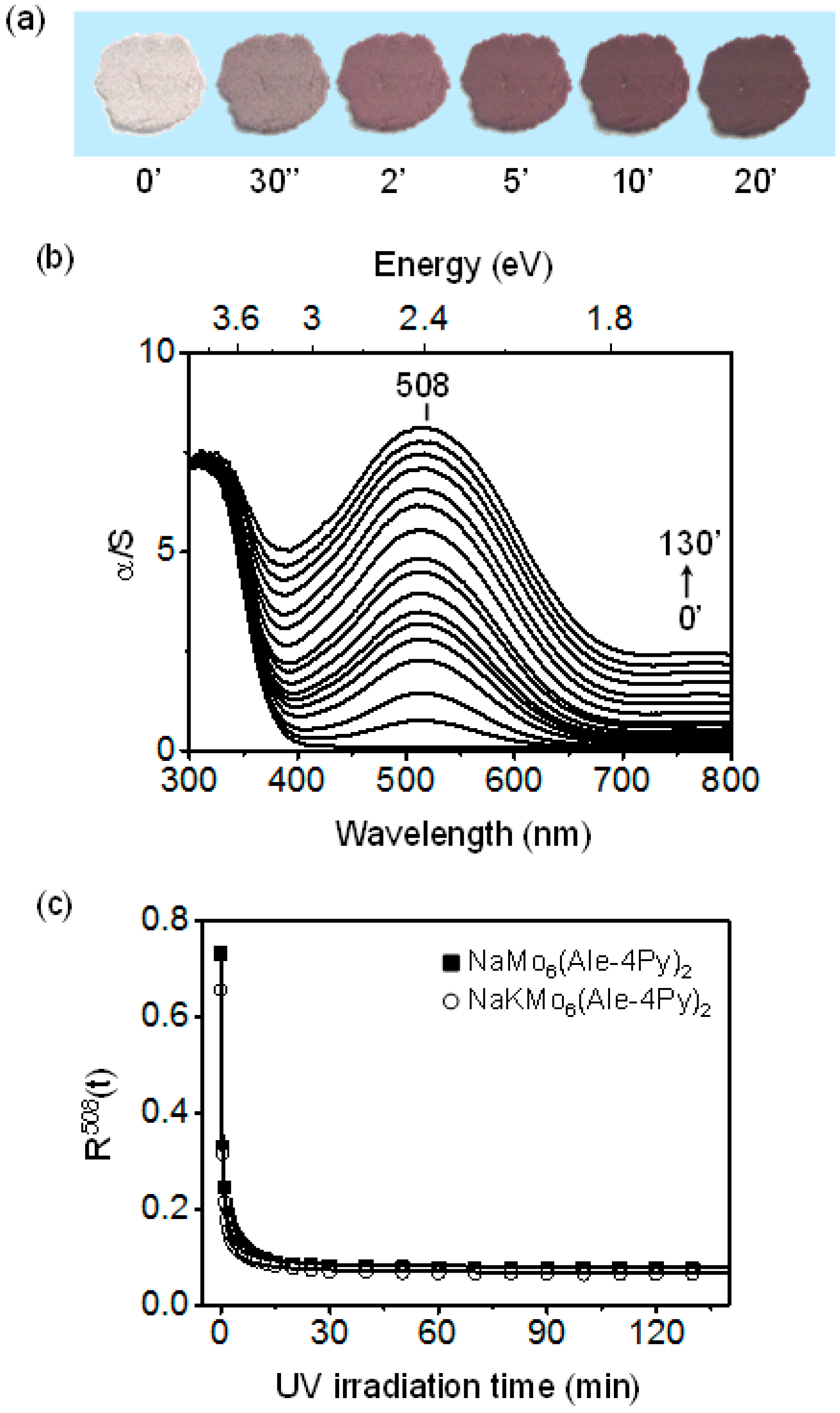
2.3. Solid-State Photochromic Properties
| Compound | λmax (nm) a | t1/2 (min) b | Ref. |
|---|---|---|---|
| NaMo6(Ale-4Py)2 | 508 | 0.37 | this work |
| NaKMo6(Ale-4Py)2 | 508 | 0.37 | this work |
| Mo6(Ale)2 | 508 | 2.87 | [18] |
| Mo6(Ale-1Ca)2 | 508 | 31.25 | [18] |
| Mo6(Ale-1Cb)2 | 508 | 4.78 | [18] |
| Mo6(Ale-2C)2 | 508 | 12.82 | [18] |
| Mo6(1C-Ale)2 Mo6(Ris)2 | 508 | 5.71 | [18] |
| Mo6(Ris)2 | 508 | 14.89 | [26] |
| Mo12(Ale)4 | 464 | 8.20 | [18] |
| Mo12(Ale-1Ca)4 | 464 | 5.44 | [18] |
| Mo12(Ale-2C)4 | 464 | 51.81 | [18] |
| (H2DABCO)2(NH4)2[Mo8O27] | 525 | 37.0 | [6] |
| (H2DABCO)2(H2pipz)[Mo8O27] | 525 | 5.6 | [6] |
| (H2pipz)3[Mo8O27] | 525 | 7.8 | [6] |
| (H2DABCO)2(HDMA)0.5Na0.75[Mo8O27] | 525 | 0.8 | [6] |
3. Experimental Section
3.1. General
3.2. Synthesis of Na2[(HO3P)2(OH)C(C3H6NHCH2(C5H4N)] (Na2H3Ale-4Py)
3.3. Synthesis of Na4[(MoVI3O8)2(O)(O3PC(O)(C3H6NH2CH2C5H4NH)PO3)2].14H2O (NaMo6(Ale-4Py))
3.4. Synthesis of Na0.5K3.5[(MoVI3O8)2(O)(O3PC(O)(C3H6NH2CH2C5H4NH)PO3)2].13H2O (NaKMo6(Ale-4Py)2)
3.5. Synthesis of (NH4)8[(MoV2O4)(MoVI2O6)2{O3PC(O)(C3H6N(CH2C5H4N)2(MoVIO3))PO3}2]·20H2O (NH4Mo6(AlePy2Mo)2)
3.6. X-ray Crystallography
| C20H58N4Mo6Na4O45P4 | C20H56N4K3.5Mo6Na0.5O44P4 |
| 1866.16 | 1904.53 |
| triclinic | triclinic |
| P-1 | P-1 |
| 9.0240(3) | 17.9501(5) |
| 19.4282(6) | 19.4745(4) |
| 21.1568(7) | 19.8487(5) |
| 103.992(2) | 76.188(1) |
| 95.5612(2) | 69.590(1) |
| 103.407(1) | 76.669(1) |
| 3454.8(2) | 6231.6(3) |
| 2 | 4 |
| 1.813 | 2.028 |
| 1.273 | 1.616 |
| 12208/865 | 36431/1558 |
| 0.0301 | 0.0429 |
| 1.023 | 1.070 |
| 0.0710 | 0.0568 |
| 0.1960 | 0.1649 |
3.7. Physical Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clemente-Juan, J.M.; Coronado, E.; Gaita-Ariño, A. Magnetic Polyoxometalates: from Molecular Magnetism to Molecular Spintronics and Quantum Computing. Chem. Soc. Rev. 2012, 41, 7464–7478. [Google Scholar] [CrossRef] [PubMed]
- Hasenknopf, B. Polyoxometalates: Introduction to a Class of Inorganic Compounds and their Biomedical Applications. Front. Biosci. 2005, 10, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-F.; Tsunashima, R. Recent Advances on Polyoxometalate-based Molecular and Composite Materials. Chem. Soc. Rev. 2012, 41, 7384–7402. [Google Scholar] [CrossRef] [PubMed]
- Kawata, S.; Kawata, Y. Three-Dimensional Optical Data Storage Using Photochromic Materials. Chem. Rev. 2000, 100, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Yamase, T. Photo- and Electrochromism of Polyoxometalates and Related Materials. Chem. Rev. 1998, 98, 307–325. [Google Scholar] [CrossRef] [PubMed]
- Dessapt, R.; Collet, M.; Coué, V.; Bujoli-Doeuff, M.; Jobic, S.; Lee, C.; Whangbo, M.-H. Kinetics of Coloration in Photochromic Organoammonium Polyoxomolybdates. Inorg. Chem. 2009, 48, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Dolbecq, A.; Dumas, E.; Mayer, C.R.; Mialane, P. Hybrid Organic-Inorganic Polyoxometalate Compounds : From Structural Diversity to Applications. Chem. Rev. 2010, 110, 6009. [Google Scholar] [CrossRef] [PubMed]
- Proust, A.; Matt, B.; Villanneau, R.; Guillemot, G.; Gouzerh, P.; Izzet, G. Functionalization and Post-functionalization : a Step Towards Polyoxometalate-based Materials. Chem. Soc. Rev. 2012, 41, 7605–7622. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, C.; Luzio, A.; Pradeep, C.P.; Miras, H.N.; Rosnes, M.H.; Song, Y.-F.; Long, D.-L.; Cronin, L.; Pignataro, B. Programmable Surface Architectures Derives from Hybrid Polyoxometalate-Based Clusters. J. Phys. Chem. C 2011, 115, 4446–4455. [Google Scholar] [CrossRef]
- Errington, R.J.; Petkar, S.S.; Horrocks, B.R.; Houlton, A.; Lie, L.H.; Patole, S.N. Covalent Immobilization of a TiW5 Polyoxometalate on Derivatized Silicon Surfaces. Angew. Chem. Int. Ed. 2005, 44, 1254–1257. [Google Scholar] [CrossRef] [PubMed]
- Geisberger, G.; Gyenge, E.B.; Hinger, D.; Bösiger, P.; Maake, C.; Patzke, G.R. Synthesis, Characterization and Bioimaging of Fluorescent Labeled Polyoxometalates. Dalton Trans. 2013, 42, 9914–9920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Huth, I.; Chamoreau, L.M.; Hasenknopf, B.; Lacôte, E.; Thorimbert, S.; Malacria, M. Insertion of Amides into a Polyoxometalate. Angew. Chem. Int. Ed. 2009, 48, 2035. [Google Scholar] [CrossRef] [PubMed]
- Du, D.-Y.; Qin, J.-S.; Li, S.-L.; Su, Z.-M.; Lan, Y.-Q. Recent Advances in Porous Polyoxometalate-based Metal-Organic Framework Materials. Chem. Soc. Rev. 2014, 43, 4615–4632. [Google Scholar] [CrossRef] [PubMed]
- Dolbecq, A.; Mialane, P.; Sécheresse, F.; Keita, B.; Nadjo, L. Functionalized Polyoxometalates with covalently linked bisphosphonate, N-donor or Carboxylate Ligands: From Electrocatalytic to Optical Properties. Chem. Commun. 2012, 48, 8299–8316. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Bassil, B.S.; Röschenthaler, G.-V.; Kortz, U. Diphosphates and Diphosphonates in Polyoxometalate Chemistry. Chem. Soc. Rev. 2012, 41, 7590–7604. [Google Scholar] [CrossRef] [PubMed]
- Compain, J.-D.; Mialane, P.; Marrot, J.; Sécheresse, F.; Zhu, W.; Oldfield, E.; Dolbecq, A. Tetra- to Dodecanuclear Oxomolybdate Complexes with Functionalized Bisphosphonate Ligands: Activity in Killing Tumor Cells. Chem. Eur. J. 2010, 16, 13741–13748. [Google Scholar] [CrossRef] [PubMed]
- El Moll, H.; Zhu, W.; Oldfield, E.; Rodriguez-Albelo, L.M.; Mialane, P.; Marrot, J.; Vila, N.; Mbomekallé, I.-M.; Rivière, E.; Duboc, C.; Dolbecq, A. Polyoxometalates Functionalized by Bisphosphonate Ligands: Synthesis, Structural, Magnetic, and Spectroscopic Characterizations and Activity on Tumor Cell Lines. Inorg. Chem. 2012, 51, 7921–7931. [Google Scholar] [CrossRef] [PubMed]
- El Moll, H.; Dolbecq, A.; Mbomekallé, I.-M.; Marrot, J.; Deniard, P.; Dessapt, R.; Mialane, P. Tuning the Photochromic Properties of Molybdenum Bisphosphonate Polyoxometalates. Inorg. Chem. 2012, 51, 2291–2302. [Google Scholar] [CrossRef] [PubMed]
- Dolbecq, A.; Compain, J.-D.; Mialane, P.; Marrot, J.; Sécheresse, F.; Keita, B.; Brudna Holzle, L.R.; Miserque, F.; Nadjo, L. Hexa- and Dodecanuclear Polyoxomolybdate Cyclic Compounds: Application toward the Facile Synthesis of Nanoparticles and Film Electrodeposition. Chem. Eur. J. 2009, 15, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Raad, F.S.; Vankova, N.; Bassil, B.S.; Heine, T.; Kortz, U. Polyoxomolybdodiphosphonates : Examples Incorporating Ethylenepyridines. Inorg. Chem. 2011, 50, 11667–11675. [Google Scholar] [CrossRef] [PubMed]
- Modec, B.; Brenčič, J.V. From Small {Mo2O4}2+ Aggregates to Infinite Solids. J. Clust. Sci. 2002, 13, 279–302. [Google Scholar] [CrossRef]
- Pope, M.T.; Müller, A. Polyoxometalate Chemistry: An Old Field with New Dimensions in Several Disciplines. Angew. Chem. Int. Ed. Engl. 1991, 30, 34–48. [Google Scholar] [CrossRef]
- Sergienko, V.S. Structural Chemistry of 1-Hydroxyethylidenediphosphonic Acid Complexes. Russ. J. Coord. Chem. 2001, 27, 681–710. [Google Scholar] [CrossRef]
- Tan, H.; Chen, W.; Liu, D.; Feng, X.; Li, Y.; Yan, A.; Wang, E. Two Diphosphonate-Functionalized Asymmetric Polyoxomolybdates with Catalytic Activity for Oxidation of Benzyl Alcohol to Benzaldehyde. Dalton Trans. 2011, 40, 8414–8418. [Google Scholar] [CrossRef] [PubMed]
- Coué, V.; Dessapt, R.; Bujoli-Doeuff, M.; Evain, M.; Jobic, S. Synthesis, characterizations and photochromic properties of hybrid organic-inorganic materials based on molybdate, DABCO and piperazine. Inorg. Chem. 2007, 46, 2824–2835. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, Z.; Ma, P.; Wang, J.; Niu, J. Assembly of Dimeric and Tetrameric Complexes of Polyoxomolybdobisphosphonates Built from [(Mo3O8){O3PC(O)(CH2–3-C5NH5)PO3}]2− Subunits. Cryst. Growth Des. 2013, 13, 2540–2547. [Google Scholar] [CrossRef]
- Kubícek, V.; Kotek, J.; Hermann, P.; Lukeš, I. Aminoalkylbis(phosphonates): Their Complexation Properties in Solution and in the Solid State. Eur. J. Inorg. Chem. 2007, 333–344. [Google Scholar] [CrossRef]
- De Rosales, R.T.M.; Finucane, C.; Mather, S.J.; Blower, P.J. Bifunctional Bisphosphonate Complexes for the Diagnosis and Therapy of Bone Metastases. Chem. Commun. 2009, 4847–4849. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SADABS; Program for Scaling and Correction of Area detector data; University of Göttingen: Germany, 1997. [Google Scholar]
- Blessing, R. An Empirical Correction for Absorption Anisotropy. Acta Crystallogr. 1995, A51, 33–38. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELX-TL, version 5.03. Software Package for the Crystal Structure Determination. Siemens Analytical X-ray Instrument Division: Madison, WI, USA, 1994.
- Brese, N.E.; O’Keeffe, M. Bond Valence Parameters for Solids. Acta Crystallogr. Sect. B 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. An Article on Optics of Paint Layers. Z. Techn. Physik 1931, 12, 593–601. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oms, O.; Benali, T.; Marrot, J.; Mialane, P.; Puget, M.; Serier-Brault, H.; Deniard, P.; Dessapt, R.; Dolbecq, A. Fully Oxidized and Mixed-Valent Polyoxomolybdates Structured by Bisphosphonates with Pendant Pyridine Groups: Synthesis, Structure and Photochromic Properties. Inorganics 2015, 3, 279-294. https://doi.org/10.3390/inorganics3020279
Oms O, Benali T, Marrot J, Mialane P, Puget M, Serier-Brault H, Deniard P, Dessapt R, Dolbecq A. Fully Oxidized and Mixed-Valent Polyoxomolybdates Structured by Bisphosphonates with Pendant Pyridine Groups: Synthesis, Structure and Photochromic Properties. Inorganics. 2015; 3(2):279-294. https://doi.org/10.3390/inorganics3020279
Chicago/Turabian StyleOms, Olivier, Tarik Benali, Jérome Marrot, Pierre Mialane, Marin Puget, Hélène Serier-Brault, Philippe Deniard, Rémi Dessapt, and Anne Dolbecq. 2015. "Fully Oxidized and Mixed-Valent Polyoxomolybdates Structured by Bisphosphonates with Pendant Pyridine Groups: Synthesis, Structure and Photochromic Properties" Inorganics 3, no. 2: 279-294. https://doi.org/10.3390/inorganics3020279
APA StyleOms, O., Benali, T., Marrot, J., Mialane, P., Puget, M., Serier-Brault, H., Deniard, P., Dessapt, R., & Dolbecq, A. (2015). Fully Oxidized and Mixed-Valent Polyoxomolybdates Structured by Bisphosphonates with Pendant Pyridine Groups: Synthesis, Structure and Photochromic Properties. Inorganics, 3(2), 279-294. https://doi.org/10.3390/inorganics3020279






