Theoretical Insights into the Regiodivergence in Ni-Catalyzed [2+2+2] Cycloaddition of Unsymmetric Diynes and CO2
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ni-Catalyzed Coupling of CO2 and Diyne 1 with Monodentate Ligand (PR3)
2.1.1. Oxidative Coupling of the C≡C Moiety and CO2
2.1.2. Oxidative Coupling of the Two C≡C Moieties
2.2. Ni-Catalyzed Coupling of CO2 and Diyne 1 with Bisdentate Ligand (PN-Ligand)
2.2.1. Oxidative Coupling of the C≡C Moiety and CO2
2.2.2. Oxidative Coupling of the Two C≡C Moieties
2.3. Ni-Catalyzed Coupling of CO2 and Diyne 4 with Monodentate Ligand (PR3)
2.3.1. Oxidative Coupling of the C≡C Moiety and CO2
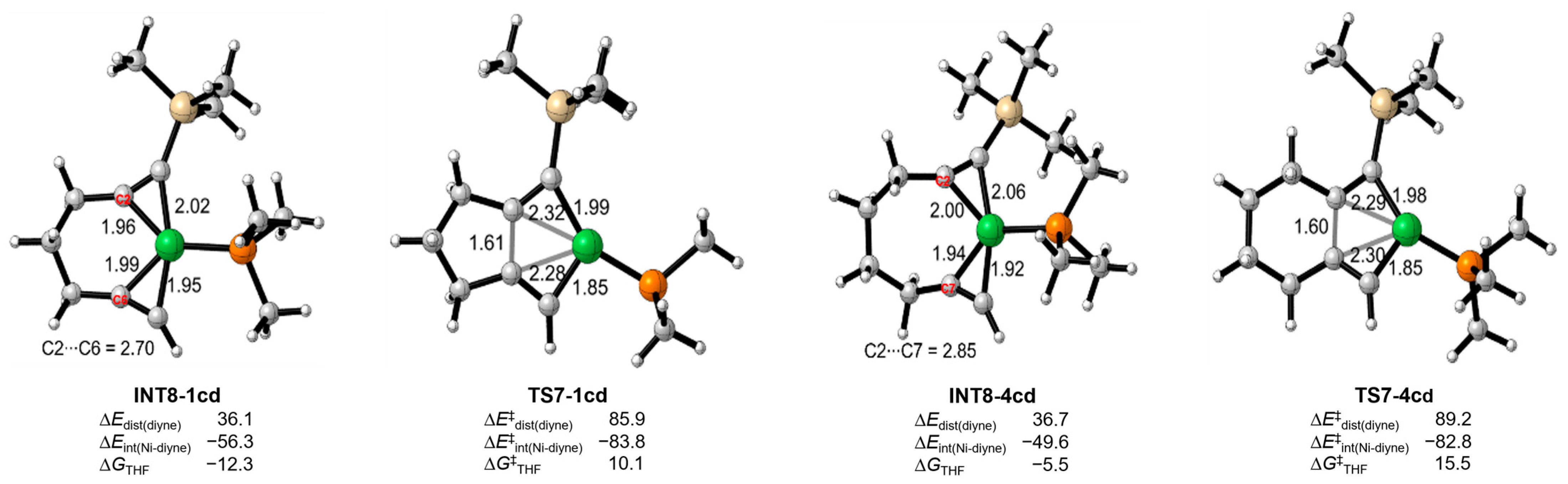
2.3.2. Oxidative Coupling of the Two C≡C Moieties
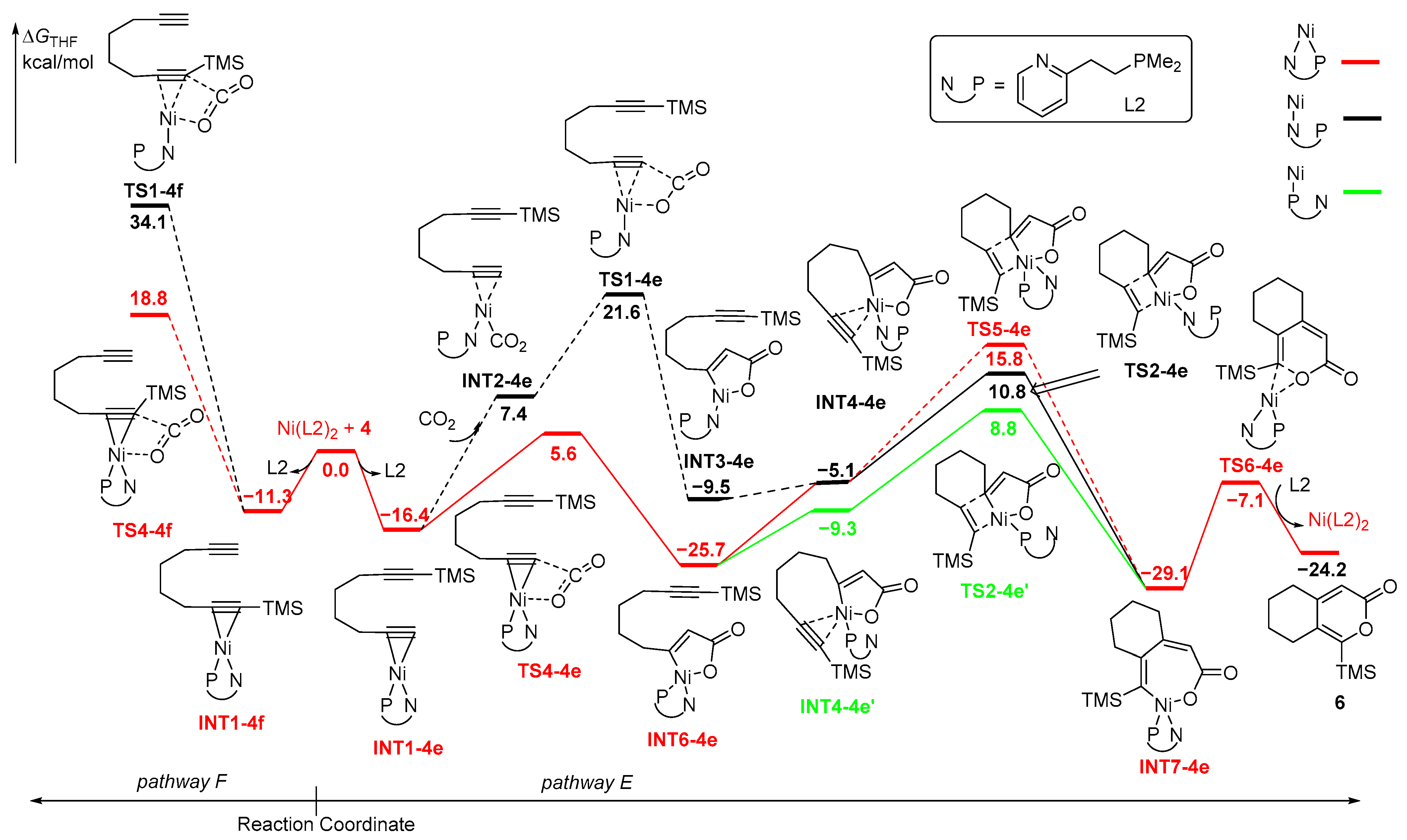
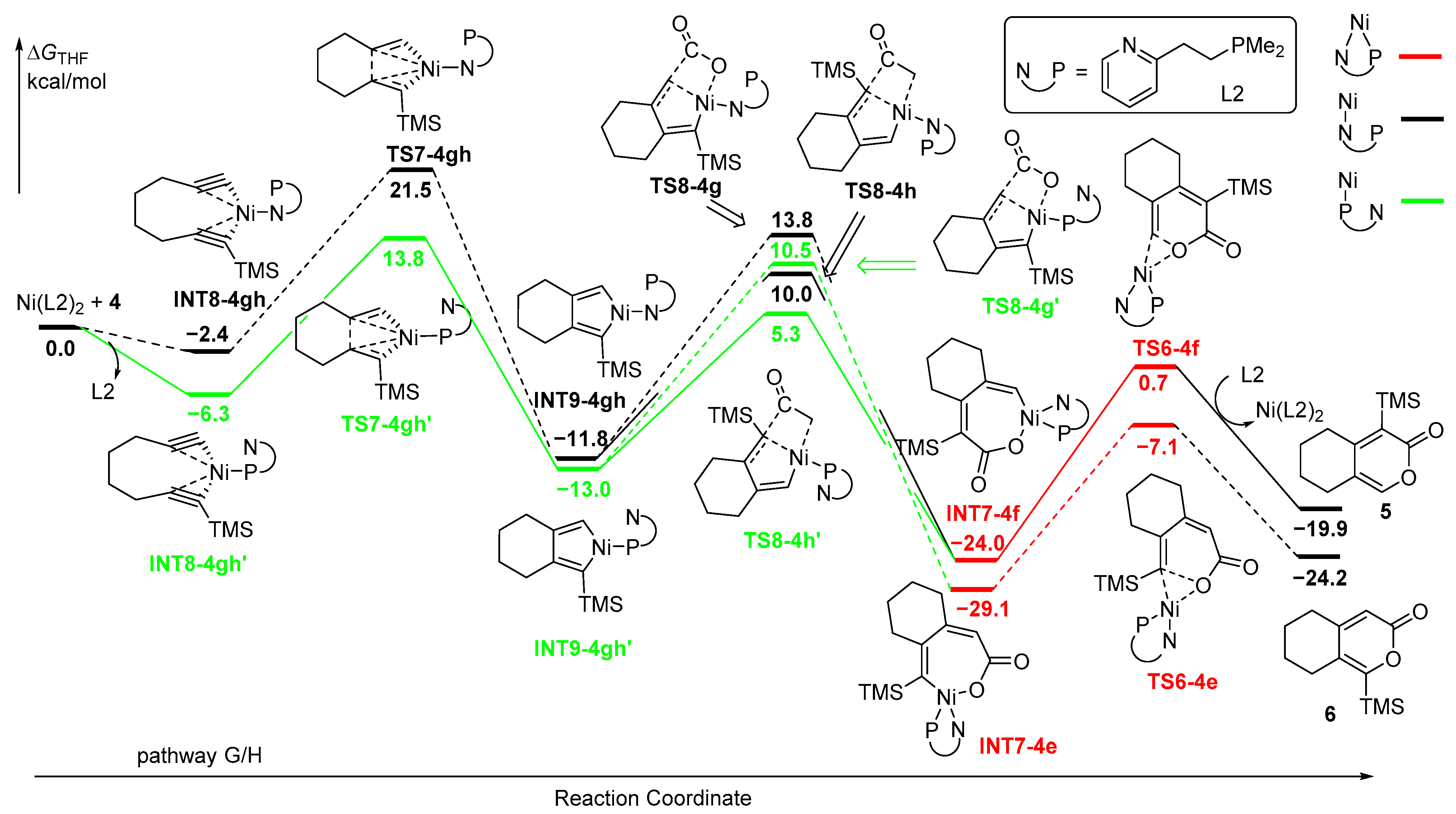
2.4. Ni-Catalyzed Coupling of CO2 and Diyne 4 with Bisdentate Ligand (PN-Ligand)
2.4.1. Oxidative Coupling of the C≡C Moiety and CO2
2.4.2. Oxidative Coupling of the Two C≡C Moieties
2.5. The Regiodivergence in Ni-Catalyzed Coupling of CO2 and Diyne 1 and 4
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakakura, T.; Choi, J.; Yasuda, H. Transformation of Carbon Dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef] [PubMed]
- Darensbourg, D.J. Making Plastics from Carbon Dioxide: Salen Metal Complexes as Catalysts for the Production of Polycarbonates from Epoxides and CO2. Chem. Rev. 2007, 107, 2388–2410. [Google Scholar] [CrossRef] [PubMed]
- Correa, A.; Martin, R. Metal-Catalyzed Carboxylation of Organometallic Reagents with Carbon Dioxide. Angew. Chem. Int. Ed. 2009, 48, 6201–6204. [Google Scholar] [CrossRef]
- Boogaerts, I.I.F.; Nolan, S.P. Direct C–H carboxylation with complexes of the coinage metals. Chem. Commun. 2011, 47, 3021–3024. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Jia, H.; Muckerman, J.T.; Fujita, E. Thermodynamics and kinetics of CO2, CO, and H+ binding to the metal centre of CO2 reduction catalysts. Chem. Soc. Rev. 2012, 41, 2036–2051. [Google Scholar] [CrossRef]
- Li, Y.N.; Ma, R.; He, L.-N.; Diao, Z.-F. Homogeneous hydrogenation of carbon dioxide to methanol. Catal. Sci. Technol. 2014, 4, 1498. [Google Scholar] [CrossRef]
- Ran, C.-K.; Chen, X.-W.; Gui, Y.-Y.; Li, J.; Son, L.; Ren, K.; Yu, D.-G. Recent advances in asymmetric synthesis with CO2. Sci. China Chem. 2020, 63, 1336–1351. [Google Scholar] [CrossRef]
- Pradhan, S.; Das, S. Recent Advances on the Carboxylations of C(sp3)–H Bonds Using CO2 as the Carbon Source. Synlett 2023, 34, 1327–1342. [Google Scholar]
- Burkart, M.D.; Hazari, N.; Tway, C.L.; Zeitler, E.L. Opportunities and challenges for Catalysis in Carbon Dioxide Utilization. ACS Catal. 2019, 9, 7937–7956. [Google Scholar] [CrossRef]
- Wang, S.; Xi, C. Recent advances in nucleophile-triggered CO2-incorporated cyclization leading to heterocycles. Chem. Soc. Rev. 2019, 48, 382–404. [Google Scholar] [CrossRef]
- Zhang, Z.; Gong, L.; Zhou, X.-Y.; Yan, S.-S.; Li, J.; Yu, D.-G. Radical-Type Difunctionalization of Alkenes with CO2. Acta Chim. Sin. 2019, 77, 783–793. [Google Scholar] [CrossRef]
- Song, L.; Jiang, Y.-X.; Gui, Y.-Y.; Zhou, X.-Y.; Yu, D.-G. CO2 = CO + [O]: Recent advances in carbonylation of C–H bonds with CO2. Chem. Commun. 2020, 56, 8355–8367. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; He, X.; Wang, N.; Li, H.-R.; He, L.-N. Photochemical and Electrochemical Carbon Dioxide Utilization with Organic Compounds. Chin. J. Chem. 2018, 36, 644–659. [Google Scholar] [CrossRef]
- Dabral, S.; Schauba, T. The Use of Carbon Dioxide (CO2) as a Building Block in Organic Synthesis from an Industrial Perspective. Adv. Synth. Catal. 2019, 361, 223–246. [Google Scholar] [CrossRef]
- Ran, C.-K.; Liao, L.-L.; Gao, T.-Y.; Gui, Y.-Y.; Yu, D.-G. Recent progress and challenges in carboxylation with CO2. Curr. Opin. Green Sust. Chem. 2021, 32, 100525. [Google Scholar] [CrossRef]
- Bertuzzi, G.; Cerveri, A.; Lombardi, L.; Bandini, M. Tandem functionalization-carboxylation reactions of πsystems with CO2. Chin. J. Chem. 2021, 39, 3116–3126. [Google Scholar] [CrossRef]
- Tortajada, A.; Juliá-Hernández, F.; Börjesson, M.; Moragas, T.; Martin, R. Transition-Metal-Catalyzed Carboxylation Reactions with Carbon Dioxide. Angew. Chem. Int. Ed. 2018, 57, 15948–15982. [Google Scholar] [CrossRef]
- Chen, Y.-G.; Xu, X.-T.; Zhang, K.; Li, Y.-Q.; Zhang, L.-P.; Fang, P.; Mei, T.-S. Transition-Metal-Catalyzed Carboxylation of Organic Halides and Their Surrogates with Carbon Dioxide. Synthesis 2018, 50, 35–48. [Google Scholar]
- Zhang, L.; Li, Z.; Takimoto, M.; Hou, Z. Carboxylation Reactions with Carbon Dioxide Using N-Heterocyclic Carbene Copper Catalysts. Chem. Rec. 2020, 20, 494–512. [Google Scholar] [CrossRef]
- Cokoja, M.; Bruckmeier, C.; Rieger, B.; Herrmann, W.A.; Kühn, F.E. Transformation of carbon dioxide with homogeneous transition-metal catalysts: A molecular solution to a global challenge? Angew. Chem. Int. Ed. 2011, 50, 8510–8557. [Google Scholar] [CrossRef]
- Tsuji, Y.; Fujihara, T. Carbon dioxide as a carbon source in organic transformation: Carbon–carbon bond forming reactions by transition-metal catalysts. Chem. Commun. 2012, 48, 9956–9964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hou, Z. N-Heterocyclic carbene (NHC)–copper-catalysed transformations of carbon dioxide. Chem. Sci. 2013, 4, 3395. [Google Scholar] [CrossRef]
- Song, J.; Liu, Q.; Liu, H.; Jiang, X. Recent Advances in Palladium-Catalyzed Carboxylation with CO2. Eur. J. Org. Chem. 2018, 6, 696–713. [Google Scholar] [CrossRef]
- Sang, R.; Hu, Y.; Razzaq, R.; Mollaert, G.; Atia, H.; Bentrup, U.; Sharif, M.; Neumann, H.; Junge, H.; Jackstell, R.; et al. A practical concept for catalytic carbonylations using carbon dioxide. Nat. Commun. 2022, 13, 4432. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Sang, R.; Sponholz, P.; Junge, H.; Beller, M. Reversible hydrogenation of carbon dioxide to formic acid using a Mn-pincer complex in the presence of lysine. Nat. Energy 2022, 7, 438. [Google Scholar] [CrossRef]
- Marx, M.; Frauendorf, H.; Spannenberg, A.; Neumann, H.; Beller, M. Revisiting Reduction of CO2 to Oxalate with First-Row Transition Metals: Irreproducibility, Ambiguous Analysis, and Conflicting Reactivity. JACS Au 2022, 2, 731. [Google Scholar] [CrossRef]
- Qiao, C.; Shi, W.; Brandolese, A.; Benet-Buchholz, J.; Escudero-Adán, E.; Kleij, A.W. A Novel Catalytic Route to Polymerizable Bicyclic Cyclic Carbonate Monomers from Carbon Dioxide. Angew. Chem. Int. Ed. 2022, 61, e202205053. [Google Scholar] [CrossRef]
- Tortajada, A.; Börjesson, M.; Martin, R. Nickel-Catalyzed Reductive Carboxylation and Amidation Reactions. Acc. Chem. Res. 2021, 54, 3941–3952. [Google Scholar] [CrossRef]
- Louie, J.; Gibby, J.E.; Farnworth, M.V.; Tekavec, T.N. Efficient Nickel-Catalyzed [2+2+2] Cycloaddition of CO2 and Diynes. J. Am. Chem. Soc. 2002, 124, 15188–15189. [Google Scholar] [CrossRef]
- Tsuda, T.; Morikawa, S.; Sumiya, R.; Saegusa, T. Nickel(0)-catalyzed cycloaddition of diynes and carbon dioxide to give bicyclic α-pyrones. J. Org. Chem. 1988, 53, 3140–3145. [Google Scholar] [CrossRef]
- Tekavec, T.N.; Arif, A.; Louie, J. Regioselectivity in Nickel(0) catalyzed cycloadditions of carbon dioxide with diynes. Tetrahedron 2004, 60, 7431–7437. [Google Scholar] [CrossRef]
- Takimoto, M.; Kawamura, M.; Mori, M. Nickel(0)-Mediated Sequential Addition of Carbon Dioxide and Aryl Aldehydes into Terminal Allenes. Org. Lett. 2003, 5, 2599–2601. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Morikawa, S.; Hasegawa, N.; Saegusa, T. Nickel(0)-catalyzed cycloaddition of silyl diynes with carbon dioxide to silyl bicyclic α-pyrones. J. Org. Chem. 1990, 55, 2978–2981. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- Williams, C.M.; Johnson, J.B.; Rovis, T. Nickel-Catalyzed Reductive Carboxylation of Styrenes Using CO2. J. Am. Chem. Soc. 2008, 130, 14936–14937. [Google Scholar] [CrossRef]
- Li, S.; Yuan, W.; Ma, S. Highly Regio- and Stereoselective Three-Component Nickel-Catalyzed syn-Hydrocarboxylation of Alkynes with Diethyl Zinc and Carbon Dioxide. Angew. Chem. Int. Ed. 2011, 50, 2578–2582. [Google Scholar] [CrossRef]
- Walter, D.; Braunlich, G. Aktivierung von CO2 an übergangsmetallzentren: Zum ablauf der homogen-katalytischen Bildung von 2-Pyron aus Kohlendioxid und Hex-3-in an Nickel(0)-Fragmenten. J. Org. Chem. 1992, 436, 109–119. [Google Scholar] [CrossRef]
- Diccianni, J.B.; Diao, T. Mechanisms of nickel-catalyzed cross-coupling reactions. Trends Chem. 2019, 1, 830–844. [Google Scholar] [CrossRef]
- Dieter, R.K.; Fishpaugh, J.R. A versatile synthesis of α-pyrones. J. Org. Chem. 1983, 48, 4439–4441. [Google Scholar] [CrossRef]
- Huang, K.; Sun, C.-L.; Shi, Z.-J. Transition-metal-catalyzed C–C bond formation through the fixation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 2435–2452. [Google Scholar] [CrossRef]
- Fan, T.; Chen, X.; Lin, Z. Theoretical studies of reactions of carbon dioxide mediated and catalysed by transition metal complexes. Chem. Commun. 2012, 48, 10808–10828. [Google Scholar] [CrossRef] [PubMed]
- Pápai, I.; Schubert, G.; Mayer, I.; Besenyei, G.; Aresta, M. Mechanistic details of nickel(0)-assisted oxidative coupling of CO2 with C2H4. Organometallics 2004, 23, 5252–5259. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Bi, S.; Liu, Y. Theoretical investigation on the regioselectivity of Ni(COD)2-catalyzed [2+2+2] cycloaddition of unsymmetric diynes and CO2. J. Organomet. Chem. 2014, 758, 45–54. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K. Formulation of the reaction coordinate. J. Phys. Chem. 1970, 74, 4161–4163. [Google Scholar] [CrossRef]
- Ishida, K.; Morokuma, K.; Komornicki, A. The intrinsic reaction coordinate. An ab initio calculation for HNC → HCN and H− + CH4 → CH4 + H−. J. Chem. Phys. 1977, 66, 2153–2156. [Google Scholar] [CrossRef]
- González, C.; Schlegel, H.B. An improved algorithm for reaction path following. J. Chem. Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
- González, C.; Schlegel, H.B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Legault, C.Y. CYLView, 1.0b; Université de Sherbrooke: Montreal, QC, Canada, 2009; Available online: http://www.cylview.org (accessed on 18 January 2024).


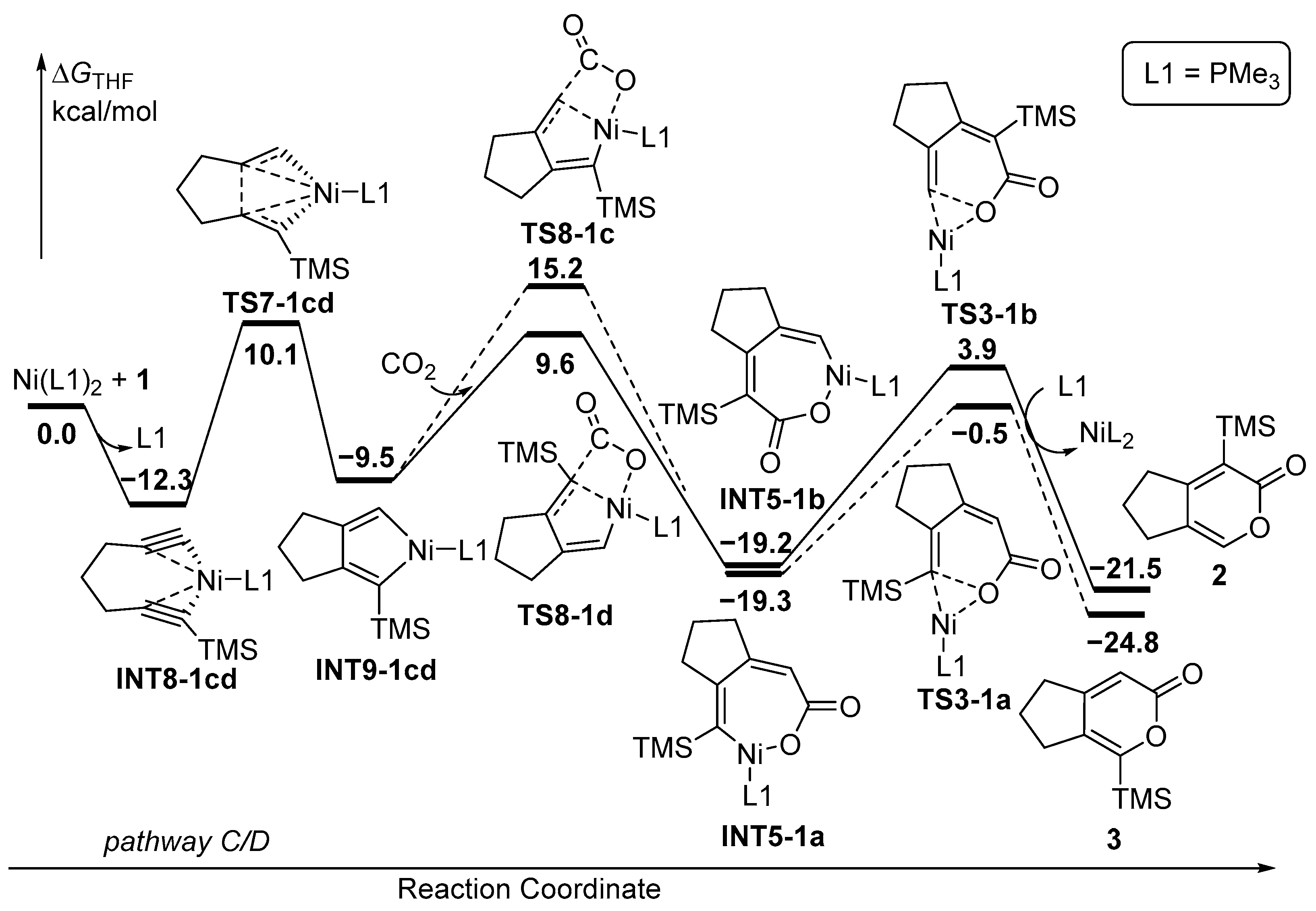
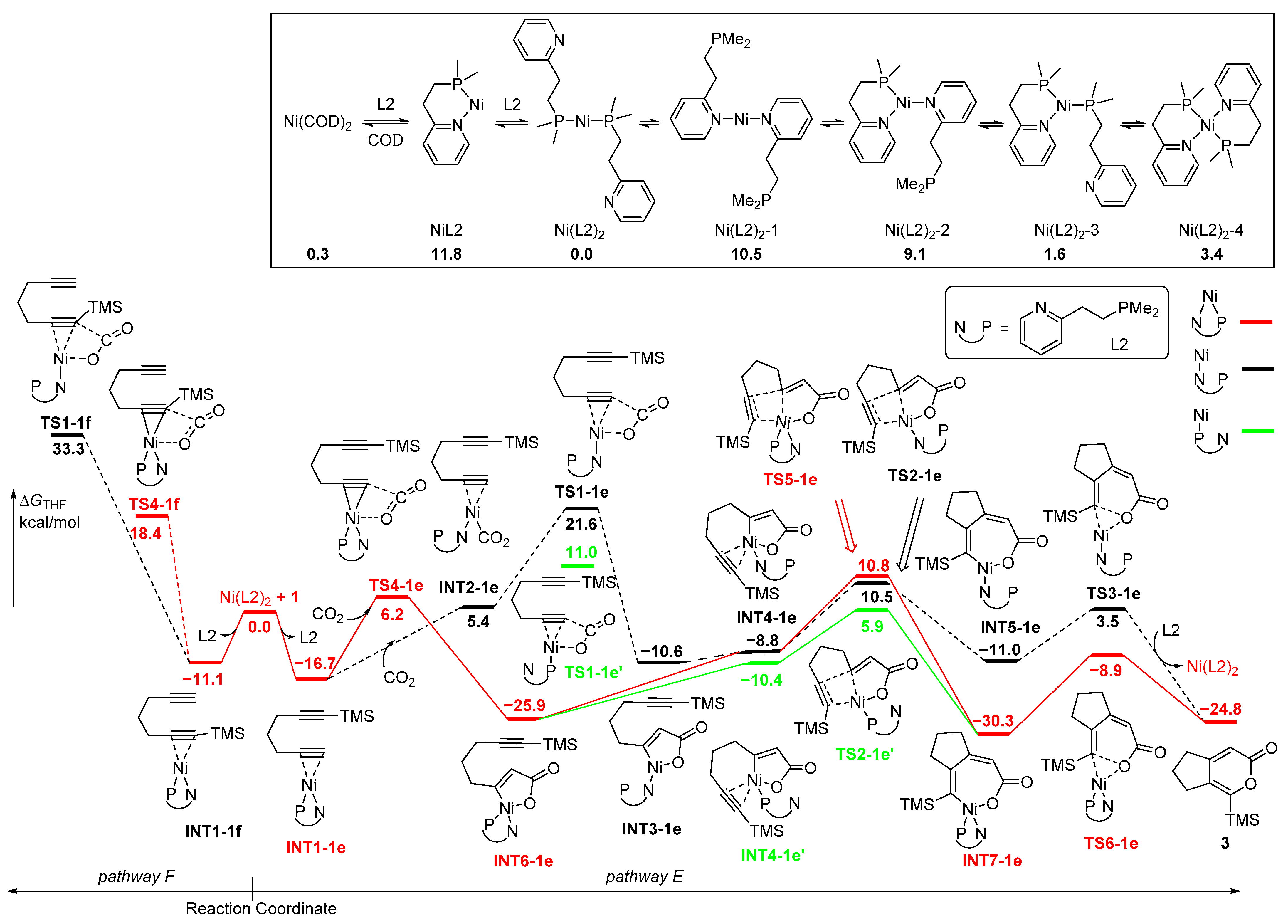
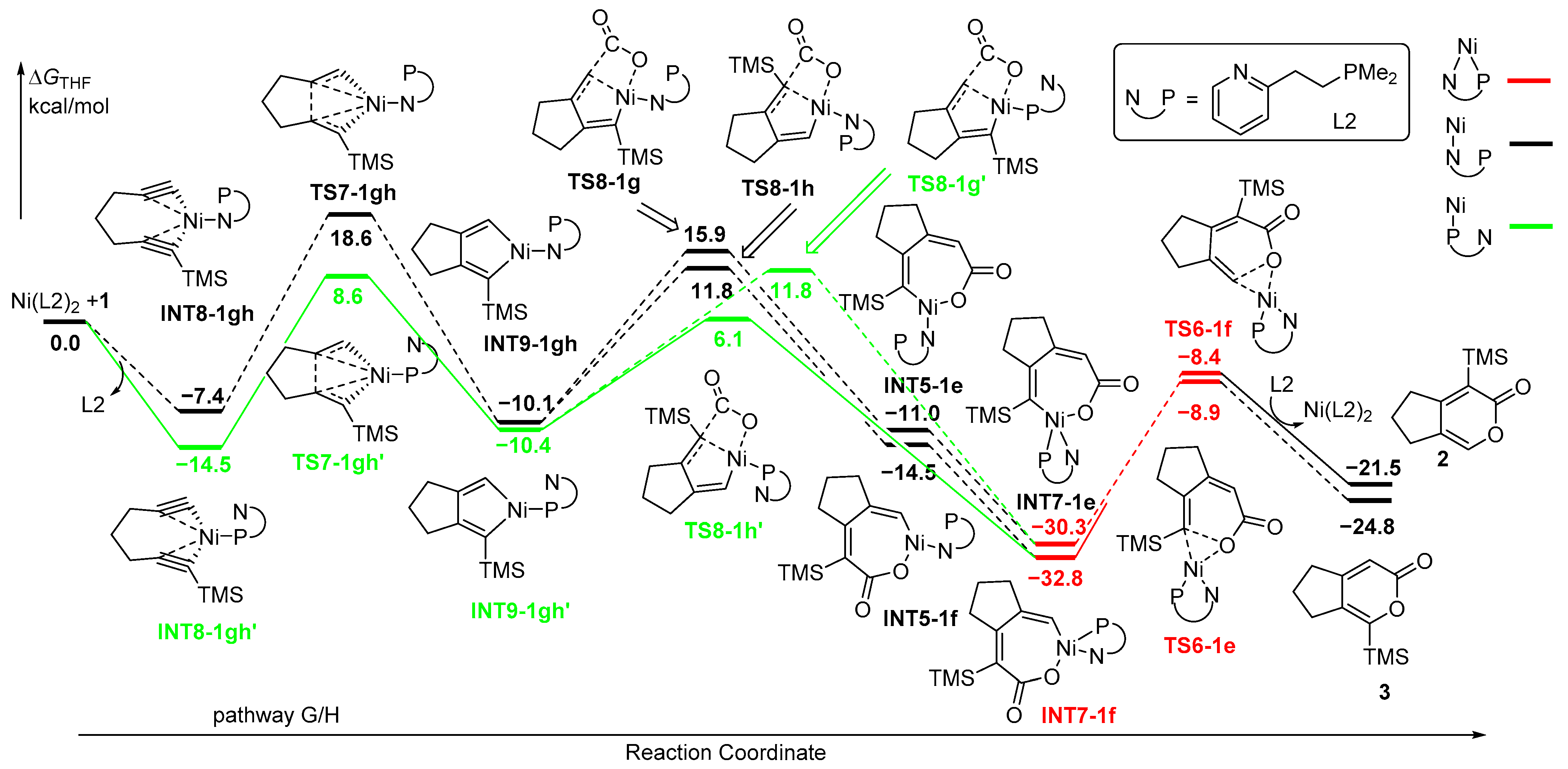

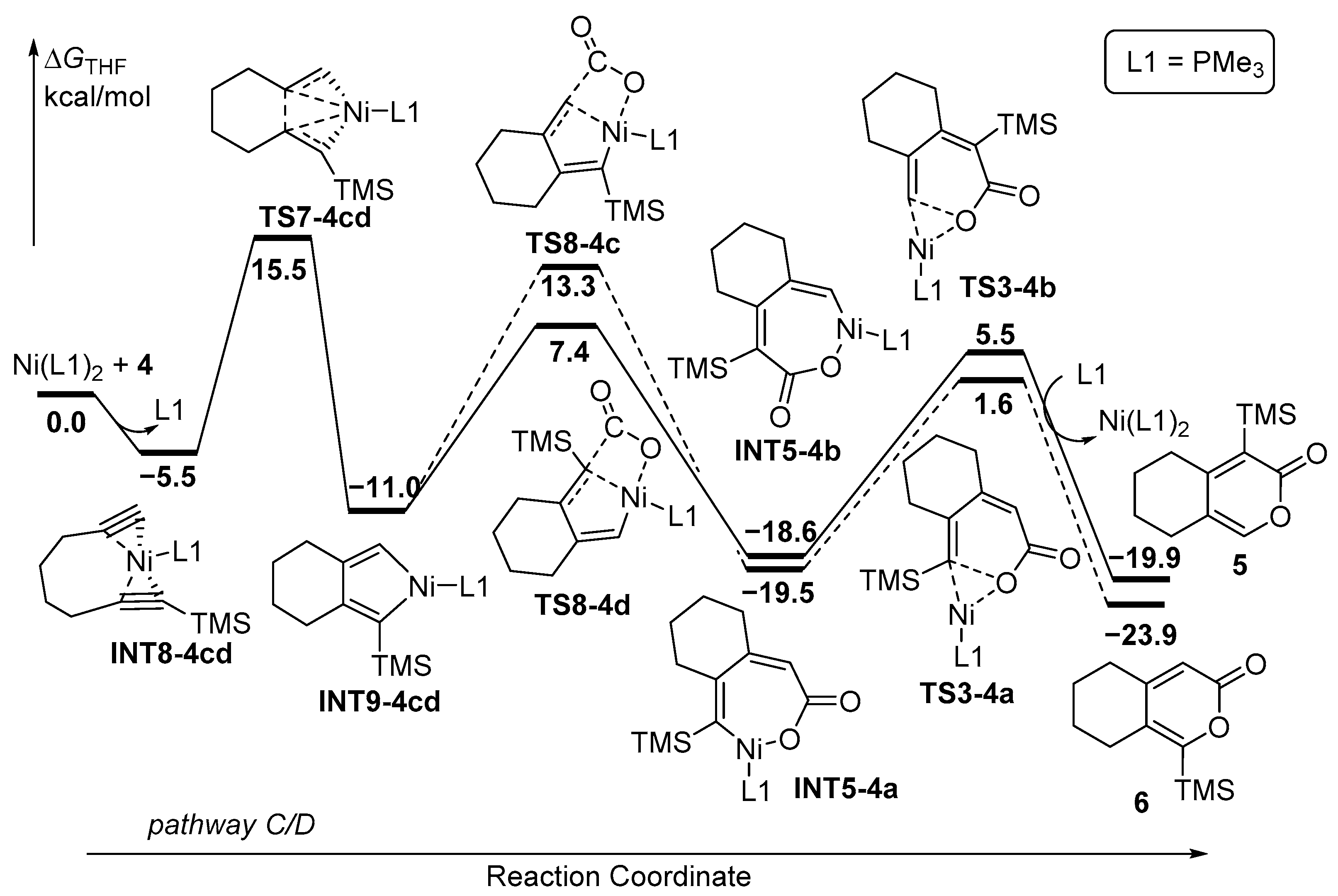
| Substrate | Ligand | ΔG‡ (2 or 5) (kcal/mol) | ΔG‡ (3 or 6) (kcal/mol) | ΔΔG‡ (2–3 or 5–6) (kcal/mol) | Cal. 2/3 or 5/6 | Exp. 2/3 or 5/6 | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | L1 | 10.1 | 13.1 | −3.0 | >99/1 | 100/0 | [33] |
| 1 | L2 | 8.6 | 6.2 | 2.0 | 3/97 | 8/92 | [33] |
| 4 | L1 | 15.5 | 13.7 | 1.8 | 4/96 | 0/100 | [33] |
| 4 | L2 | 13.8 | 8.8 | 5.0 | <1/99 | 0/100 | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Huang, Q.; Yang, C.; Li, X. Theoretical Insights into the Regiodivergence in Ni-Catalyzed [2+2+2] Cycloaddition of Unsymmetric Diynes and CO2. Inorganics 2024, 12, 39. https://doi.org/10.3390/inorganics12020039
Zhang K, Huang Q, Yang C, Li X. Theoretical Insights into the Regiodivergence in Ni-Catalyzed [2+2+2] Cycloaddition of Unsymmetric Diynes and CO2. Inorganics. 2024; 12(2):39. https://doi.org/10.3390/inorganics12020039
Chicago/Turabian StyleZhang, Kun, Qiwen Huang, Cun Yang, and Xinyao Li. 2024. "Theoretical Insights into the Regiodivergence in Ni-Catalyzed [2+2+2] Cycloaddition of Unsymmetric Diynes and CO2" Inorganics 12, no. 2: 39. https://doi.org/10.3390/inorganics12020039







