Mild and Fast Construction of Ni-Based Electrodes for Industrial-Grade Water Splitting
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Chemicals and Materials
2.2. Electrode Characterization
2.3. Preparation of NF@NiB Catalytic Electrode
2.4. Electrochemical Measurements
3. Results
3.1. Characterization of NF@NiB
3.2. Electrochemical Analysis
3.2.1. HER and OER Electrochemical Measurements of NF@NiB Electrode
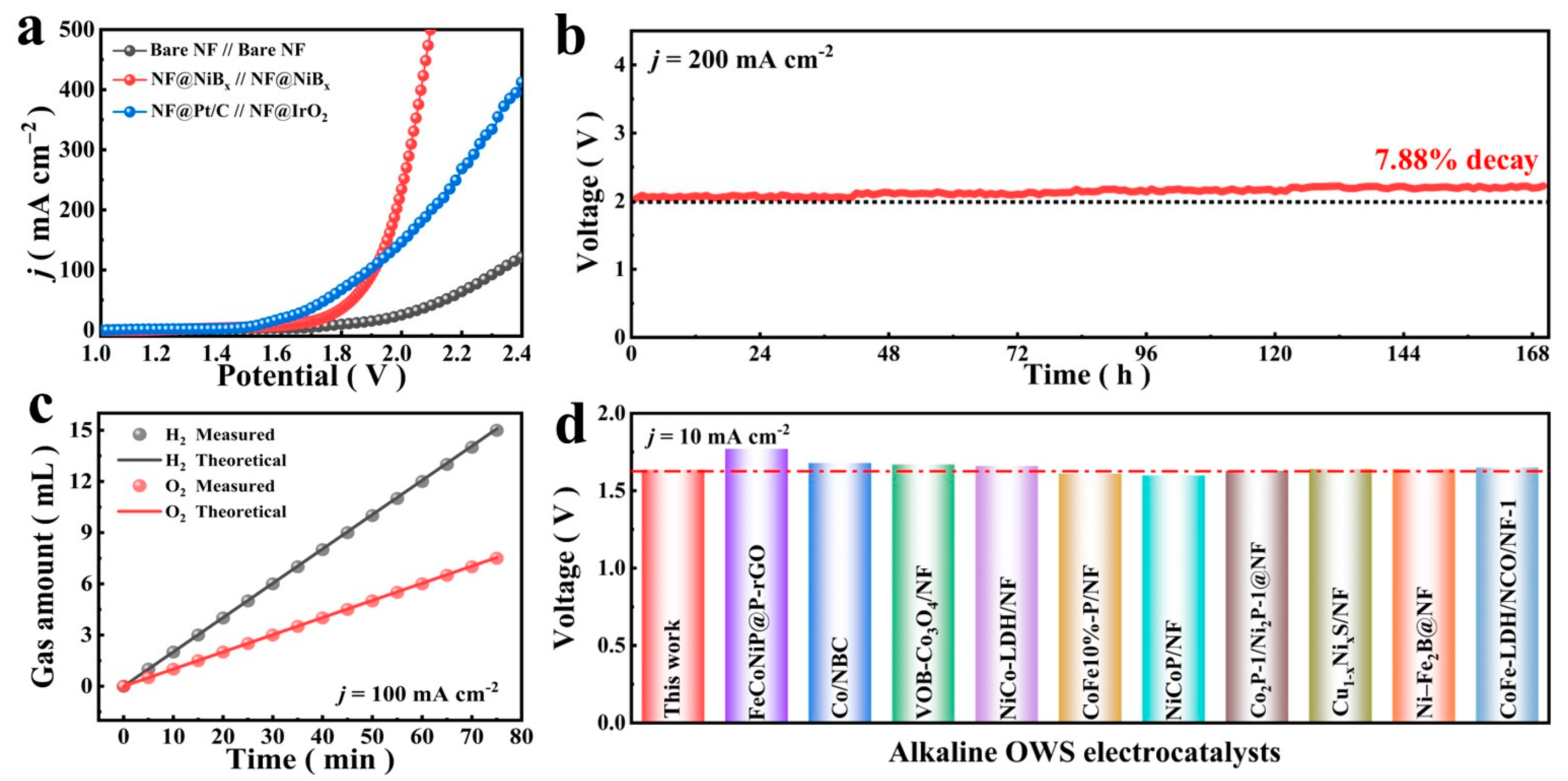
3.2.2. OWS Electrochemical Measurements of NF@NiB Electrode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Glenk, G.; Reichelstein, S. Economics of converting renewable power to hydrogen. Nat. Energy 2019, 4, 216–222. [Google Scholar] [CrossRef]
- Priamushko, T.; Guillet-Nicolas, R.; Kleitz, F. Mesoporous nanocast electrocatalysts for oxygen reduction and oxygen evolution reactions. Inorganics 2019, 7, 98. [Google Scholar] [CrossRef]
- Tamboli, M.S.; Patil, S.A.; Tamboli, A.M.; Patil, S.S.; Truong, N.T.N.; Lee, K.; Praveen, C.S.; Shrestha, N.K.; Park, C.; Kale, B.B. Polyaniline-wrapped MnMoO4 as an active catalyst for hydrogen production by electrochemical water splitting. Dalton Trans. 2022, 51, 6027–6035. [Google Scholar] [CrossRef]
- Pan, H.; Wang, Y.; Lu, Z.; Huang, X.; Chen, X. Free-standing Co/Zn sulfide supported on Cu-foam for efficient overall water splitting. New J. Chem. 2022, 46, 11149–11157. [Google Scholar] [CrossRef]
- Lin, H.Y.; Yang, G.; Liu, P.F. Unveiling the reconstructed active phase of Ni3Se2 model for water splitting. New J. Chem. 2023, 47, 1666–1671. [Google Scholar] [CrossRef]
- Zou, W.; Xiang, J.; Tang, H. Three-dimensional nano-framework CoP/Co2P/Co3O4 heterojunction as a trifunctional electrocatalyst for metal–air battery and water splitting. New J. Chem. 2022, 46, 8786–8793. [Google Scholar] [CrossRef]
- Dar, M.; Majid, K.; Wahid, M. Enhanced alkaline bifunctional electrocatalytic water splitting achieved through N and S dual-doped carbon shell reinforced Co9S8 microplates. New J. Chem. 2022, 46, 22427–22440. [Google Scholar] [CrossRef]
- Jia, L.; Wagner, P.; Chen, J. Electrocatalyst derived from NiCu-MOF arrays on graphene oxide modified carbon cloth for water splitting. Inorganics 2022, 10, 53. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; Zhang, F.; McElhenny, B.; Luo, D.; Karim, A.; Chen, S.; Ren, Z. Heterogeneous Bimetallic Phosphide Ni2P-Fe2P as an Efficient Bifunctional Catalyst for Water/Seawater Splitting. Adv. Funct. Mater. 2021, 31, 2006484. [Google Scholar] [CrossRef]
- Wang, S.; Yang, P.; Sun, X.; Xing, H.; Hu, J.; Chen, P.; Cui, Z.; Zhu, W.; Ma, Z. Synthesis of 3D heterostructure Co-doped Fe2P electrocatalyst for overall seawater electrolysis. Appl. Catal. B-Environ. 2021, 297, 120386. [Google Scholar] [CrossRef]
- Yang, Y.; Qian, Y.; Li, H.; Zhang, Z.; Mu, Y.; Do, D.; Zhou, B.; Dong, J.; Yan, W.; Qin, Y.; et al. O-coordinated W-Mo dual-atom catalyst for pH-universal electrocatalytic hydrogen evolution. Sci. Adv. 2020, 6, eaba6586. [Google Scholar] [CrossRef]
- Umer, M.; Umer, S.; Zafari, M.; Ha, M.; Anand, R.; Hajibabaei, A.; Abbas, A.; Lee, G.; Kim, K.S. Machine learning assisted high-throughput screening of transition metal single atom based superb hydrogen evolution electrocatalysts. J. Mater. Chem. A 2022, 10, 6679–6689. [Google Scholar] [CrossRef]
- Song, F.; Li, W.; Sun, Y. Metal–organic frameworks and their derivatives for photocatalytic water splitting. Inorganics 2017, 5, 40. [Google Scholar] [CrossRef]
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.; Fowler, M.; Chen, Z. Electrically rechargeable zinc–air batteries: Progress, challenges, and perspectives. Adv. Mater. 2017, 29, 1604685. [Google Scholar] [CrossRef]
- Fan, J.; Fu, C.; Liang, R.; Lv, H.; Fang, C.; Guo, Y.; Hao, W. Mild construction of “midas touch” metal–organic framework-based catalytic electrodes for highly efficient overall seawater splitting. Small 2022, 18, 2203588. [Google Scholar] [CrossRef]
- Jin, H.; Liu, X.; Chen, S.; Vasileff, A.; Li, L.; Jiao, Y.; Song, L.; Zheng, Y.; Qiao, S.-Z. Heteroatom-doped transition metal electrocatalysts for hydrogen evolution reaction. ACS Energy Lett. 2019, 4, 805–810. [Google Scholar] [CrossRef]
- Liu, T.; Li, P.; Yao, N.; Cheng, G.; Chen, S.; Luo, W.; Yin, Y. CoP-doped MOF-based electrocatalyst for pH-universal hydrogen evolution reaction. Angew. Chem. Int. Ed. 2019, 58, 4679–4684. [Google Scholar] [CrossRef]
- He, S.; Liu, Y.; Peng, S.; Lin, L. Carbonaceous FexP synthesized via carbothermic reduction of dephosphorization slag as hydrogen evolution catalyst for water splitting. Inorganics 2022, 10, 70. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Patil, S.A.; Han, J.; Cho, S.; Inamdar, A.I.; Kim, H.; Im, H. Chemical etching induced microporous nickel backbones decorated with metallic Fe@hydroxide nanocatalysts: An efficient and sustainable OER anode toward industrial alkaline water-splitting. J. Mater. Chem. A 2022, 10, 8989–9000. [Google Scholar] [CrossRef]
- Jo, Y.; Cho, S.; Seo, J.; Ahmed, A.T.A.; Lee, C.H.; Seok, J.H.; Hou, B.; Patil, S.A.; Park, Y.; Shrestha, N.K.; et al. Experimental and Theoretical Insights into the Borohydride-Based Reduction-Induced Metal Interdiffusion in Fe-Oxide@NiCo2O4 for Enhanced Oxygen Evolution. ACS Appl. Mater. Interfaces 2021, 13, 53725–53735. [Google Scholar] [CrossRef] [PubMed]
- Laghari, A.J.; Aftab, U.; Tahira, A.; Shah, A.A.; Gradone, A.; Solangi, M.Y.; Samo, A.H.; Kumar, M.; Abro, M.I.; Akhtar, M.W.; et al. MgO as promoter for electrocatalytic activities of Co3O4–MgO composite via abundant oxygen vacancies and Co2+ ions towards oxygen evolution reaction. Int. J. Hydrog. Energy 2023, 48, 12672–12682. [Google Scholar] [CrossRef]
- Ye, S.; Luo, F.; Zhang, Q.; Zhang, P.; Xu, T.; Wang, Q.; He, D.; Guo, L.; Zhang, Y.; He, C.; et al. Highly stable single Pt atomic sites anchored on aniline-stacked graphene for hydrogen evolution reaction. Energy Environ. Sci. 2019, 12, 1000–1007. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, C.; Weng, S.; Lv, H.; Li, P.; Deng, S.; Hao, W. Construction of an “environment-friendly” CuBx@PU self-supporting electrode toward efficient seawater electrolysis. Green Chem. 2022, 24, 5918–5929. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Y.; Sheng, W.; Xu, Z.J.; Jaroniec, M.; Qiao, S.-Z. Strategies for design of electrocatalysts for hydrogen evolution under alkaline conditions. Mater. Today 2020, 36, 125–138. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Yu, F.; Pang, H.; Zhou, X.; Li, D.; Ma, W.; Zhou, Q.; Mo, Y.; Zhou, H. Engineering Multilevel Collaborative Catalytic Interfaces with Multifunctional Iron Sites Enabling High-Performance Real Seawater Splitting. ACS Nano 2023, 17, 1681–1692. [Google Scholar] [CrossRef]
- Nsanzimana, J.M.V.; Gong, L.; Dangol, R.; Reddu, V.; Jose, V.; Xia, B.Y.; Yan, Q.; Lee, J.-M.; Wang, X. Tailoring of metal boride morphology via anion for efficient water oxidation. Adv. Energy Mater. 2019, 9, 1901503. [Google Scholar] [CrossRef]
- Guo, Y.; Park, T.; Yi, J.W.; Henzie, J.; Kim, J.; Wang, Z.; Jiang, B.; Bando, Y.; Sugahara, Y.; Tang, J.; et al. Nanoarchitectonics for transition-metal-sulfide-based electrocatalysts for water splitting. Adv. Mater. 2019, 31, 1807134. [Google Scholar] [CrossRef]
- Hao, W.; Wu, R.; Huang, H.; Ou, X.; Wang, L.; Sun, D.; Ma, X.; Guo, Y. Fabrication of practical catalytic electrodes using insulating and eco-friendly substrates for overall water splitting. Energy Environ. Sci. 2020, 13, 102–110. [Google Scholar] [CrossRef]
- Shi, Y.; Li, M.; Yu, Y.; Zhang, B. Recent advances in nanostructured transition metal phosphides: Synthesis and energy-related applications. Energy Environ. Sci. 2020, 13, 4564–4582. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Yang, P.; Wang, D.; Xu, H.; Wang, B.; Meng, F.; Zhang, J.; An, M. Electrodeposition: Synthesis of advanced transition metal-based catalyst for hydrogen production via electrolysis of water. J. Energy Chem. 2021, 57, 547–566. [Google Scholar] [CrossRef]
- Chodankar, N.R.; Ji, S.-H.; Han, Y.-K.; Kim, D.-H. Dendritic nanostructured waste copper wires for high-energy alkaline battery. Nano-Micro Lett. 2020, 12, 1. [Google Scholar] [CrossRef]
- Hao, W.; Yao, D.; Xu, Q.; Wang, R.; Zhang, C.; Guo, Y.; Sun, R.; Huang, M.; Chen, Z. Highly efficient overall-water splitting enabled via grafting boron-inserted Fe-Ni solid solution nanosheets onto unconventional skeleton. Appl. Catal. B-Environ. 2021, 292, 120188. [Google Scholar] [CrossRef]
- Yu, T.; Xu, Q.; Qian, G.; Chen, J.; Zhang, H.; Luo, L.; Yin, S. Amorphous CoOx-Decorated Crystalline RuO2 Nanosheets as Bifunctional Catalysts for Boosting Overall Water Splitting at Large Current Density. ACS Sustain. Chem. Eng. 2020, 8, 17520–17526. [Google Scholar] [CrossRef]
- Zhang, H.; Qian, G.; Chen, X.; Jiang, W.; Yu, T.; Wang, Y.; Luo, L.; Yin, S. V2O3-Decorated Spinel CoFe2O4 with Carbon-Encapsulated Mesoporous Nanosheets for Efficient Water Splitting. ACS Sustain. Chem. Eng. 2021, 9, 980–986. [Google Scholar] [CrossRef]
- Liang, J.; Liu, Q.; Li, T.; Luo, Y.; Lu, S.; Shi, X.; Zhang, F.; Asiri, A.M.; Sun, X. Magnetron sputtering enabled sustainable synthesis of nanomaterials for energy electrocatalysis. Green Chem. 2021, 23, 2834–2867. [Google Scholar] [CrossRef]
- Wu, J.; Hou, M.; Chen, Z.; Hao, W.; Pan, X.; Yang, H.; Cen, W.; Liu, Y.; Huang, H.; Menezes, P.W.; et al. Composition engineering of amorphous nickel boride nanoarchitectures enabling highly efficient electrosynthesis of hydrogen peroxide. Adv. Mater. 2022, 34, 2202995. [Google Scholar] [CrossRef]
- Ledendecker, M.; Krick, S.; Papp, C.; Steinrück, H.-P.; Antonietti, M.; Shalom, M. The synthesis of nanostructured Ni5P4 films and their use as a non-noble bifunctional electrocatalyst for full water splitting. Angew. Chem. Int. Ed. 2015, 54, 12361–12365. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Yu, Z.; Hou, Y.; Jiang, R.; Wang, M.; Huang, J.; Chen, J.; Zhang, Y.; Zhu, H. Functional group scission-induced lattice strain in chiral macromolecular metal-organic framework arrays for electrocatalytic overall water splitting. Appl. Catal. B-Environ. 2022, 307, 121151. [Google Scholar] [CrossRef]
- Qian, G.; Yu, G.; Lu, J.; Luo, L.; Wang, T.; Zhang, C.; Ku, R.; Yin, S.; Chen, W.; Mu, S. Ultra-thin N-doped-graphene encapsulated Ni nanoparticles coupled with MoO2 nanosheets for highly efficient water splitting at large current density. J. Mater. Chem. A 2020, 8, 14545–14554. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, J.; Yin, S.; Luo, L.; Jing, S.; Brouzgou, A.; Chen, J.; Shen, P.K.; Tsiakaras, P. One-pot synthesized boron-doped RhFe alloy with enhanced catalytic performance for hydrogen evolution reaction. Appl. Catal. B-Environ. 2018, 230, 58–64. [Google Scholar] [CrossRef]
- Ren, X.; Tian, Y.; Shaik, F.; Yang, J.; Liu, R.; Guo, K.; Jiang, B. An efficient electrocatalyst based on vertically aligned heteroatom(B/N/P/O/S)-doped graphene array integrated with FeCoNiP nanoparticles for overall water splitting. Adv. Sust. Syst. 2022, 6, 2100436. [Google Scholar] [CrossRef]
- Liu, M.-R.; Hong, Q.-L.; Li, Q.-H.; Du, Y.; Zhang, H.-X.; Chen, S.; Zhou, T.; Zhang, J. Cobalt boron imidazolate framework derived cobalt nanoparticles encapsulated in B/N codoped nanocarbon as efficient bifunctional electrocatalysts for overall water splitting. Adv. Funct. Mater. 2018, 28, 1801136. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, S.; Ma, Z.; Kundu, M.; Tang, B.; Li, J.; Wang, X. Oxygen vacancies engineered self-supported B doped Co3O4 nanowires as an efficient multifunctional catalyst for electrochemical water splitting and hydrolysis of sodium borohydride. Chem. Eng. J. 2021, 404, 126474. [Google Scholar] [CrossRef]
- Liu, W.; Bao, J.; Guan, M.; Zhao, Y.; Lian, J.; Qiu, J.; Xu, L.; Huang, Y.; Qian, J.; Li, H. Nickel–cobalt-layered double hydroxide nanosheet arrays on Ni foam as a bifunctional electrocatalyst for overall water splitting. Dalton Trans. 2017, 46, 8372–8376. [Google Scholar] [CrossRef]
- Liu, B.; Li, S.; Wang, T.; Yang, Y.; Wang, L.; Zhang, X.; Liu, Z.; Niu, L. Construction of CoFe bimetallic phosphide microflowers electrocatalyst for highly efficient overall water splitting. Catal. Commun. 2023, 175, 106607. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Fei, T.; Mao, C.; Song, Y.; Zhou, Y.; Dong, G. NiCoP/NF 1D/2D biomimetic architecture for markedly enhanced overall water splitting. Chem. Electro. Chem. 2021, 8, 3064–3072. [Google Scholar] [CrossRef]
- Zhao, H.; Liang, J.; Zhao, Y. Construction of hierarchical Co2P/Ni2P heterostructures on Ni foam as efficient bifunctional electrocatalyst for overall water splitting. J. Alloys Compd. 2022, 907, 164479. [Google Scholar] [CrossRef]
- Li, Y.; Su, H.; Fu, J.; Du, X. The 3D ultra-thin Cu1-xNixS/NF nanosheet as a highly efficient and stable electrocatalyst for overall water splitting. Int. J. Hydrog. Energy 2019, 44, 11744–11753. [Google Scholar] [CrossRef]
- Yao, R.; Wu, Y.; Zhao, Q.; Li, J.; Liu, G. Autogenous growth of highly active bifunctional Ni–Fe2B nanosheet arrays toward efficient overall water splitting. Int. J. Hydrog. Energy 2022, 47, 8303–8313. [Google Scholar] [CrossRef]
- Yao, H.; Wang, S.; Cao, Y.; Chen, R.; Lu, Z.; Hu, J.; Xie, J.; Hao, A. High-performance bifunctional electrocatalysts of CoFe-LDH/NiCo2O4 heterostructure supported on nickel foam for effective overall water splitting. J. Alloys Compd. 2022, 926, 166846. [Google Scholar] [CrossRef]
- Yuan, W.; Cui, Z.; Zhu, S.; Li, Z.; Wu, S.; Liang, Y. Structure engineering of electrodeposited NiMo films for highly efficient and durable seawater splitting. Electrochim. Acta 2021, 365, 137366. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Z.; Li, Z.; Han, M.; Cheng, Y.; Zheng, Z. Standing NiFe LDH nanosheets on stainless steel fibers felt: A synergistic impact on the oxygen evolution reaction (OER) for the water splitting. Catal. Commun. 2022, 164, 106425. [Google Scholar] [CrossRef]
- Xiao, Z.; Yang, M.; Wang, J.; Xu, Z.; Zhang, S.; Tang, A.; Gao, R.; Yang, H. FeNiP/MoOx integrated electrode grown on monocrystalline NiMoO4 nanorods with multi-interface for accelerating alkaline hydrogen evolution reaction. Appl. Catal. B-Environ. 2022, 303, 120913. [Google Scholar] [CrossRef]
- Kafle, A.; Gupta, D.; Nagaiah, T.C. Facile fabrication of NiFeB deposited flexible carbon cloth electrode towards overall water splitting in alkaline and saline solutions. Electrochim. Acta 2023, 441, 141779. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Liang, R.; Shao, Y.; Hao, W. Mild and Fast Construction of Ni-Based Electrodes for Industrial-Grade Water Splitting. Inorganics 2023, 11, 170. https://doi.org/10.3390/inorganics11040170
Lu Z, Liang R, Shao Y, Hao W. Mild and Fast Construction of Ni-Based Electrodes for Industrial-Grade Water Splitting. Inorganics. 2023; 11(4):170. https://doi.org/10.3390/inorganics11040170
Chicago/Turabian StyleLu, Zikang, Rikai Liang, Yuqi Shao, and Weiju Hao. 2023. "Mild and Fast Construction of Ni-Based Electrodes for Industrial-Grade Water Splitting" Inorganics 11, no. 4: 170. https://doi.org/10.3390/inorganics11040170





