Study of Cyclohexane and Methylcyclohexane Functionalization Promoted by Manganese(III) Compounds
Abstract
:1. Introduction
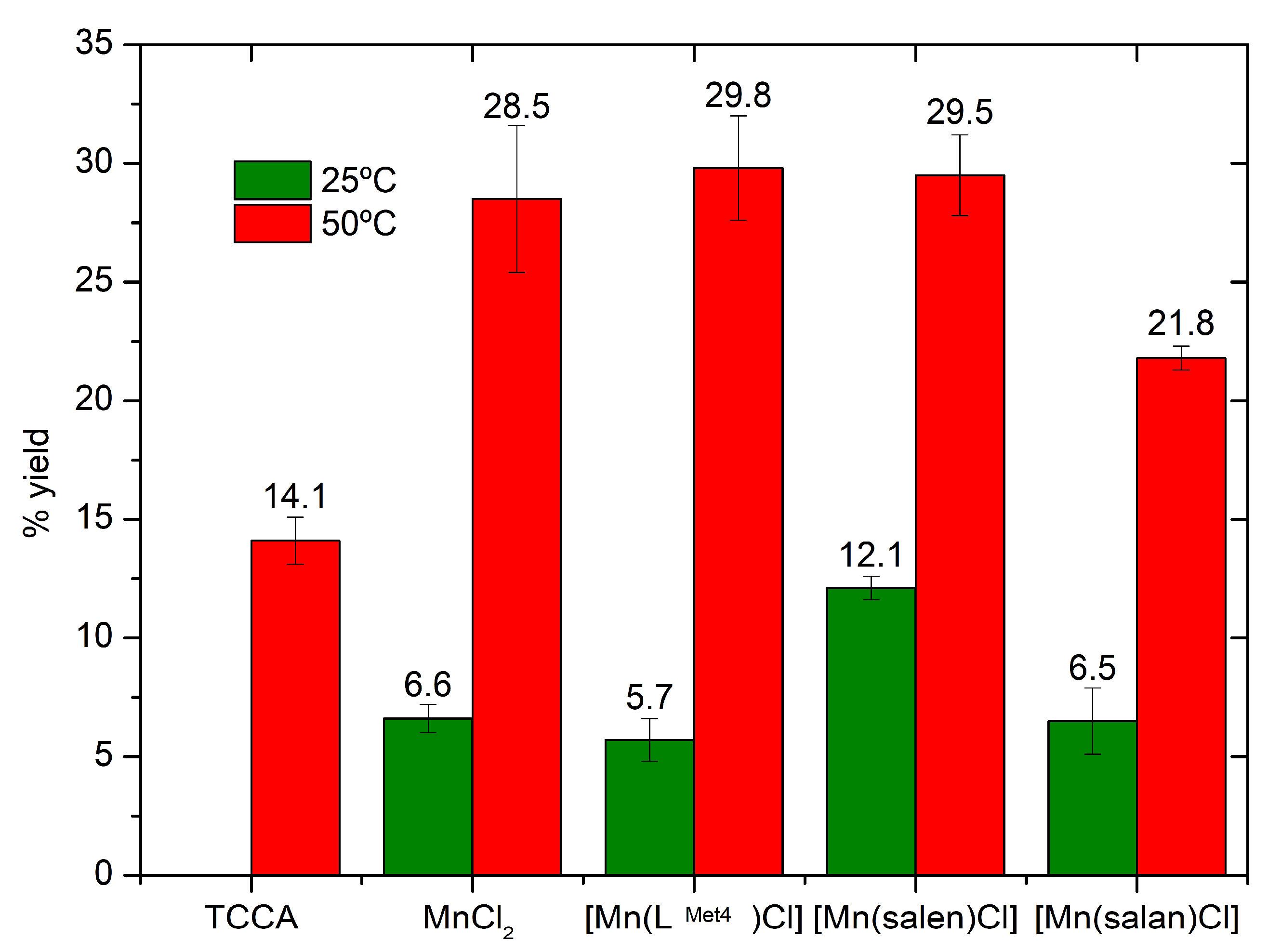
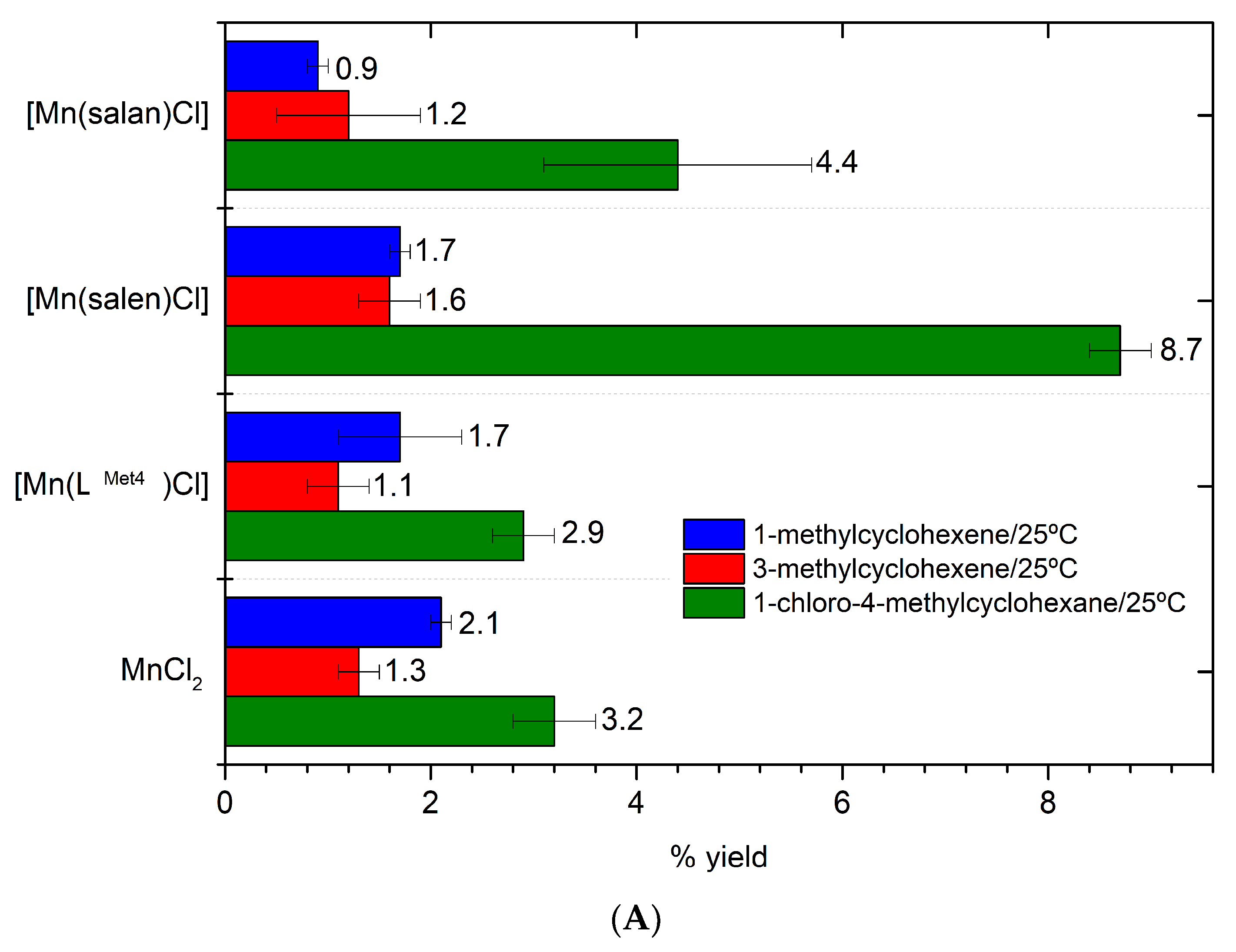
2. Results
3. Discussion
4. Materials and Methods
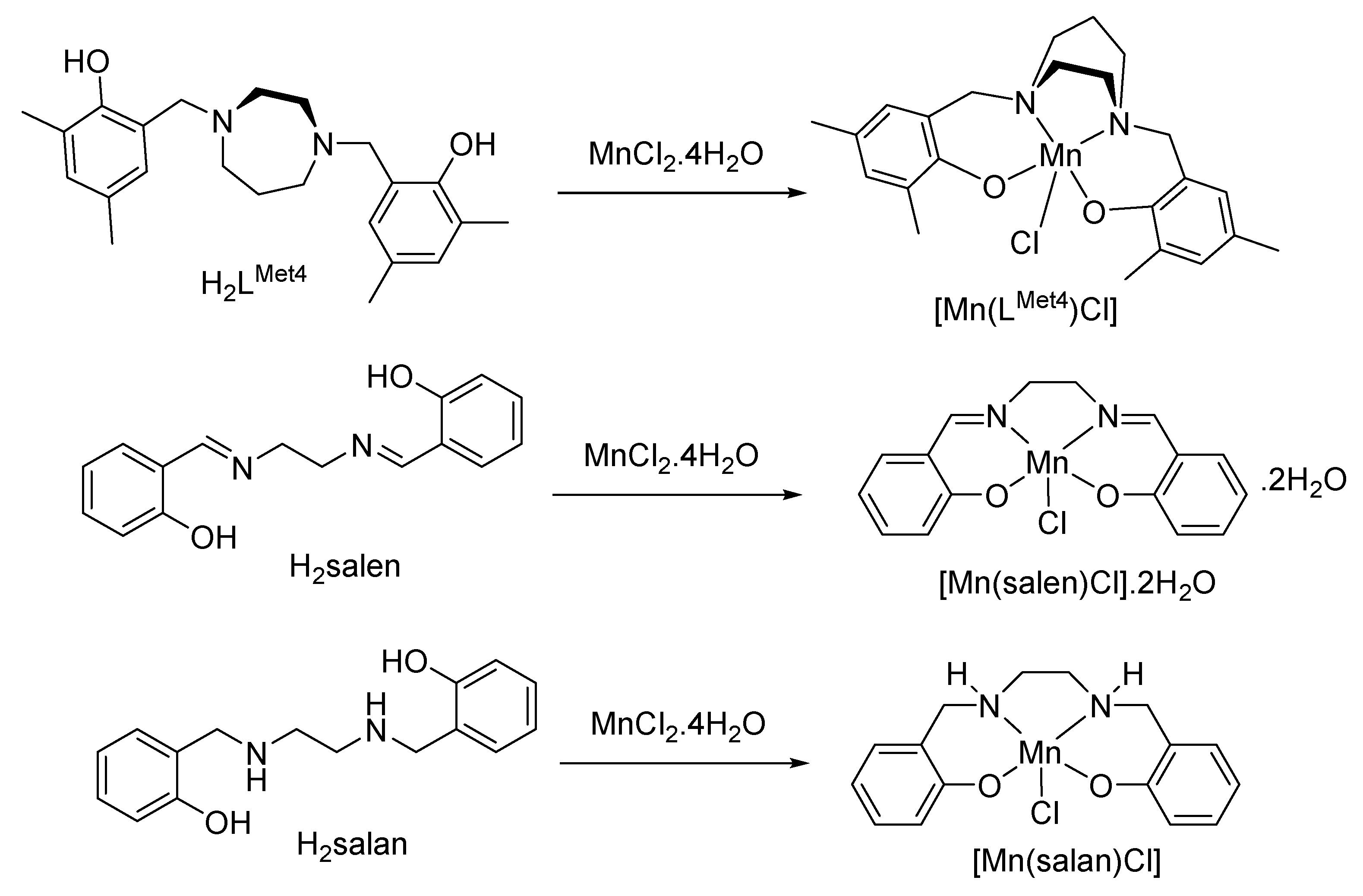
4.1. Syntheses
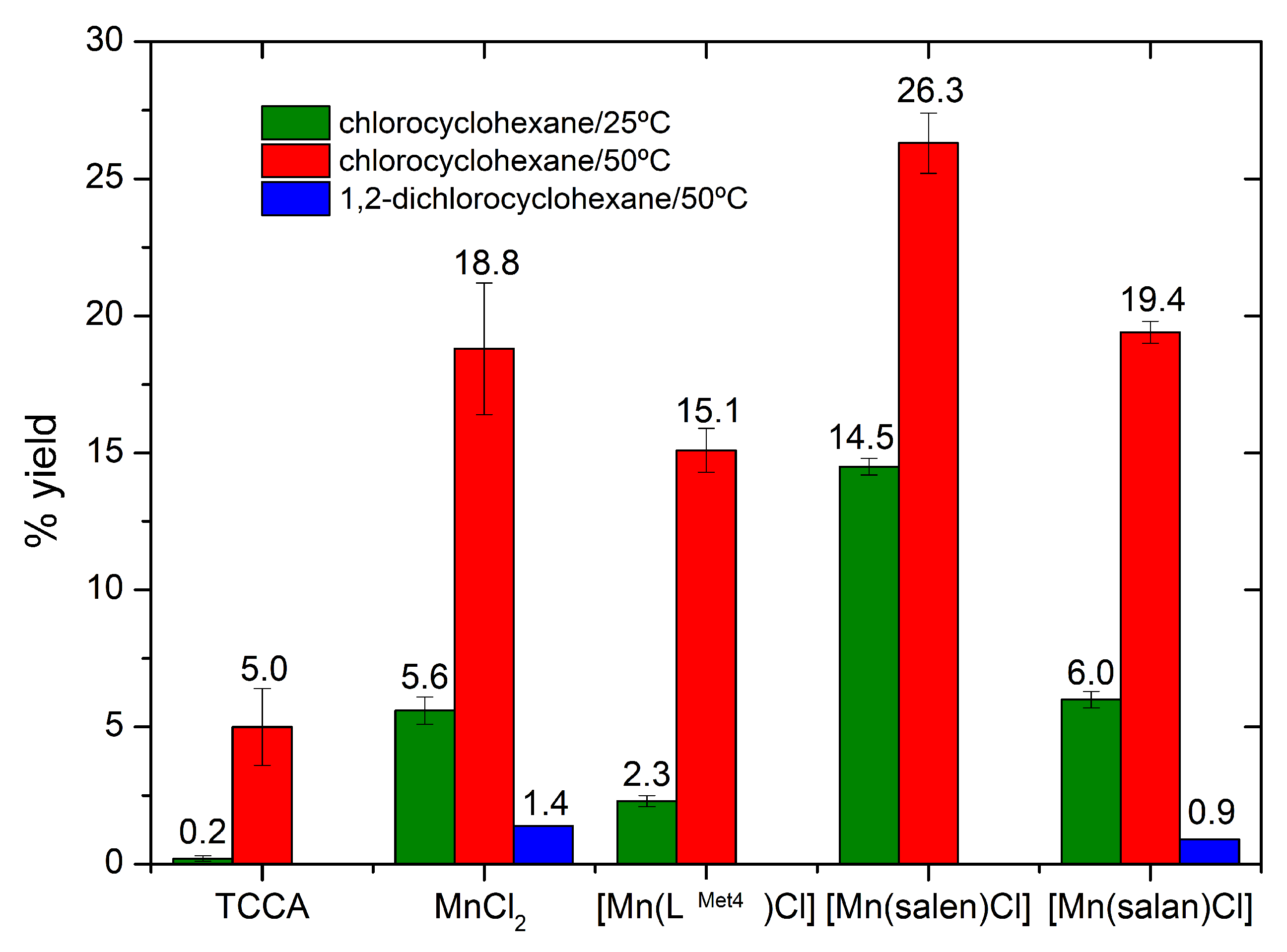
4.2. Catalytic Activity
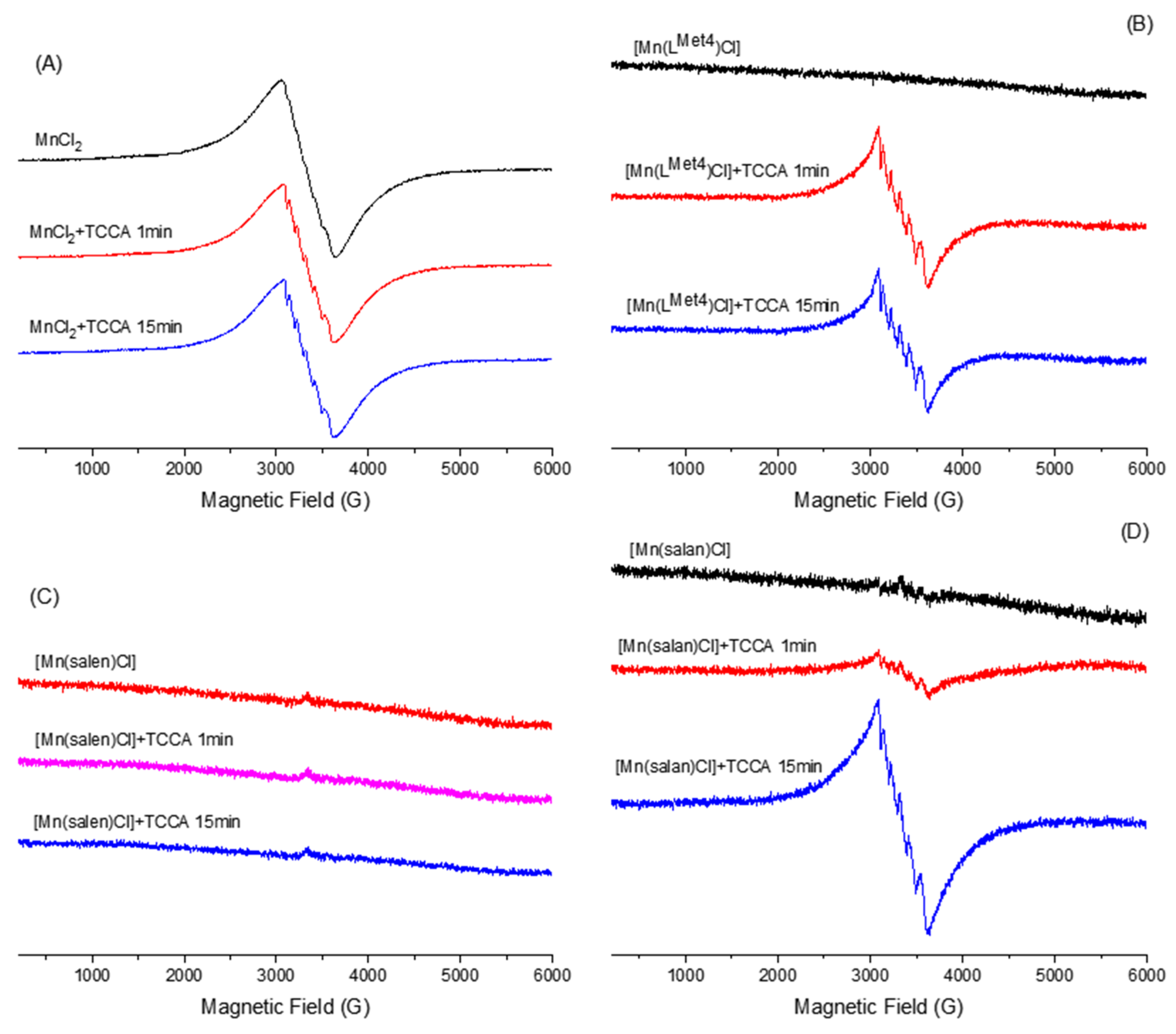
4.3. EPR Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schwartz, N.A.; Boaz, N.C.; Kalman, S.E.; Zhuang, T.; Goldberg, J.M.; Fu, R.; Nielsen, R.J.; Goddard, W.A.; Groves, J.T.; Gunnoe, T.B. Mechanism of Hydrocarbon Functionalization by an Iodate/Chloride System: The Role of Ester Protection. ACS Catal. 2018, 8, 3138–3149. [Google Scholar] [CrossRef] [Green Version]
- Oloo, W.; Que, L. Hydrocarbon Oxidations Catalyzed by Bio-Inspired Nonheme Iron and Copper Catalysts. In Homogeneous Catalytic Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; pp. 763–778. [Google Scholar] [CrossRef]
- Roduner, E.; Kaim, W.; Sarkar, B.; Urlacher, V.B.; Pleiss, J.; Gläser, R.; Einicke, W.-D.; Sprenger, G.A.; Beifuß, U.; Klemm, E.; et al. Selective Catalytic Oxidation of C-H Bonds with Molecular Oxygen. Chemcatchem 2012, 5, 82–112. [Google Scholar] [CrossRef]
- Qiu, Y.; Hartwig, J.F. Mechanism of Ni-Catalyzed Oxidations of Unactivated C(sp3)–H Bonds. J. Am. Chem. Soc. 2020, 142, 19239–19248. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Zhu, L.; Liu, H.; Fu, Y.; Yin, S.-F.; Yang, W. Microporous cobaltporphyrin covalent polymer mediated Co3O4@PNC nanocomposites for efficient catalytic C-H bond activation. Appl. Catal. A Gen. 2021, 614, 118035. [Google Scholar] [CrossRef]
- Kanbur, U.; Paterson, A.L.; Rodriguez, J.; Kocen, A.L.; Yappert, R.; Hackler, R.A.; Wang, Y.-Y.; Peters, B.; Delferro, M.; LaPointe, A.M.; et al. Zirconium-Catalyzed C–H Alumination of Polyolefins, Paraffins, and Methane. J. Am. Chem. Soc. 2023, 145, 2901–2910. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, P.; Yang, J.; Zhu, Y.-A.; Chen, D. C–H bond activation in light alkanes: A theoretical perspective. Chem. Soc. Rev. 2021, 50, 4299–4358. [Google Scholar] [CrossRef]
- Canta, M.; Rodriguez, M.; Costas, M. Recent Advances in the Selective Oxidation of Alkyl C–H Bonds Catalyzed by Iron Coordination Complexes; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 1–28. [Google Scholar] [CrossRef]
- Maldonado-Domínguez, M.; Srnec, M. Quantifiable polarity match effect on C–H bond cleavage reactivity and its limits in reaction design. Dalton Trans. 2023, 52, 1399–1412. [Google Scholar] [CrossRef]
- Horn, R.; Schlögl, R. Methane Activation by Heterogeneous Catalysis. Catal. Lett. 2014, 145, 23–39. [Google Scholar] [CrossRef] [Green Version]
- Fawcett, A.; Keller, M.J.; Herrera, Z.; Hartwig, J.F. Site Selective Chlorination of C(sp3 )−H Bonds Suitable for Late-Stage Functionalization. Angew. Chem. 2021, 133, 8357–8364. [Google Scholar] [CrossRef]
- Chen, X.; Peng, M.; Xiao, D.; Liu, H.; Ma, D. Fully Exposed Metal Clusters: Fabrication and Application in Alkane Dehydrogenation. ACS Catal. 2022, 12720–12743. [Google Scholar] [CrossRef]
- Kuznetsov, M.L.; Pombeiro, A.J. Metal-free and iron(II)-assisted oxidation of cyclohexane to adipic acid with ozone: A theoretical mechanistic study. J. Catal. 2021, 399, 52–66. [Google Scholar] [CrossRef]
- Veski, R.; Veski, S. Aliphatic dicarboxylic acids from oil shale organic matter—Historic review. Oil Shale 2019, 36. [Google Scholar] [CrossRef]
- Lee, Y.; Lin, K.-Y.A.; Kwon, E.E.; Lee, J. Renewable routes to monomeric precursors of nylon 66 and nylon 6 from food waste. J. Clean. Prod. 2019, 227, 624–633. [Google Scholar] [CrossRef]
- Bauer, E.B. Recent Advances in Iron Catalyzed Oxidation Reactions of Organic Compounds. Isr. J. Chem. 2017, 57, 1131–1150. [Google Scholar] [CrossRef]
- Siedlecka, R. Selectivity in the Aliphatic C–H Bonds Oxidation (Hydroxylation) Catalyzed by Heme- and Non-Heme Metal Complexes—Recent Advances. Catalysts 2023, 13, 121. [Google Scholar] [CrossRef]
- Zima, A.M.; Lyakin, O.Y.; Bryliakov, K.P.; Talsi, E.P. Low-Spin and High-Spin Perferryl Intermediates in Non-Heme Iron Catalyzed Oxidations of Aliphatic C−H Groups. Chem.–A Eur. J. 2021, 27, 7781–7788. [Google Scholar] [CrossRef] [PubMed]
- Tonucci, L.; Mascitti, A.; Ferretti, A.M.; Coccia, F.; D’Alessandro, N. The Role of Nanoparticle Catalysis in the Nylon Production. Catalysts 2022, 12, 1206. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; López-Francés, A.; Navalon, S.; Garcia, H. Porous Metal Organic Frameworks as Multifunctional Catalysts for Cyclohexane Oxidation. Chemcatchem 2022, 14. [Google Scholar] [CrossRef]
- Yang, J.; Liu, J.; Neumann, H.; Franke, R.; Jackstell, R.; Beller, M. Direct synthesis of adipic acid esters via palladium-catalyzed carbonylation of 1,3-dienes. Science 2019, 366, 1514–1517. [Google Scholar] [CrossRef]
- Alnefaie, R.S.; Abboud, M.; Alhanash, A.; Hamdy, M.S. Efficient Oxidation of Cyclohexane over Bulk Nickel Oxide under Mild Conditions. Molecules 2022, 27, 3145. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Poelman, H.; Marin, G.B.; Galvita, V.V. Approaches for Selective Oxidation of Methane to Methanol. Catalysts 2020, 10, 194. [Google Scholar] [CrossRef] [Green Version]
- Zima, A.M.; Lyakin, O.Y.; Bryliakov, K.P.; Talsi, E.P. On the nature of the active intermediates in iron-catalyzed oxidation of cycloalkanes with hydrogen peroxide and peracids. Mol. Catal. 2018, 455, 6–13. [Google Scholar] [CrossRef]
- Ayad, M.; Gebbink, R.J.M.K.; Le Mest, Y.; Schollhammer, P.; Le Poul, N.; Pétillon, F.Y.; Mandon, D. Mononuclear iron(ii) complexes containing a tripodal and macrocyclic nitrogen ligand: Synthesis, reactivity and application in cyclohexane oxidation catalysis. Dalton Trans. 2018, 47, 15596–15612. [Google Scholar] [CrossRef] [PubMed]
- Rydel-Ciszek, K.; Pacześniak, T.; Zaborniak, I.; Błoniarz, P.; Surmacz, K.; Sobkowiak, A.; Chmielarz, P. Iron-Based Catalytically Active Complexes in Preparation of Functional Materials. Processes 2020, 8, 1683. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Alegria, E.C.B.A.; Matias, I.A.S.; Duarte, T.A.G.; Pombeiro, A.J.L. Catalytic Performance of Fe(II)-Scorpionate Complexes towards Cyclohexane Oxidation in Organic, Ionic Liquid and/or Supercritical CO2 Media: A Comparative Study. Catalysts 2017, 7, 230. [Google Scholar] [CrossRef] [Green Version]
- Kal, S.; Xu, S.; Que, L. Bio-inspired Nonheme Iron Oxidation Catalysis: Involvement of Oxoiron(V) Oxidants in Cleaving Strong C−H Bonds. Angew. Chem. Int. Ed. 2020, 59, 7332–7349. [Google Scholar] [CrossRef]
- Lyakin, O.Y.; Bryliakov, K.P.; Talsi, E.P. Non-heme oxoiron(V) intermediates in chemo-, regio- and stereoselective oxidation of organic substrates. Coord. Chem. Rev. 2019, 384, 126–139. [Google Scholar] [CrossRef]
- Guo, M.; Corona, T.; Ray, K.; Nam, W. Heme and Nonheme High-Valent Iron and Manganese Oxo Cores in Biological and Abiological Oxidation Reactions. ACS Central. Sci. 2018, 5, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.M.; Dias, L.D.; Calvete, M.J.F. Metalloporphyrins: Bioinspired Oxidation Catalysts. ACS Catal. 2018, 8, 10784–10808. [Google Scholar] [CrossRef]
- Tabor, E.; Połtowicz, J.; Pamin, K.; Basąg, S.; Kubiak, W.W. Influence of substituents in meso-aryl groups of iron μ-oxo porphyrins on their catalytic activity in the oxidation of cycloalkanes. Polyhedron 2016, 119, 342–349. [Google Scholar] [CrossRef]
- Shul’Pin, G.B.; Nesterov, D.S.; Shul’Pina, L.S.; Pombeiro, A.J. A hydroperoxo-rebound mechanism of alkane oxidation with hydrogen peroxide catalyzed by binuclear manganese(IV) complex in the presence of an acid with involvement of atmospheric dioxygen. Inorganica Chim. Acta 2017, 455, 666–676. [Google Scholar] [CrossRef]
- Wang, W.; Xu, D.; Sun, Q.; Sun, W. Efficient Aliphatic C−H Bond Oxidation Catalyzed by Manganese Complexes with Hydrogen Peroxide. Chem.–Asian J. 2018, 13, 2458–2464. [Google Scholar] [CrossRef] [PubMed]
- Ottenbacher, R.V.; Talsi, E.P.; Bryliakov, K.P. Direct Selective Oxidative Functionalization of C–H Bonds with H2O2: Mn-Aminopyridine Complexes Challenge the Dominance of Non-Heme Fe Catalysts. Molecules 2016, 21, 1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hull, J.F.; Balcells, D.; Sauer, E.L.O.; Raynaud, C.; Brudvig, G.W.; Crabtree, R.H.; Eisenstein, O. Manganese Catalysts for C−H Activation: An Experimental/Theoretical Study Identifies the Stereoelectronic Factor That Controls the Switch between Hydroxylation and Desaturation Pathways. J. Am. Chem. Soc. 2010, 132, 7605–7616. [Google Scholar] [CrossRef] [Green Version]
- Srour, H.; Le Maux, P.; Chevance, S.; Simonneaux, G. Metal-catalyzed asymmetric sulfoxidation, epoxidation and hydroxylation by hydrogen peroxide. Coord. Chem. Rev. 2013, 257, 3030–3050. [Google Scholar] [CrossRef] [Green Version]
- Salomão, G.C.; Olsen, M.H.; Drago, V.; Fernandes, C.; Filho, L.C.; Antunes, O. Oxidation of cyclohexane promoted by [Fe(III)(Salen)Cl] and [Mn(III)(Salen)Cl]. Catal. Commun. 2007, 8, 69–72. [Google Scholar] [CrossRef]
- Silva, A.C.; Fernández, T.L.; Carvalho, N.M.; Herbst, M.H.; Bordinhão, J.; Horn, A., Jr.; Wardell, J.L.; Oestreicher, E.G.; Antunes, O. Oxidation of cyclohexane catalyzed by bis-(2-pyridylmethyl)amine Cu(II) complexes. Appl. Catal. A Gen. 2007, 317, 154–160. [Google Scholar] [CrossRef]
- Carvalho, N.M.; Horn, A., Jr.; Antunes, O. Cyclohexane oxidation catalyzed by mononuclear iron(III) complexes. Appl. Catal. A Gen. 2006, 305, 140–145. [Google Scholar] [CrossRef]
- Zhang, W.; Loebach, J.L.; Wilson, S.R.; Jacobsen, E.N. Enantioselective epoxidation of unfunctionalized olefins catalyzed by salen manganese complexes. J. Am. Chem. Soc. 1990, 112, 2801–2803. [Google Scholar] [CrossRef]
- Jacobsen, E.N.; Zhang, W.; Muci, A.R.; Ecker, J.R.; Deng, L. Highly enantioselective epoxidation catalysts derived from 1,2-diaminocyclohexane. J. Am. Chem. Soc. 1991, 113, 7063–7064. [Google Scholar] [CrossRef]
- Doctrow, S.R.; Huffman, K.; Marcus, C.B.; Tocco, G.; Malfroy, E.; Adinolfi, C.A.; Kruk, H.; Baker, K.; Lazarowych, N.; Mascarenhas, J.; et al. Salen−Manganese Complexes as Catalytic Scavengers of Hydrogen Peroxide and Cytoprotective Agents: Structure−Activity Relationship Studies. J. Med. Chem. 2002, 45, 4549–4558. [Google Scholar] [CrossRef] [PubMed]
- Tovmasyan, A.; Maia, C.G.; Weitner, T.; Carballal, S.; Sampaio, R.S.; Lieb, D.; Ghazaryan, R.; Ivanovic-Burmazovic, I.; Ferrer-Sueta, G.; Radi, R.; et al. A comprehensive evaluation of catalase-like activity of different classes of redox-active therapeutics. Free. Radic. Biol. Med. 2015, 86, 308–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segat, B.B.; Menezes, L.B.; Cervo, R.; Cargnelutti, R.; Tolentino, H.; Latini, A.; Horn, A., Jr.; Fernandes, C. Scavenging of reactive species probed by EPR and ex-vivo nanomolar reduction of lipid peroxidation of manganese complexes. J. Inorg. Biochem. 2023, 239. [Google Scholar] [CrossRef]
- Sasmal, S.; Rana, S.; Lahiri, G.K.; Maiti, D. Manganese-salen catalyzed oxidative benzylic chlorination. J. Chem. Sci. 2018, 130, 88. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Groves, J.T. Manganese Porphyrins Catalyze Selective C−H Bond Halogenations. J. Am. Chem. Soc. 2010, 132, 12847–12849. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.-L.; Wang, K.; Wang, B.; Su, J.; Zou, X.; Xie, Y.; Li, J.-R.; Zhou, H.-C. A Base-Resistant Metalloporphyrin Metal–Organic Framework for C–H Bond Halogenation. J. Am. Chem. Soc. 2016, 139, 211–217. [Google Scholar] [CrossRef]
- Liu, W.; Groves, J.T. Manganese Catalyzed C–H Halogenation. Accounts Chem. Res. 2015, 48, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Carney, J.R.; Dillon, B.R.; Thomas, S.P. Recent Advances of Manganese Catalysis for Organic Synthesis. Eur. J. Org. Chem. 2016, 2016, 3912–3929. [Google Scholar] [CrossRef]
- Li, G.; Dilger, A.K.; Cheng, P.T.; Ewing, W.R.; Groves, J.T. Selective C−H Halogenation with a Highly Fluorinated Manganese Porphyrin. Angew. Chem. Int. Ed. 2017, 57, 1251–1255. [Google Scholar] [CrossRef]
- Lin, R.; Amrute, A.P.; Pérez-Ramírez, J. Halogen-Mediated Conversion of Hydrocarbons to Commodities. Chem. Rev. 2017, 117, 4182–4247. [Google Scholar] [CrossRef]
- Wu, W.; Fu, Z.; Wen, X.; Wang, Y.; Zou, S.; Meng, Y.; Liu, Y.; Kirk, S.R.; Yin, D. Light-triggered oxy-chlorination of cyclohexane by metal chlorides. Appl. Catal. A Gen. 2014, 469, 483–489. [Google Scholar] [CrossRef]
- Borowski, T.; Noack, H.; Radoń, M.; Zych, K.; Siegbahn, P.E.M. Mechanism of Selective Halogenation by SyrB2: A Computational Study. J. Am. Chem. Soc. 2010, 132, 12887–12898. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.K.-W.; Law, B.Y.-K.; Yao, X.-J.; Chen, X.; Xu, S.W.; Liu, L.; Leung, E.L.-H. Advanced research technology for discovery of new effective compounds from Chinese herbal medicine and their molecular targets. Pharmacol. Res. 2016, 111, 546–555. [Google Scholar] [CrossRef]
- Crowe, C.; Molyneux, S.; Sharma, S.V.; Zhang, Y.; Gkotsi, D.S.; Connaris, H.; Goss, R.J.M. Halogenases: A palette of emerging opportunities for synthetic biology–synthetic chemistry and C–H functionalisation. Chem. Soc. Rev. 2021, 50, 9443–9481. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lin, J.-H.; Xiao, J.-C. Halogenation through Deoxygenation of Alcohols and Aldehydes. Org. Lett. 2018, 20, 3061–3064. [Google Scholar] [CrossRef]
- Chen, J.; Lin, J.-H.; Xiao, J.-C. Dehydroxylation of alcohols for nucleophilic substitution. Chem. Commun. 2018, 54, 7034–7037. [Google Scholar] [CrossRef]
- Hennecke, U. New Catalytic Approaches towards the Enantioselective Halogenation of Alkenes. Chem.–Asian J. 2012, 7, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, C.; Yang, X. Recent Advances in Hydrochlorination of Alkenes. Chin. J. Org. Chem. 2021, 41, 3808. [Google Scholar] [CrossRef]
- Ding, L.; Tang, J.; Cui, M.; Bo, C.; Chen, X.; Qiao, X. Optimum Design and Analysis Based on Independent Reaction Amount for Distillation Column with Side Reactors: Production of Benzyl Chloride. Ind. Eng. Chem. Res. 2011, 50, 11143–11152. [Google Scholar] [CrossRef]
- Kolvari, E.; Ghorbani-Choghamarani, A.; Salehi, P.; Shirini, F.; Zolfigol, M.A. Application of N-halo reagents in organic synthesis. J. Iran. Chem. Soc. 2007, 4, 126–174. [Google Scholar] [CrossRef]
- Gomes, C.A.; Lube, L.M.; Fernandes, C.; Franco, R.W.A.; Resende, J.A.L.C.; Horn, A. A new system for cyclohexane functionalization employing iron(iii) catalysts and trichloroisocyanuric acid. New J. Chem. 2017, 41, 11498–11502. [Google Scholar] [CrossRef]
- Melo, I.L.; Lube, L.M.; Neves, E.S.; Terra, W.S.; Fernandes, C.; Matos, C.R.; Franco, R.W.; Resende, J.A.; Valente, D.C.; Horta, B.A.; et al. Experimental and theoretical studies of a greener catalytic system for saturated hydrocarbon chlorination composed by trichloroisocyanuric acid and a copper(II) compound. Appl. Catal. A Gen. 2018, 562, 150–158. [Google Scholar] [CrossRef]
- Combe, S.H.; Hosseini, A.; Parra, A.; Schreiner, P.R. Mild Aliphatic and Benzylic Hydrocarbon C–H Bond Chlorination Using Trichloroisocyanuric Acid. J. Org. Chem. 2017, 82, 2407–2413. [Google Scholar] [CrossRef]
- Ye, J.; Wang, Y.; Liu, R.; Zhang, G.; Zhang, Q.; Chen, J.; Liang, X. A Highly Enantioselective Phase-Transfer Catalyzed Epoxidation of Enones with a Mild Oxidant, Trichloroisocyanuric Acid. Cheminform 2004, 35, 2714–2715. [Google Scholar] [CrossRef]
- Wengert, M.; Sanseverino, A.M.; De Mattos, M.C.S. Trichloroisocyanuric Acid: An Alternate Green Route for the Transformation of Alkenes into Epoxides. J. Braz. Chem. Soc. 2002, 13, 700–703. [Google Scholar] [CrossRef] [Green Version]
- Wilson, G.R.; Dubey, A. Synthesis and characterization of Trichloroisocyanouric acid functionalized mesoporous silica nanocomposite (SBA/TCCA) for the Acylation of Indole. J. Chem. Sci. 2016, 128, 1285–1290. [Google Scholar] [CrossRef] [Green Version]
- Duguta, G.; Muddam, B.; Kamatala, C.R.; Chityala, Y. Symmetric trichloro triazine adducts with N, N’-dimethyl formamide and N, N’-dimethyl acetamide as green Vilsmeier–Haack reagents for effective formylation and acylation of Indoles. Chem. Data Collect. 2020, 28, 100382. [Google Scholar] [CrossRef]
- Mendonca, G.; de Mattos, M. Green Chlorination of Organic Compounds Using Trichloroisocyanuric Acid (TCCA). Curr. Org. Synth. 2014, 10, 820–836. [Google Scholar] [CrossRef]
- Gaspa, S.; Carraro, M.; Pisano, L.; Porcheddu, A.; De Luca, L.V.G. Trichloroisocyanuric Acid: A Versatile and Efficient Chlorinating and Oxidizing Reagent. Eur. J. Org. Chem. 2019, 2019, 3544–3552. [Google Scholar] [CrossRef]
- Chattaway, F.D.; Wadmore, J.M. XX—The constitution of hydrocyanic, cyanic, and cyanuric acids. J. Chem. Soc., Trans. 1902, 81, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Tilstam, U.; Weinmann, H. Trichloroisocyanuric Acid: A Safe and Efficient Oxidant. Org. Process. Res. Dev. 2002, 6, 384–393. [Google Scholar] [CrossRef]
- Tozetti, S.D.F.; de Almeida, L.S.; Esteves, P.M.; de Mattos, M.C.S. Trihaloisocyanuric acids/NaX: An environmentaly friendly system for vicinal dihalogenation of alkenes without using molecular halogen. J. Braz. Chem. Soc. 2007, 18, 675–677. [Google Scholar] [CrossRef] [Green Version]
- Blödorn, G.B.; Duarte, L.F.B.; Roehrs, J.A.; Silva, M.S.; Neto, J.S.S.; Alves, D. Trichloroisocyanuric Acid (TCCA): A Suitable Reagent for the Synthesis of Selanyl-benzo[b]chalcogenophenes. Eur. J. Org. Chem. 2022, 2022. [Google Scholar] [CrossRef]
- Sankaralingam, M.; Palaniandavar, M. Tuning the olefin epoxidation by manganese(iii) complexes of bisphenolate ligands: Effect of Lewis basicity of ligands on reactivity. Dalton Trans. 2013, 43, 538–550. [Google Scholar] [CrossRef]
- Deawati, Y.; Onggo, D.; Mulyani, I.; Hastiawan, I.; Kurnia, D.; Lönnecke, P.; Schmorl, S.; Kersting, B.; Hey-Hawkins, E. Synthesis, crystal structures, and superoxide dismutase activity of two new multinuclear manganese(III)-salen-4,4′-bipyridine complexes. Inorganica Chim. Acta 2018, 482, 353–357. [Google Scholar] [CrossRef]
- Abbasi, V.; Hosseini-Monfared, H.; Hosseini, S.M. Mn(III)-salan/graphene oxide/magnetite nanocomposite as a highly selective catalyst for aerobic epoxidation of olefins. Appl. Organomet. Chem. 2016, 31, e3554. [Google Scholar] [CrossRef]
- Stefan, M.; Nistor, S.; Barascu, J. Accurate determination of the spin Hamiltonian parameters for Mn2+ ions in cubic ZnS nanocrystals by multifrequency EPR spectra analysis. J. Magn. Reson. 2011, 210, 200–209. [Google Scholar] [CrossRef]
- Vezin, H.; Lamour, E.; Routier, S.; Villain, F.; Bailly, C.; Bernier, J.-L.; Catteau, J.P. Free radical production by hydroxy-salen manganese complexes studied by ESR and XANES. J. Inorg. Biochem. 2002, 92, 177–182. [Google Scholar] [CrossRef]
- Irie, R.; Hashihayata, T.; Katsuki, T.; Akita, M.; Moro-oka, Y. X-Ray Structures of Chiral (Salen)manganese(III) Complexes: Proof of Pliability oh the Salen Ligand. Chem. Soc. Japan 1998, 1041–1042. [Google Scholar] [CrossRef]
- Jacobsen, H.; Cavallo, L. A Possible Mechanism for Enantioselectivity in the Chiral Epoxidation of Olefins with [Mn(salen)] Catalysts. Chem.–A Eur. J. 2001, 7, 800–807. [Google Scholar] [CrossRef]
- Zhang, W.; Jacobsen, E.N. ChemInform Abstract: Asymmetric Olefin Epoxidation with Sodium Hypochlorite Catalyzed by Easily Prepared Chiral Mn(III) Salen Complexes. Cheminform 1991, 22. [Google Scholar] [CrossRef]
- Motati, D.R.; Uredi, D.; Watkins, E.B. A general method for the metal-free, regioselective, remote C–H halogenation of 8-substituted quinolines. Chem. Sci. 2018, 9, 1782–1788. [Google Scholar] [CrossRef] [Green Version]
- Brady, A.P.; Sancier, K.M.; Sirine, G. Equilibria in Solutions of Cyanuric Acid and its Chlorinated Derivatives. J. Am. Chem. Soc. 1963, 85, 3101–3104. [Google Scholar] [CrossRef]
- Kurahashi, T.; Kikuchi, A.; Tosha, T.; Shiro, Y.; Kitagawa, T.; Fujii, H. Transient Intermediates from Mn(salen) with Sterically Hindered Mesityl Groups: Interconversion between MnIV-Phenolate and MnIII-Phenoxyl Radicals as an Origin for Unique Reactivity. Inorg. Chem. 2008, 47, 1674–1686. [Google Scholar] [CrossRef]
- Nesterov, D.S.; Nesterova, O.V.; Pombeiro, A.J.L. Homo- and heterometallic polynuclear transition metal catalysts for alkane C H bonds oxidative functionalization: Recent advances. Coord. Chem. Rev. 2018, 355, 199–222. [Google Scholar] [CrossRef]
- Groves, J.T.; Kruper, W.J., Jr.; Haushalter, R.C. Hydrocarbon oxidations with oxometalloporphinates. Isolation and reactions of a (porphinato)manganese(V) complex. J. Am. Chem. Soc. 1980, 102, 6375–6377. [Google Scholar] [CrossRef]
- Mayilmurugan, R.; Sankaralingam, M.; Suresh, E.; Palaniandavar, M. Novel square pyramidal iron(iii) complexes of linear tetradentate bis(phenolate) ligands as structural and reactive models for intradiol-cleaving 3,4-PCD enzymes: Quinone formation vs. intradiol cleavage. Dalton Trans. 2010, 39, 9611–9625. [Google Scholar] [CrossRef] [PubMed]
- Haikarainen, A.; Sipilä, J.; Pietikäinen, P.; Pajunen, A.; Mutikainen, I. Synthesis and characterization of bulky salen-type complexes of Co, Cu, Fe, Mn and Ni with amphiphilic solubility properties. J. Chem. Soc. Dalton Trans. 2001, 991–995. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, D.; Yu, R.; Chen, S.; Zhao, D. Tetrahydrosalen Uranyl(VI) Complexes: Crystal Structures and Solution Binding Study. Eur. J. Inorg. Chem. 2018, 2018, 1185–1191. [Google Scholar] [CrossRef]
- Boucher, L.J. Manganese Schiff's base complexes—I: Synthesis and spectroscopy of some anion complexes of (4-sec-butylsalicylaldehydeethylenediiminato) manganese(III). J. Inorg. Nucl. Chem. 1973, 35, 3731–3738. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neves, E.S.; Fernandes, C.; Horn, A., Jr. Study of Cyclohexane and Methylcyclohexane Functionalization Promoted by Manganese(III) Compounds. Inorganics 2023, 11, 105. https://doi.org/10.3390/inorganics11030105
Neves ES, Fernandes C, Horn A Jr. Study of Cyclohexane and Methylcyclohexane Functionalization Promoted by Manganese(III) Compounds. Inorganics. 2023; 11(3):105. https://doi.org/10.3390/inorganics11030105
Chicago/Turabian StyleNeves, Eduardo S., Christiane Fernandes, and Adolfo Horn, Jr. 2023. "Study of Cyclohexane and Methylcyclohexane Functionalization Promoted by Manganese(III) Compounds" Inorganics 11, no. 3: 105. https://doi.org/10.3390/inorganics11030105



