Research Progress on Modifications of Zeolite Y for Improved Catalytic Properties
Abstract
:1. Introduction
2. High SiO2/Al2O3 Ratio Y Zeolites
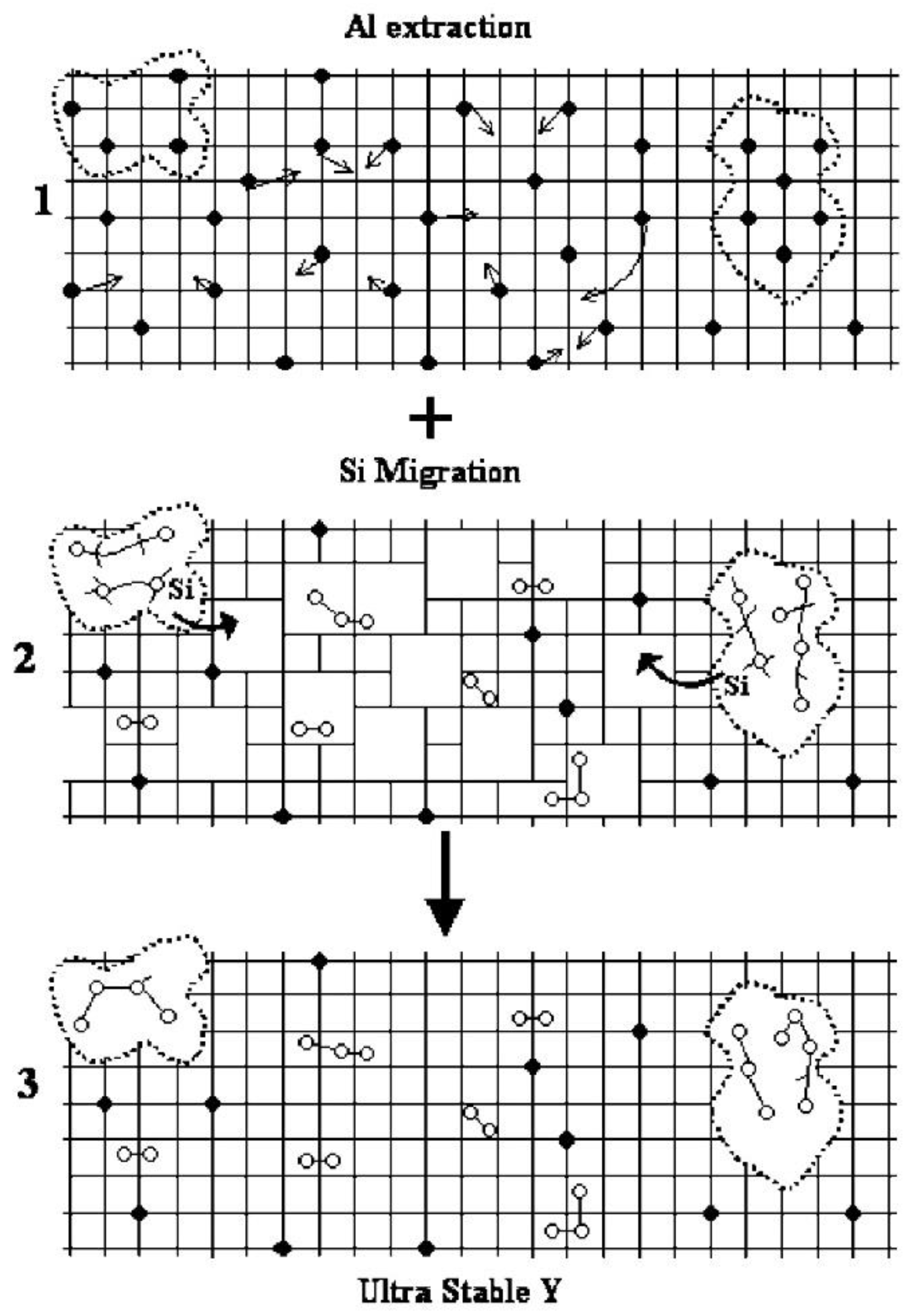
2.1. Post-Treatment Method
2.2. Direct Synthesis Method
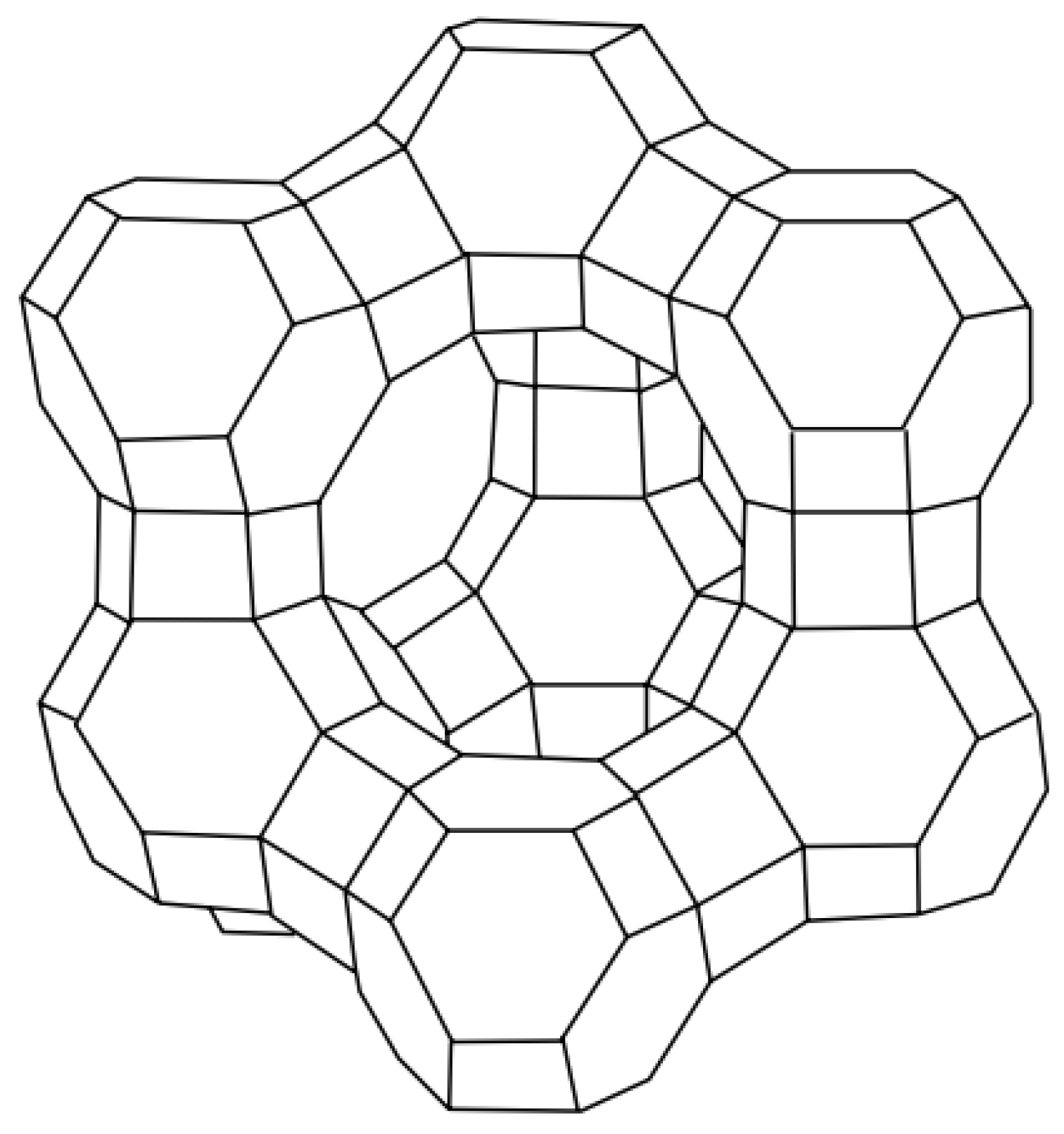
3. Hierarchically Porous Y Zeolite
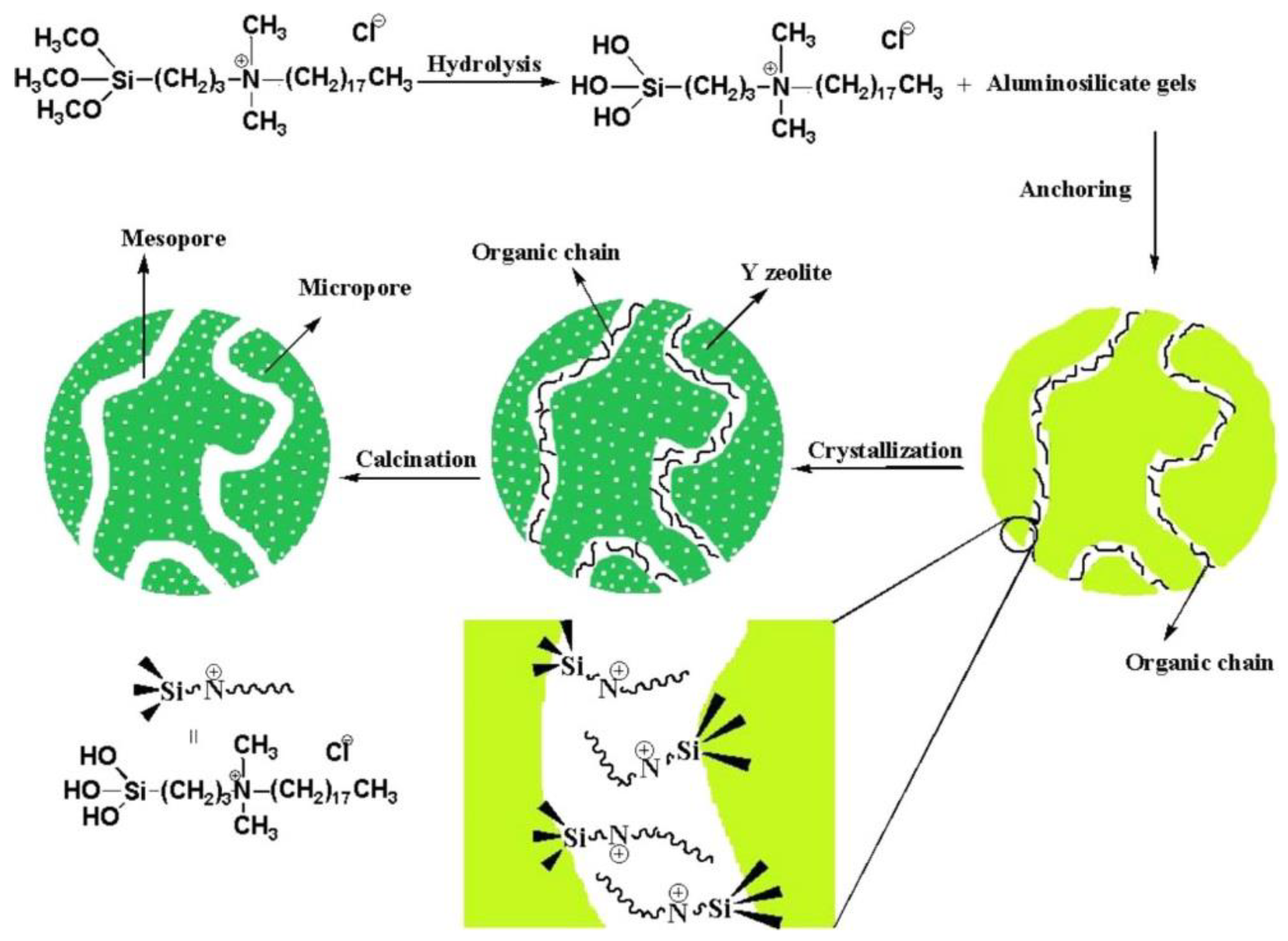
3.1. Bottom-Up Method
3.2. Top-Down Method
4. Heteroatomic Y Molecular Sieve
4.1. Direct Synthesis Method
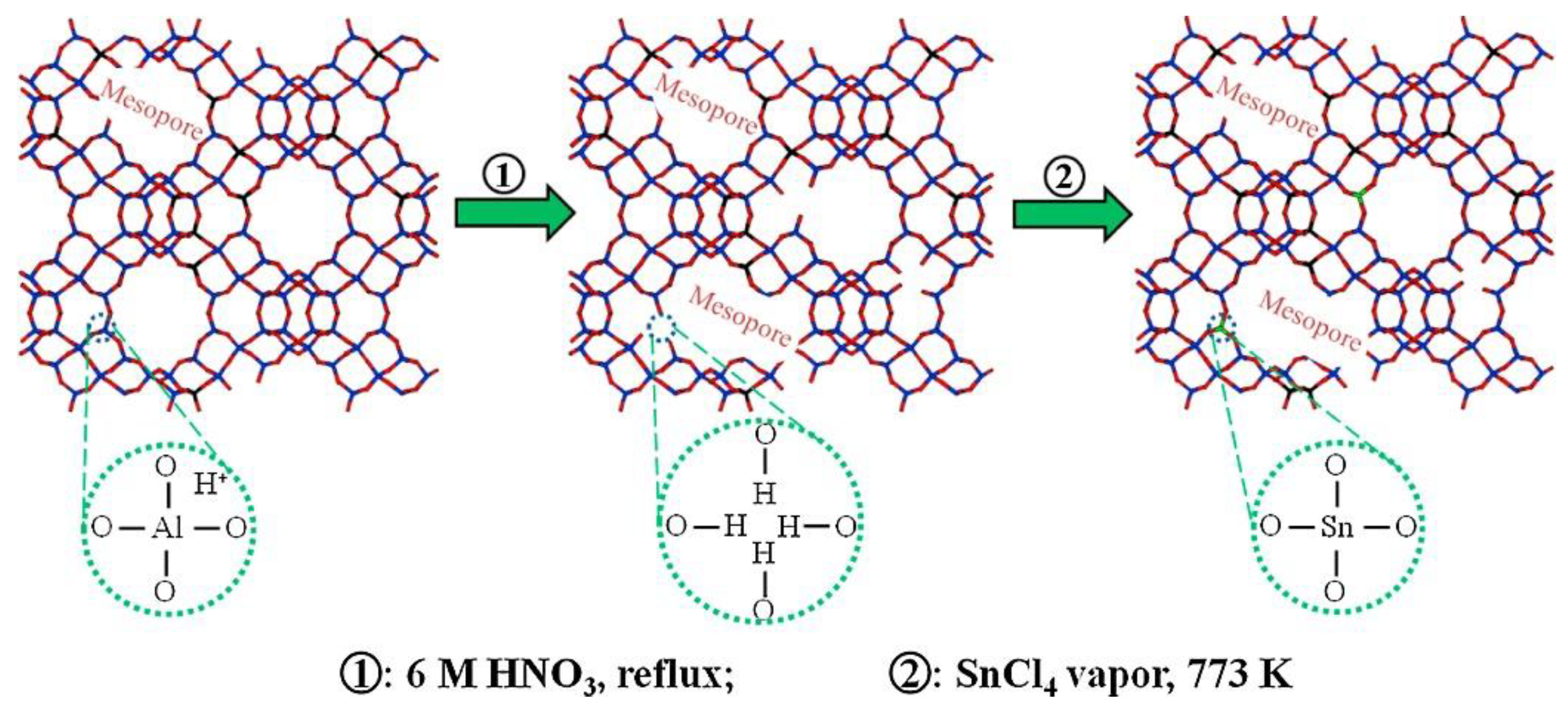
4.2. Post-Treatment Method
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, R.R.; Pang, W.Q.; Yu, J.H.; Huo, Q.S.; Chen, J.S. Molecular Sieves and Porous Materials Chemistry; Science Press: Beijing, China, 2014. [Google Scholar]
- Corma, A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem. Rev. 1997, 97, 2373–2419. [Google Scholar] [CrossRef] [PubMed]
- Corma, A. State of the art and future challenges of zeolites as catalysts. J. Catal. 2003, 216, 298–312. [Google Scholar] [CrossRef]
- Sun, M.H.; Huang, S.Z.; Chen, L.H.; Li, Y.; Yang, X.Y.; Yuan, Z.Y.; Su, B.L. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem. Soc. Rev. 2016, 45, 3479–3563. [Google Scholar] [CrossRef] [PubMed]
- Lutz, W. Zeolite Y: Synthesis, modification, and properties—A case revisited. Adv. Mater. Sci. Eng. 2014, 1, 724248. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Nishimura, Y.; Honna, K.; Matsubayashi, N.; Shimada, H. Role of HY Zeolite Mesopores in Hydrocracking of Heavy Oils. J. Catal. 2001, 200, 288–297. [Google Scholar] [CrossRef]
- Martins, L.; Hölderich, W.; Cardoso, D. Methylammonium-FAU zeolite: Investigation of the basic sites in base catalyzed reactions and its performance. J. Catal. 2008, 258, 14–24. [Google Scholar] [CrossRef]
- Cheng, Z.; Gao, E.; Wan, H. Novel synthesis of FAU-type zeolite membrane with high performance. Chem. Commun. 2004, 15, 1718–1719. [Google Scholar] [CrossRef] [PubMed]
- Hamad, B.; Perard, A.; Figueras, F.; Rataboul, F.; Prakash, S.; Essayem, N. Zirconia modified by Cs cationic exchange: Physico-chemical and catalytic evidences of basicity enhancement. J. Catal. 2010, 269, 122–130. [Google Scholar] [CrossRef]
- Vogt, E.T.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef] [Green Version]
- Vermeiren, W.; Gilson, J.-P. Impact of zeolites on the petroleum and petrochemical industry. Top. Catal. 2009, 52, 1131–1161. [Google Scholar] [CrossRef]
- De Jong, K.P.; Zečević, J.; Friedrich, H.; De Jongh, P.E.; Bulut, M.; Van Donk, S.; Kenmogne, R.; Finiels, A.; Hulea, V.; Fajula, F. Zeolite Y crystals with trimodal porosity as ideal hydrocracking catalysts. Angew. Chem. Int. Ed. 2010, 49, 10074–10078. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Zhou, Y.; Wei, Q.; Cui, Q.; Dai, Y. Physicochemical properties of small grain Y molecular sieves with different silica-alumina ratios and their hydrocracking performance. Petrochem. Technol. 2013, 10, 1080–1085. [Google Scholar]
- Zhao, X.; Xu, A.; Wang, B.; Zhao, H.; Shen, B.; Zhang, L.; Shi, X.; Liu, C. Synthesis and modification of high-silica NaY molecular sieve. Petrochem. Technol. Appl. 2009, 2, 107–111. [Google Scholar]
- Liu, X.; Qian, L.; Yan, Z. Review of chemical modification methods of Y-type molecular sieve. J. Petrochem. Univ. 1997, 4, 27–31. [Google Scholar]
- Bazyari, A.; Khodadadi, A.A.; Hosseinpour, N.; Mortazavi, Y. Effects of steaming-made changes in physicochemical properties of Y-zeolite on cracking of bulky 1,3,5-triisopropylbenzene and coke formation. Fuel Process. Technol. 2009, 90, 1226–1233. [Google Scholar] [CrossRef]
- Beyerlein, R.A.; McVicker, G.B. Defect Structure and Acid Catalysis of High Silica, FAU-Framework Zeolites: Effects of Aluminum Removal and of Basic Metal Oxide Addition. Stud. Surf. Sci. Catal. 2001, 134, 3–40. [Google Scholar]
- Li, S.; Zheng, A.; Su, Y.; Zhang, H.; Chen, L.; Yang, J.; Ye, C.; Deng, F. Brønsted/Lewis Acid Synergy in Dealuminated HY Zeolite: A Combined Solid-State NMR and Theoretical Calculation Study. J. Am. Chem. Soc. 2007, 129, 11161–11171. [Google Scholar] [CrossRef]
- Yurtaeva, A.S.; Sorokina, T.P.; Plekhova, K.S.; Potapenko, O.V.; Gulyaeva, T.I.; Talsi, V.P.; Doronin, V.P. Effect of modification conditions on the physicochemical characteristics of Y zeolite as a component of a petrochemical cracking catalyst. Pet. Chem. 2021, 61, 325–331. [Google Scholar] [CrossRef]
- López-Fonseca, R.; De Rivas, B.; Gutiérrez-Ortiz, J.I.; Aranzabal, A.; González-Velasco, J.R. Enhanced activity of zeolites by chemical dealumination for chlorinated VOC abatement. Appl. Catal. B Environ. 2003, 41, 31–42. [Google Scholar] [CrossRef]
- Yan, Z.; Ma, D.; Zhuang, J.; Liu, X.; Liu, X.; Han, X.; Bao, X.; Chang, F.; Xu, L.; Liu, Z. On the acid-dealumination of USY zeolite: A solid state NMR investigation. J. Mol. Catal. A Chem. 2003, 194, 153–167. [Google Scholar] [CrossRef]
- Maghfirah, A.; Susanti, Y.; Fajar, A.T.; Mukti, R.R.; Kadja, G.T. The role of tetraalkylammonium for controlling dealumination of zeolite Y in acid media. Mater. Res. Express 2019, 6, 094002. [Google Scholar] [CrossRef]
- Yu, Y.; Feng, A.; Yu, Y.; Mi, L.; Chen, B.; Sun, F.; Song, L. A Method of Preparing Y Zeolite with High Crystallinity and High Silicon to Aluminum Ratio. China, CN 108892150A, 27 November 2018. [Google Scholar]
- Yakimov, A.V.; Zasukhin, D.S.; Vorobkalo, V.A.; Ponomareva, O.A.; Knyazeva, E.E.; Zaikovskii, V.I.; Kolozhvari, B.A.; Ivanova, I.I. Dealumination of Nanosized Zeolites Y. Pet. Chem. 2019, 59, 540–545. [Google Scholar] [CrossRef]
- Hosseini, M.; Zanjanchi, M.A.; Ghalami-Choobar, B.; Golmojdeh, H. Ultrasound-assisted dealumination of zeolite Y. J. Chem. Sci. 2015, 127, 25–31. [Google Scholar] [CrossRef]
- Buttersack, C.; König, A.; Gläser, R. Stability of a highly dealuminated Y-zeolite in liquid aqueous media. Microporous Mesoporous Mater. 2019, 281, 148–160. [Google Scholar] [CrossRef]
- Wang, W.; Liu, B.; Gao, S.; Li, M. Hydrocracking performance of combined modified Y-type molecular sieve. J. Fuel Chem. Technol. 2009, 37, 454–458. [Google Scholar]
- Delprato, F.; Delmotte, L.; Guth, J.L.; Huve, L. Synthesis of new silica-rich cubic and hexagonal faujasites using crown-etherbased supramolecules as templates. Zeolites 1990, 10, 546–552. [Google Scholar] [CrossRef]
- Zhu, L.; Ren, L.; Zeng, S.; Yang, C.; Zhang, H.; Meng, X.; Rigutto, M.; Van Der Made, A.; Xiao, F.S. High temperature synthesis of high silica zeolite Y with good crystallinity in the presence of N-methylpyridinium iodide. Chem. Commun. 2013, 49, 10495–10497. [Google Scholar] [CrossRef]
- Yuan, D.; He, D.; Xu, S.; Song, Z.; Zhang, M.; Wei, Y.; He, Y.; Xu, S.; Liu, Z.; Xu, Y. Imidazolium-based ionic liquids as novel organic SDA to synthesize high-silica Y zeolite. Microporous Mesoporous Mater. 2015, 204, 1–7. [Google Scholar] [CrossRef]
- He, D.; Yuan, D.; Xu, Y.; Liu, Z.; Song, Z. A Method of Preparing Y-Type Molecular Sieve with High Silica-Alumina Ratio. China CN109502604A, 22 March 2019. [Google Scholar]
- He, D.; Yuan, D.; Song, Z.; Tong, Y.; Wu, Y.; Xu, S.; Xu, Y.; Liu, Z. Hydrothermal synthesis of high silica zeolite Y using tetraethylammonium hydroxide as a structure-directing agent. Chem. Commun. 2016, 52, 12765–12768. [Google Scholar] [CrossRef]
- Yan, W.; Zhu, K.; Mi, Z. A Preparation Method of High Silicon-Aluminum Ratio Y-Type Molecular Sieve. China, CN 110963502A, 4 July 2020. [Google Scholar]
- Li, R.; Linares, N.; Sutjianto, J.G.; Chawla, A.; Garcia-Martinez, J.; Rimer, J.D. Ultrasmall Zeolite L Crystals Prepared from Highly-Interdispersed Alkali-Silicate Precursors. Angew. Chem. Int. Ed. 2018, 130, 11453–11458. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Wang, L.; Fan, D.; Yan, N.; Huang, S.; Xu, S.; Guo, P.; Yang, M.; Zhang, J.; Tian, P.; et al. A Bottom-Up Strategy for the Synthesis of Highly Siliceous Faujasite-Type Zeolite. Adv. Mater. 2020, 32, 2000272. [Google Scholar] [CrossRef] [PubMed]
- Oleksiak, M.D.; Muraoka, K.; Hsieh, M.F.; Conato, M.T.; Shimojima, A.; Okubo, T.; Chaikittisilp, W.; Rimer, J.D. Organic-Free Synthesis of a Highly Siliceous Faujasite Zeolite with Spatially Biased Q4 (nAl) Si Speciation. Angew. Chem. Int. Ed. 2017, 129, 13551–13556. [Google Scholar] [CrossRef]
- Ferdov, S.; Tsuchiya, K.; Tsunoji, N.; Sano, T. Comparative study between high-silica faujasites (FAU) from organic-free system and the commercial zeolite Y. Microporous Mesoporous Mater. 2019, 276, 154–159. [Google Scholar] [CrossRef]
- Wang, J.; Liu, P.; Boronat, M.; Ferri, P.; Xu, Z.; Liu, P.; Shen, B.; Wang, Z.; Yu, J. Organic-Free Synthesis of High Silica Zeolite Y via a Combined Strategy of In Situ Hydroxyl Radical Assistance and Post-Synthesis Treatment. Angew. Chem. Int. Ed. 2020, 132, 17378–17381. [Google Scholar] [CrossRef]
- Zhang, K.; Ostraat, M.L. Innovations in hierarchical zeolite synthesis. Catal. Today 2016, 264, 3–15. [Google Scholar] [CrossRef]
- Na, K.; Park, W.; Seo, Y.; Ryoo, R. Disordered Assembly of MFI Zeolite Nanosheets with a Large Volume of Intersheet Mesopores. Chem. Mater. 2011, 23, 1273–1279. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Y.; Zhu, L.; Rigutto, M.; van der Made, A.; Yang, C.; Pan, S.; Wang, L.; Zhu, L.; Jin, Y.; et al. Highly mesoporous single-crystalline zeolite beta synthesized using a nonsurfactant cationic polymer as a dual-function template. J. Am. Chem. Soc. 2014, 136, 2503–2510. [Google Scholar] [CrossRef]
- Stanislaus, A.; Marafi, A.; Rana, M.S. Recent advances in the science and technology of ultra low sulfur diesel (ULSD) production. Catal. Today 2010, 153, 1–68. [Google Scholar] [CrossRef]
- Lopez-Orozco, S.; Inayat, A.; Schwab, A.; Selvam, T.; Schwieger, W. Zeolitic Materials with Hierarchical Porous Structures. Adv. Mater. 2011, 23, 2602–2615. [Google Scholar] [CrossRef]
- Moller, K.; Bein, T. Mesoporosity—A new dimension for zeolites. Chem. Soc. Rev. 2013, 42, 3689–3707. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, I.; Boisen, A.; Gustavsson, E.; Ståhl, K.; Pehrson, S.; Dahl, S.; Carlsson, A.; Jacobsen, C.J. Carbon Nanotube Templated Growth of Mesoporous Zeolite Single Crystals. Chem. Mater. 2001, 13, 4416–4418. [Google Scholar] [CrossRef]
- Du, F.; Liu, J.; Guo, Z. Shape controlled synthesis of Cu2O and its catalytic application to synthesize amorphous carbon nanofibers. Mater. Res. Bull. 2009, 44, 25–29. [Google Scholar] [CrossRef]
- Yang, Z.X.; Xia, Y.D.; Mokaya, R. Zeolite ZSM-5 with Unique Supermicropores Synthesized Using Mesoporous Carbon as a Template. Adv. Mater. 2004, 16, 727–732. [Google Scholar] [CrossRef]
- Tao, Y.S.; Kanoh, H.; Kaneko, K. Uniform Mesopore-Donated Zeolite Y Using Carbon Aerogel Templating. J. Phys. Chem. B 2003, 107, 10974–10976. [Google Scholar] [CrossRef]
- Tao, Y.; Kanoh, H.; Hanzawa, Y.; Kaneko, K. Template synthesis and characterization of mesoporous zeolites. Colloids Surf. A Physicochem. Eng. Asp. 2004, 241, 75–80. [Google Scholar] [CrossRef]
- Abdulridha, S.; Jiao, Y.; Xu, S.; Zhang, R.; Ren, Z.; Garforth, A.A.; Fan, X. A comparative study on mesoporous Y zeolites prepared by hard-templating and post-synthetic treatment methods. Appl. Catal. A Gen. 2021, 612, 117986. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, L.; Tang, T.; Ke, Q.; Wang, S.; Hu, J.; Fang, G.; Li, J.; Xiao, F.S. Extraordinarily High Activity in the Hydrodesulfurization of 4, 6-Dimethyldibenzothiophene over Pd Supported on Mesoporous Zeolite Y. J. Am. Chem. Soc. 2011, 133, 15346–15349. [Google Scholar] [CrossRef]
- Jin, J.; Peng, C.; Wang, J.; Liu, H.; Gao, X.; Liu, H.; Xu, C. Facile Synthesis of Mesoporous Zeolite Y with Improved Catalytic Performance for Heavy Oil Fluid Catalytic Cracking. Ind. Eng. Chem. Res. 2014, 53, 3406–3411. [Google Scholar] [CrossRef]
- Zhao, J.; Yin, Y.; Li, Y.; Chen, W.; Liu, B. Synthesis and characterization of mesoporous zeolite Y by using block copolymers as templates. Chem. Eng. J. 2016, 284, 405–411. [Google Scholar] [CrossRef]
- Wang, L.; Liu, G.; Yu, H.; Zang, J.; Hong, M.; Wang, H.; Hong, L.; Ji, C.; Qiu, Y.; Song, W. A Preparation Method of Hierarchically Porous Y Molecular Sieve. China, CN 107555446A, 9 January 2018. [Google Scholar]
- Sachse, A.; Wuttke, C.; Díaz, U.; de Souza, M.O. Mesoporous Y zeolite through ionic liquid based surfactant templating. Microporous Mesoporous Mater. 2015, 217, 81–86. [Google Scholar] [CrossRef]
- Dabbawala, A.A.; Ismail, I.; Vaithilingam, B.V.; Polychronopoulou, K.; Singaravel, G.; Morin, S.; Berthod, M.; Al Wahedi, Y. Synthesis of hierarchical porous zeolite-Y for enhanced CO2 capture. Microporous Mesoporous Mater. 2020, 303, 110261. [Google Scholar] [CrossRef]
- Cui, W.; Zhu, D.; Tan, J.; Chen, N.; Fan, D.; Wang, J.; Han, J.; Wang, L.; Tian, P.; Liu, Z. Synthesis of mesoporous high-silica zeolite Y and their catalytic cracking performance. Chin. J. Catal. 2022, 43, 1945–1954. [Google Scholar] [CrossRef]
- Martins, A.; Neves, V.; Moutinho, J.; Nunes, N.; Carvalho, A.P. Friedel-Crafts acylation reaction over hierarchical Y zeolite modified through surfactant mediated technology. Microporous Mesoporous Mater. 2021, 323, 111167. [Google Scholar] [CrossRef]
- Van Donk, S.; Janssen, A.H.; Bitter, J.H.; de Jong, K.P. Generation, characterization, and impact of mesopores in zeolite catalysts. Catal. Rev. 2003, 45, 297–319. [Google Scholar] [CrossRef] [Green Version]
- Janssen, A.H.; Koster, A.J.; De Jong, K.P. Three-dimensional transmission electron microscopic observations of mesopores in dealuminated zeolite Y. Angew. Chem. Int. Edit. 2001, 40, 1102–1104. [Google Scholar] [CrossRef]
- Triantafillidis, C.S.; Vlessidis, A.G.; Evmiridis, N.P. Dealuminated H-Y zeolites: Influence of the degree and the type of dealumination method on the structural and acidic characteristics of H-Y zeolites. Ind. Eng. Chem. Res. 2000, 39, 307–319. [Google Scholar] [CrossRef]
- Li, C.; Guo, L.; Liu, P.; Gong, K.; Jin, W.; Li, L.; Zhu, X.; Liu, X.; Shen, B. Defects in AHFS-dealuminated Y zeolite: A crucial factor for mesopores formation in the following base treatment procedure. Microporous Mesoporous Mater. 2018, 255, 242–252. [Google Scholar] [CrossRef]
- Gackowski, M.; Tarach, K.; Podobiński, J.; Jarczewski, S.; Kuśtrowski, P.; Datka, J. Hierarchical zeolites Y obtained by desilication: Porosity, acidity and catalytic properties. Microporous Mesoporous Mater. 2017, 263, 282–288. [Google Scholar] [CrossRef]
- Gackowski, M.; Tarach, K.; Kuterasiński, Ł.; Podobiński, J.; Sulikowski, B.; Datka, J. Spectroscopic IR and NMR studies of hierarchical zeolites obtained by desilication of zeolite Y: Optimization of the desilication route. Microporous Mesoporous Mater. 2019, 281, 134–141. [Google Scholar] [CrossRef]
- Duan, L.; Meng, X.; Cheng, L.; Lin, C.; Zhang, Y.; Cao, S.; Qin, H. A Method for the Preparation of Hierarchically Porous Y Molecular Sieves by Oxalic Acid-ammonia Water Co-Treatment. China, CN 110117017A, 13 August 2019. [Google Scholar]
- Qin, Z.; Cychosz, K.A.; Melinte, G.; El Siblani, H.; Gilson, J.P.; Thommes, M.; Fernandez, C.; Mintova, S.; Ersen, O.; Valtchev, V. Opening the cages of faujasite-type zeolite. J. Am. Chem. Soc. 2017, 139, 17273–17276. [Google Scholar] [CrossRef]
- Feng, A.; Yu, Y.; Mi, L.; Cao, Y.; Yu, Y.; Song, L. Synthesis and characterization of hierarchical Y zeolites using NH4HF2 as dealumination agent. Microporous Mesoporous Mater. 2019, 280, 211–218. [Google Scholar] [CrossRef]
- Zhang, R.; Raja, D.; Zhang, Y.; Yan, Y.; Garforth, A.A.; Jiao, Y.; Fan, X. Sequential Microwave-assisted dealumination and hydrothermal alkaline treatments of Y zeolite for preparing hierarchical mesoporous zeolite catalysts. Top. Catal. 2020, 63, 340–350. [Google Scholar] [CrossRef]
- Gola, A.; Rebours, B.; Milazzo, E.; Lynch, J.; Benazzi, E.; Lacombe, S.; Delevoye, L.; Fernandez, C. Effect of leaching agent in the dealumination of stabilized Y zeolites. Microporous Mesoporous Mater. 2000, 40, 73–83. [Google Scholar] [CrossRef]
- Zečević, J.; Gommes, C.J.; Friedrich, H.; de Jongh, P.E.; de Jong, K.P. Mesoporosity of zeolite Y: Quantitative three-dimensional study by image analysis of electron tomograms. Angew. Chem. 2012, 51, 4213–4217. [Google Scholar] [CrossRef]
- Xu, R.; Pang, W.; Yu, J.; Huo, Q.; Chen, J. Chemistry of Zeolites and Related Porous Materials Synthesis and Structure; Science Press: Beijing, China, 2014; pp. 359–364. [Google Scholar]
- Qin, Z.; Shen, W.; Zhou, S.; Shen, Y.; Li, C.; Zeng, P.; Shen, B. Defect-assisted mesopore formation during Y zeolite dealumination: The types of defect matter. Microporous Mesoporous Mater. 2020, 303, 110248. [Google Scholar] [CrossRef]
- Perego, G.; Millini, R.; Bellussi, G. Synthesis and Characterization of Molecular Sieves Containing Transition Metals in the Framework; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Xie, Z. New Structured High Performance Porous Catalytic Materials; China Petrochemical Press: Beijing, China, 2009; pp. 1–20. [Google Scholar]
- Corma, A.; Nemeth, L.T.; Renz, M.; Valencia, S. Sn-zeolite beta as a heterogeneous chemoselective catalyst for Baeyer-Villiger oxidations. Nature 2001, 412, 423–425. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, P.; Navrotsky, A.; Kim, S.H.; Hong, S.B. Formation and dehydration enthalpies of gallosilicate materials with different framework topologies and Ga contents. Microporous Mesoporous Mater. 2009, 121, 200–207. [Google Scholar] [CrossRef]
- Bui, L.; Luo, H.; Gunther, W.R.; Román-Leshkov, Y. Domino reaction catalyzed by zeolites with Brnsted and Lewis acid sites for the production of γ-valerolactone from furfural. Angew. Chem. Int. Ed. 2013, 52, 8022–8025. [Google Scholar] [CrossRef]
- Luo, H.Y.; Bui, L.; Gunther, W.R.; Min, E.; Román-Leshkov, Y. Synthesis and Catalytic Activity of Sn-MFI Nanosheets for the Baeyer-Villiger Oxidation of Cyclic Ketones. ACS Catal. 2012, 2, 2695–2699. [Google Scholar] [CrossRef]
- Pang, T.; Yang, X.; Yuan, C.; Elzatahry, A.A.; Alghamdi, A.; He, X.; Cheng, X.; Deng, Y. Recent advance in synthesis and application of heteroatom zeolites. Chin. Chem. Lett. 2020, 32, 328–338. [Google Scholar] [CrossRef]
- Ju, Y.; Shen, Z.; Zhao, J.; Zhu, J.; Wang, X. Synthesis and characterization of transition metal heteroatomic tiy zeolites. Ind. Catal. 2005, 13, 511–513. [Google Scholar]
- Shen, Z.; Ju, Y.; Wang, X. Effect of heteroatomic Ti on the performance of TiY zeolite. J. Fuel Chem. Technol. 2006, 5, 616–619. [Google Scholar]
- Shen, Z.; Pang, X.; Gao, X.; Ju, Y.; Qi, X.; Qi, Y.; Zhang, L.; Zhou, Z. A Catalytic Cracking Catalyst of Y Zeolites Containing Framework Heteroatoms and Its Preparation Method. China CN101898144B, 13 February 2013. [Google Scholar]
- Liu, J.; Wang, Z.; Wang, W. Investigation of supports for hydrocleavage catalysts with Ni containing Y Zeolites. J. Fuel Chem. Technol. 2012, 8, 992–995. [Google Scholar]
- Zhou, W.; Liu, M.; Zhou, Y.; Wei, Q.; Zhang, Q.; Ding, S.; Zhang, Y.; Yu, T.; You, Q. 4,6-Dimethyldibenzothiophene hydrodesulfurization on nickel-modified USY-supported NiMoS catalysts: Effects of modification method. Energy Fuels 2017, 31, 7445–7455. [Google Scholar] [CrossRef]
- Huang, W.; Wei, Q.; Zhou, Y.; Liu, X.; Liu, M.; Zhang, P.; Xu, Z.; Yu, Z.; Wang, X.; Liu, H. Hydrotreating of diesel fuel over in-situ nickel modified Y zeolite supported Ni-Mo-S catalyst. Catal. Today 2022. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, M.; Zhang, Q.; Wei, Q.; Ding, S.; Zhou, Y. Synthesis of NiMo catalysts supported on gallium-containing mesoporous Y zeolites with different gallium contents and their high activities in the hydrodesulfurization of 4, 6-dimethyldibenzothiophene. ACS Catal. 2017, 7, 7665–7679. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, S.; Wei, Q.; Mu, L.; Yu, G.; Zhang, T.; Zhou, Y. Synthesis and characterization of Zr incorporated small crystal size Y zeolite supported NiW catalysts for hydrocracking of vacuum gas oil. Fuel 2019, 237, 597–605. [Google Scholar] [CrossRef]
- Skeels, G.W.; Breck, D.W. Proceedings of the Sixth International Zeolite Conference, Reno, NV, USA, 10–15 July 1983; Butterworth: Guildford, UK, 1984; p. 87. [Google Scholar]
- Gao, Z.; Xu, J. Discussion on the Substitution Law of the Liquid-Solid Phase of Y Zeolite by Heteroatoms. Acta Chim. Sin. 1995, 2, 135–140. [Google Scholar]
- Liu, X. Gallosilicate Zeolites. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 1986. [Google Scholar]
- Dwyer, J.; Karim, K. The incorporation of heteroatoms into faujastic framework by secondary synthesis using aqueous fluoride complexes. J. Chem. Soc. Chem. Commun. 1991, 14, 905–906. [Google Scholar] [CrossRef]
- Rawlence, D.J.; Karim, K.; Dwyer, J.G. Zeolites. US Patent 5238675, 24 August 1993. [Google Scholar]
- Tang, K.; Song, L.J.; Duan, L.H.; Li, X.Q.; Gui, J.Z.; Sun, Z.L. Deep desulfurization by selective adsorption on a heteroatoms zeolite prepared by secondary synthesis. Fuel Process. Technol. 2008, 89, 1–6. [Google Scholar] [CrossRef]
- Oumi, Y.; Manabe, T.; Sasaki, H.; Inuzuka, T.; Sano, T. Preparation of Ti incorporated Y zeolites by post-synthesis method under acidic conditions and their catalytic properties. Appl. Catal. A Gen. 2010, 388, 256–261. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Xu, H.; Jiang, J.; Liu, X.; Ding, J.; Wu, P. Postsynthesis of FAU-type stannosilicate as efficient heterogeneous catalyst for Baeyer-Villiger oxidation. Appl. Catal. A Gen. 2016, 519, 155–164. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, M.; Yu, Y. Density functional calculations on the distribution of Ti in a Y zeolite and its influence on acidity. RSC Adv. 2014, 4, 4324–4329. [Google Scholar] [CrossRef]
- Pagis, C.; Prates, A.R.M.; Bats, N.; Tuel, A.; Farrusseng, D. High-silica hollow Y zeolite by selective desilication of dealuminated NaY crystals in the presence of protective Al species. CrystEngComm 2018, 20, 1564–1572. [Google Scholar] [CrossRef]
- Yu, H.; Zang, J.; Liu, G.; Hong, M.; Chen, R.; Chen, T. Acid-Modified Hierarchical Porous Rare-Earth-Containing Y Zeolite as a Highly Active and Stable Catalyst for Olefin Removal. ACS Omega 2020, 5, 18028–18034. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, J.; Yu, H.; Liu, G.; Hong, M.; Liu, J.; Chen, T. Research Progress on Modifications of Zeolite Y for Improved Catalytic Properties. Inorganics 2023, 11, 22. https://doi.org/10.3390/inorganics11010022
Zang J, Yu H, Liu G, Hong M, Liu J, Chen T. Research Progress on Modifications of Zeolite Y for Improved Catalytic Properties. Inorganics. 2023; 11(1):22. https://doi.org/10.3390/inorganics11010022
Chicago/Turabian StyleZang, Jiazhong, Haibin Yu, Guanfeng Liu, Meihua Hong, Jiawei Liu, and Tiehong Chen. 2023. "Research Progress on Modifications of Zeolite Y for Improved Catalytic Properties" Inorganics 11, no. 1: 22. https://doi.org/10.3390/inorganics11010022



