Green Synthesis of Silver Nanoparticles Using Artemisia vulgaris Extract and Its Application toward Catalytic and Metal-Sensing Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Study of Optical Properties of AgNPs by UV-Vis Spectrometry
2.2. Structural and Morphological Analysis
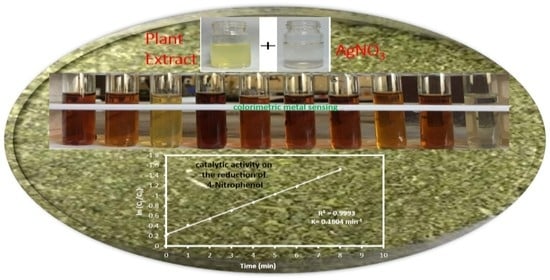
2.3. Analytical Application of Synthesized AgNPs
3. Materials and Methods
3.1. Plant Extract Preparation
3.2. Biosynthesis of Silver Nanoparticles
3.3. Colorimetric Metal Sensing
3.4. Catalytic Activity of the AgNPs
4. Conclusions
- Further optimization of protocol (effect of temperature, AgNO3 concentration, plant extract concentration) is necessary for the large-scale production of the AgNPs.
- A comprehensive study is required to prepare the AgNPs-based sensor and application in catalysis.
- The toxicity should be tested for commercialization of the AgNPs.
- Besides the study of sensing and catalytic activity of AgNPs, other properties and their possible application should be studied.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kharissova, O.V.; Dias, H.V.R.; Kharisov, B.I.; Pérez, B.O.; Pérez, V.M.J. The greener synthesis of nanoparticles. Trends Biotechnol. 2013, 31, 240–248. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Rauwel, P. Emerging Trends in Nanoparticle Synthesis Using Plant Extracts for Biomedical Applications. Glob. J. Nanomed. 2017, 1, 5555562. [Google Scholar]
- Phuyal, S.; Lamichhane, G.; Gupta, A.; Khadayat, K.; Adhikari, A.; Marahatha, R.; Khadka, S.; Parajuli, N. Biosynthesis of Silver Nanoparticles from Rhododendron arboreum for Metal Sensing, Antibacterial Assessment, and Photocatalytic Degradation. J. Nanomater. 2022, 2022, 2110636. [Google Scholar] [CrossRef]
- Chapagain, A.; Acharya, D.; Das, A.K.; Chhetri, K.; Oli, H.B.; Yadav, A.P. Alkaloid of Rhynchostylis retusa as Green Inhibitor for Mild Steel Corrosion in 1 M H2SO4 Solution. Electrochem 2022, 3, 211–224. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Bharath, L.V. Mechanism of plant-mediated synthesis of silver nanoparticles-A review on biomolecules involved, characterisation and antibacterial activity. Chem. Biol. Interact. 2017, 273, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Nadzir, M.M.; Idris, F.N.; Hat, K. Green synthesis of silver nanoparticle using Gynura procumbens aqueous extracts. AIP Conf. Proc. 2019, 2124, 030018. [Google Scholar]
- Patil, S.V.; Borase, H.P.; Patil, C.D.; Salunke, B.K. Biosynthesis of silver nanoparticles using latex from few euphorbian plants and their antimicrobial potential. Appl. Biochem. Biotechnol. 2012, 167, 776–790. [Google Scholar] [CrossRef]
- Logaranjan, K.; Devi, S.; Pandian, K. Biogenic Synthesis of Silver Nanoparticles Using Fruit Extract of Ficus Carica and Study Its Antimicrobial Activity. Nano Biomed. Eng. 2012, 4, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Aravinthan, A.; Govarthanan, M.; Selvam, K.; Praburaman, L.; Selvankumar, T.; Balamurugan, R.; Kamala-Kannan, S.; Kim, J.-H. Sunroot mediated synthesis and characterization of silver nanoparticles and evaluation of its antibacterial and rat splenocyte cytotoxic effects. Int. J. Nanomed. 2015, 10, 1977. [Google Scholar]
- Sable, N.; Gaikwad, S.; Bonde, S.; Gade, A.; Rai, M. Phytofabrication of silver nanoparticles by using aquatic plant Hydrilla verticillata. Nusant. Biosci. 2012, 4, 45–49. [Google Scholar] [CrossRef]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; De, S.P.; Misra, A. Green synthesis of AgNPs using latex of Jatropha curcas. Colliod Surf. A 2009, 39, 134–139. [Google Scholar] [CrossRef]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Hajraj, A.; Ezzat, K.; Ikram, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar]
- MubarakAli, D.; Thajuddin, N.; Jeganathan, K.; Gunasekaran, M. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf. B Biointerfaces 2011, 85, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Roni, M.; Murugan, K.; Panneerselvam, C.; Subramaniam, J.; Hwang, J.-S. Evaluation of leaf aqueous extract and synthesized silver nanoparticles using Nerium oleander against Anopheles stephensi (Diptera: Culicidae). Parasitol. Res. 2013, 112, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Ramteke, C.; Chakrabarti, T.; Sarangi, B.K.; Pandey, R.-A. Synthesis of Silver Nanoparticles from the Aqueous Extract of Leaves of Ocimum sanctum for Enhanced Antibacterial Activity. J. Chem. 2013, 2013, 278925. [Google Scholar] [CrossRef] [Green Version]
- Jacob, S.J.P.; Finub, J.S.; Narayanan, A. Synthesis of silver nanoparticles using Piper longum leaf extracts and its cytotoxic activity against Hep-2 cell line. Colloids Surf. B Biointerfaces 2012, 91, 212–214. [Google Scholar] [CrossRef]
- Karthiga, D.; Anthony, S.P. Selective colorimetric sensing of toxic metal cations by green synthesized silver nanoparticles over a wide pH range. RSC Adv. 2013, 3, 16765–16774. [Google Scholar] [CrossRef]
- Nipane, S.V.; Mahajan, P.G.; Gokavi, G.S. Green synthesis of silver nanoparticle in calotropis procera flower extract and its application for Fe2+ sensing in aqueous solution. Int. J. Recent Innov. Trends Comput. Commun 2016, 4, 98–107. [Google Scholar]
- Hoyos, L.E.S.; Sánchez-Mendieta, V.; Vilchis-Nestor, A.R.; Camacho-López, M.A. Biogenic silver nanoparticles as sensors of Cu2+ and Pb2+ in aqueous solutions. Univers. J. Mater. Sci. 2017, 5, 29–37. [Google Scholar]
- Wu, T.; Liu, C.; Tan, K.J.; Hu, P.P.; Huang, C.Z. Highly selective light scattering imaging of chromium (III) in living cells with silver nanoparticles. Anal. Bioanal. Chem. 2010, 397, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Synthesis of silver nanoparticles using Sacha inchi (Plukenetia volubilis L.) leaf extracts. Saudi J. Biol. Sci. 2014, 21, 605–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, S.P.; Lahtinen, M.; Sillanpää, M. Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 34–41. [Google Scholar] [CrossRef]
- Raman, C.; Goldsmith, M.R.; Agunbiade, T.A. Short Views on Insect Genomics and Proteomics; Springer: Berlin/Heidelberg, Germany, 2015; pp. 112–115. [Google Scholar]
- Govindarajan, M.; Rajeswary, M.; Veerakumar, K.; Muthukumaran, U.; Hoti, S.L.; Benelli, G. Green synthesis and characterization of silver nanoparticles fabricated using Anisomeles indica: Mosquitocidal potential against malaria, dengue and Japanese encephalitis vectors. Exp. Parasitol. 2016, 161, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Alzoubi, F.Y.; Bidier, S.A.A. Characterization and Aggregation of Silver Nanoparticles Dispersed in an Aqueous Solution. Chin. J. Phys. 2013, 51, 378–387. [Google Scholar]
- Vanaja, M.; Annadurai, G. Coleus aromaticus leaf extract mediated synthesis of silver nanoparticles and its bactericidal activity. Appl. Nanosci. 2013, 3, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Gautam, K.P.; Acharya, D.; Bhatta, I.; Subedi, V.; Das, M.; Neupane, S.; Kunwar, J.; Chhetri, K.; Yadav, A.P. Nickel Oxide-Incorporated Polyaniline Nanocomposites as an Efficient Electrode Material for Supercapacitor Application. Inorganics 2022, 10, 86. [Google Scholar] [CrossRef]
- Prakash, P.; Gnanaprakasam, P.; Emmanuel, R.; Arokiyaraj, S.; Saravanan, M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf. B Biointerfaces 2013, 108, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Litvin, V.A.; Minaev, B.F. Spectroscopy study of silver nanoparticles fabrication using synthetic humic substances and their antimicrobial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 108, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, Z.; Qu, W.; Shao, H.; Jiang, X. Colorimetric detection of mercury, lead and copper ions simultaneously using protein-functionalized gold nanoparticles. Biosens. Bioelectron. 2011, 26, 4064–4069. [Google Scholar] [CrossRef] [PubMed]
- Alshawi, J.M.S.; Mohammed, M.Q.; Alesary, H.F.; Ismail, H.K.; Barton, S. Voltammetric Determination of Hg2+, Zn2+, and Pb2+ Ions Using a PEDOT/NTA-Modified Electrode. ACS Omega 2022, 7, 20405–20419. [Google Scholar] [CrossRef] [PubMed]
- Katsikas, L.; Gutiérrez, M.; Henglein, A. Bimetallic colloids: Silver and mercury. J. Phys. Chem. 1996, 100, 11203–11206. [Google Scholar] [CrossRef]
- Dotzauer, D.M.; Dai, J.; Sun, L.; Bruening, M.L. Catalytic membranes prepared using layer-by-layer adsorption of polyelectrolyte/metal nanoparticle films in porous supports. Nano Lett. 2006, 6, 2268–2272. [Google Scholar] [CrossRef]
- Qian, H.; He, Q.; Zheng, J.; Li, S.; Zhang, S. Catechol-functionalized microporous organic polymer as supported media for Pd nanoparticles and its high catalytic activity. Polymer 2014, 55, 550–555. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari, A.; Lamichhane, L.; Adhikari, A.; Gyawali, G.; Acharya, D.; Baral, E.R.; Chhetri, K. Green Synthesis of Silver Nanoparticles Using Artemisia vulgaris Extract and Its Application toward Catalytic and Metal-Sensing Activity. Inorganics 2022, 10, 113. https://doi.org/10.3390/inorganics10080113
Adhikari A, Lamichhane L, Adhikari A, Gyawali G, Acharya D, Baral ER, Chhetri K. Green Synthesis of Silver Nanoparticles Using Artemisia vulgaris Extract and Its Application toward Catalytic and Metal-Sensing Activity. Inorganics. 2022; 10(8):113. https://doi.org/10.3390/inorganics10080113
Chicago/Turabian StyleAdhikari, Achyut, Laxman Lamichhane, Anup Adhikari, Gobinda Gyawali, Debendra Acharya, Ek Raj Baral, and Kisan Chhetri. 2022. "Green Synthesis of Silver Nanoparticles Using Artemisia vulgaris Extract and Its Application toward Catalytic and Metal-Sensing Activity" Inorganics 10, no. 8: 113. https://doi.org/10.3390/inorganics10080113