Composites Based on Polylactide Doped with Amorphous Europium(III) Complex as Perspective Thermosensitive Luminescent Materials
Abstract
:1. Introduction
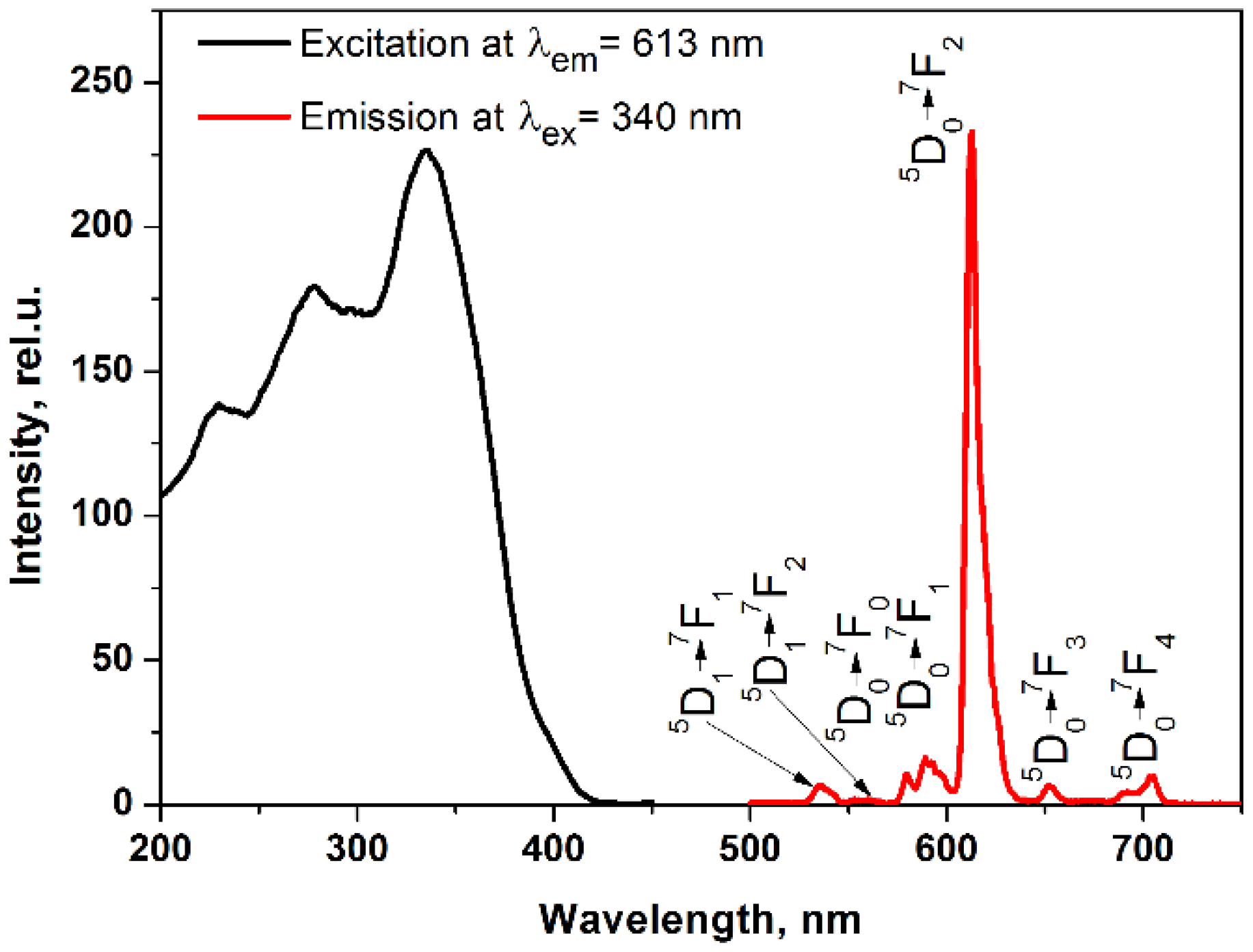
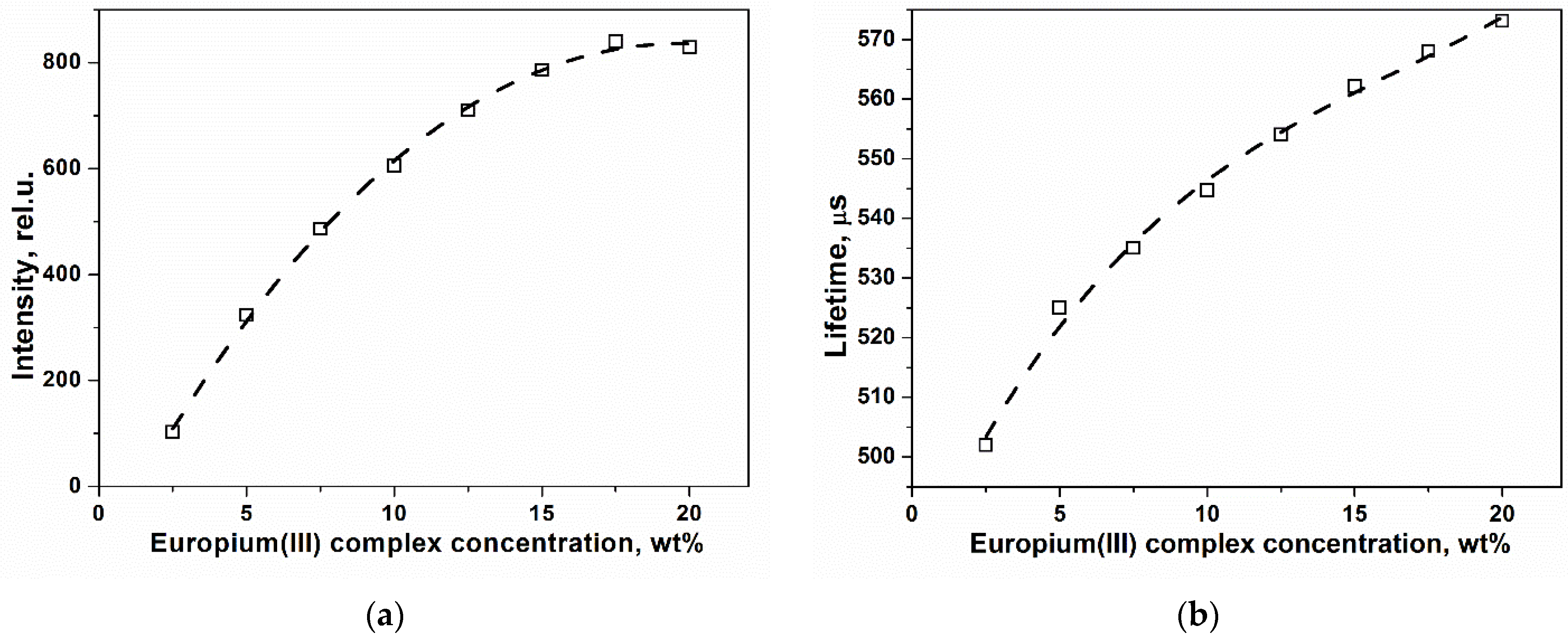
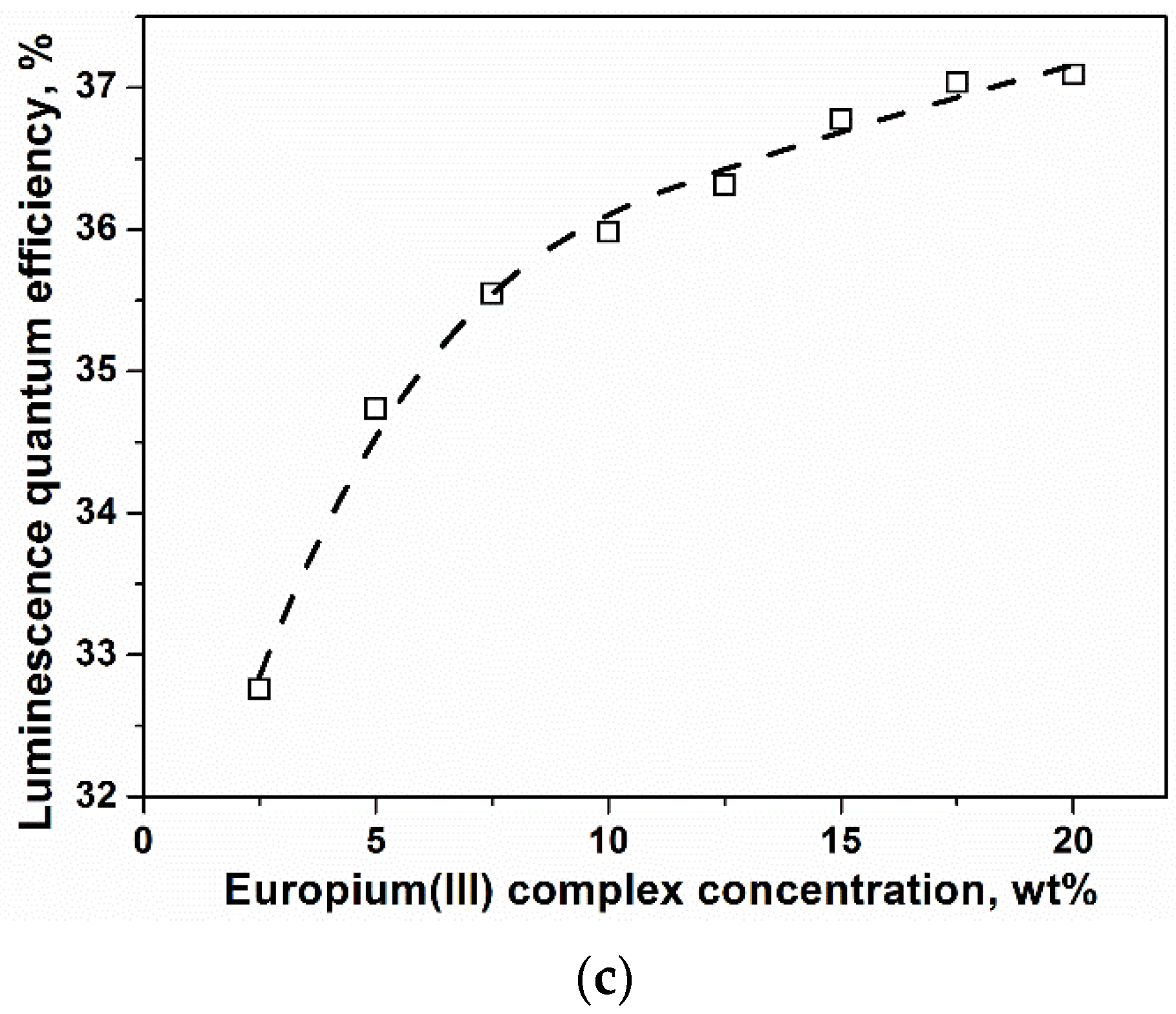
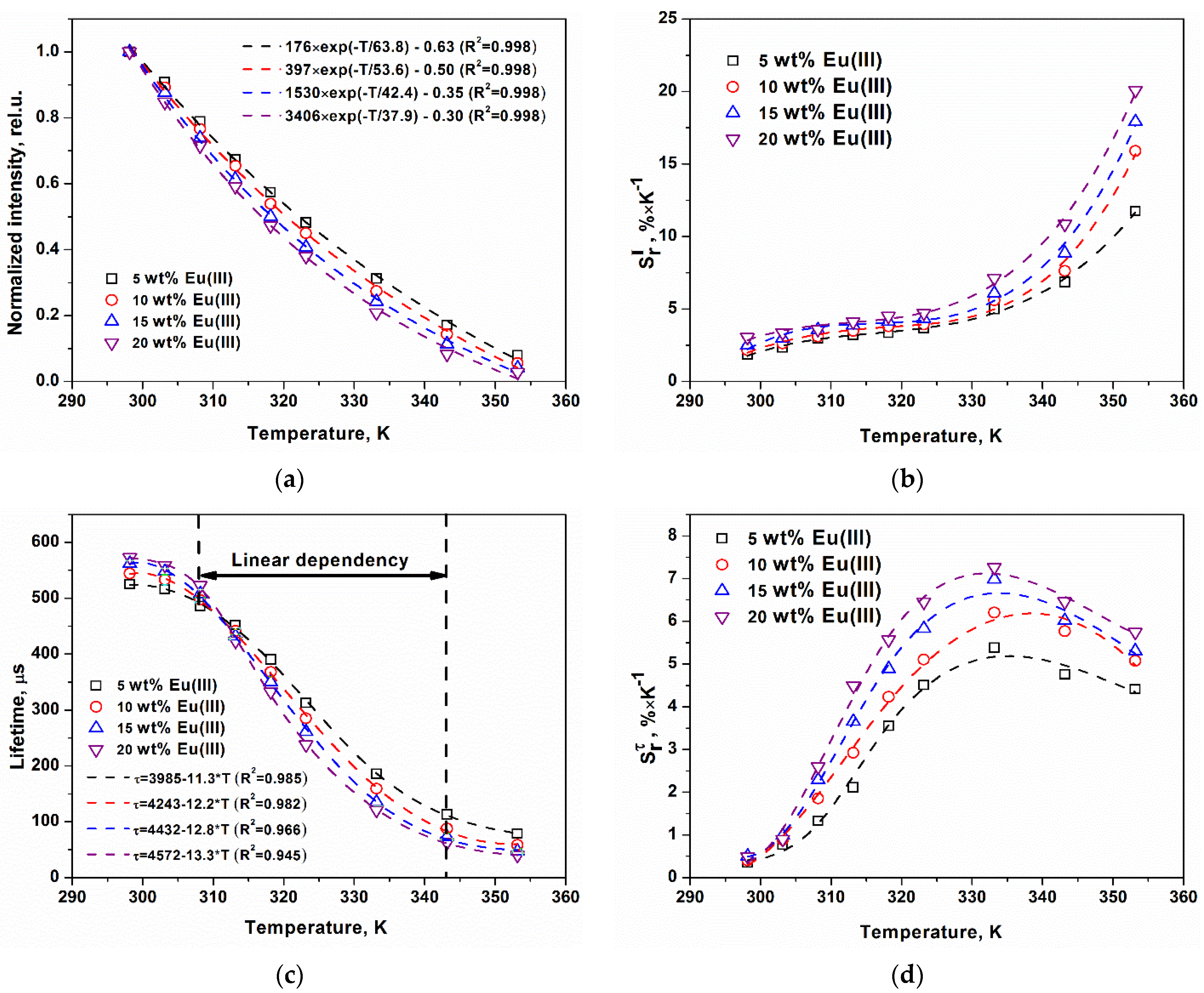
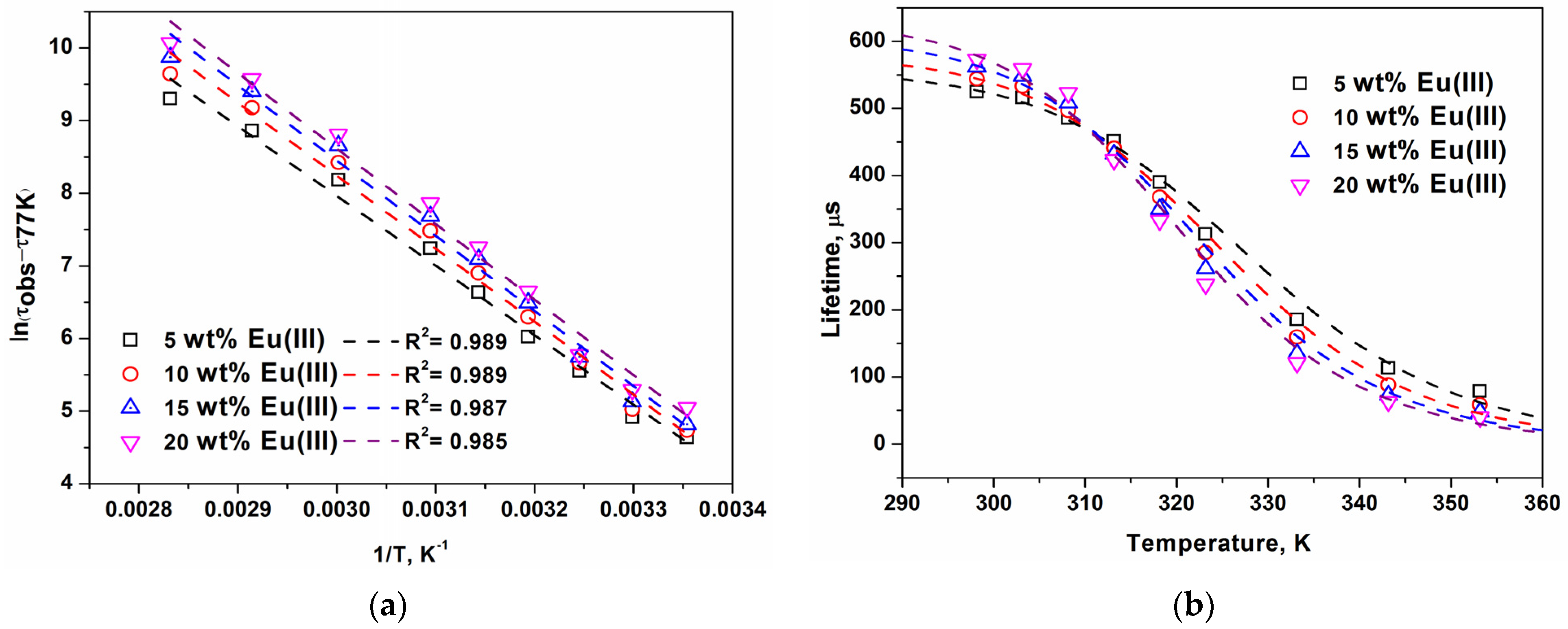
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Characterization Techniques
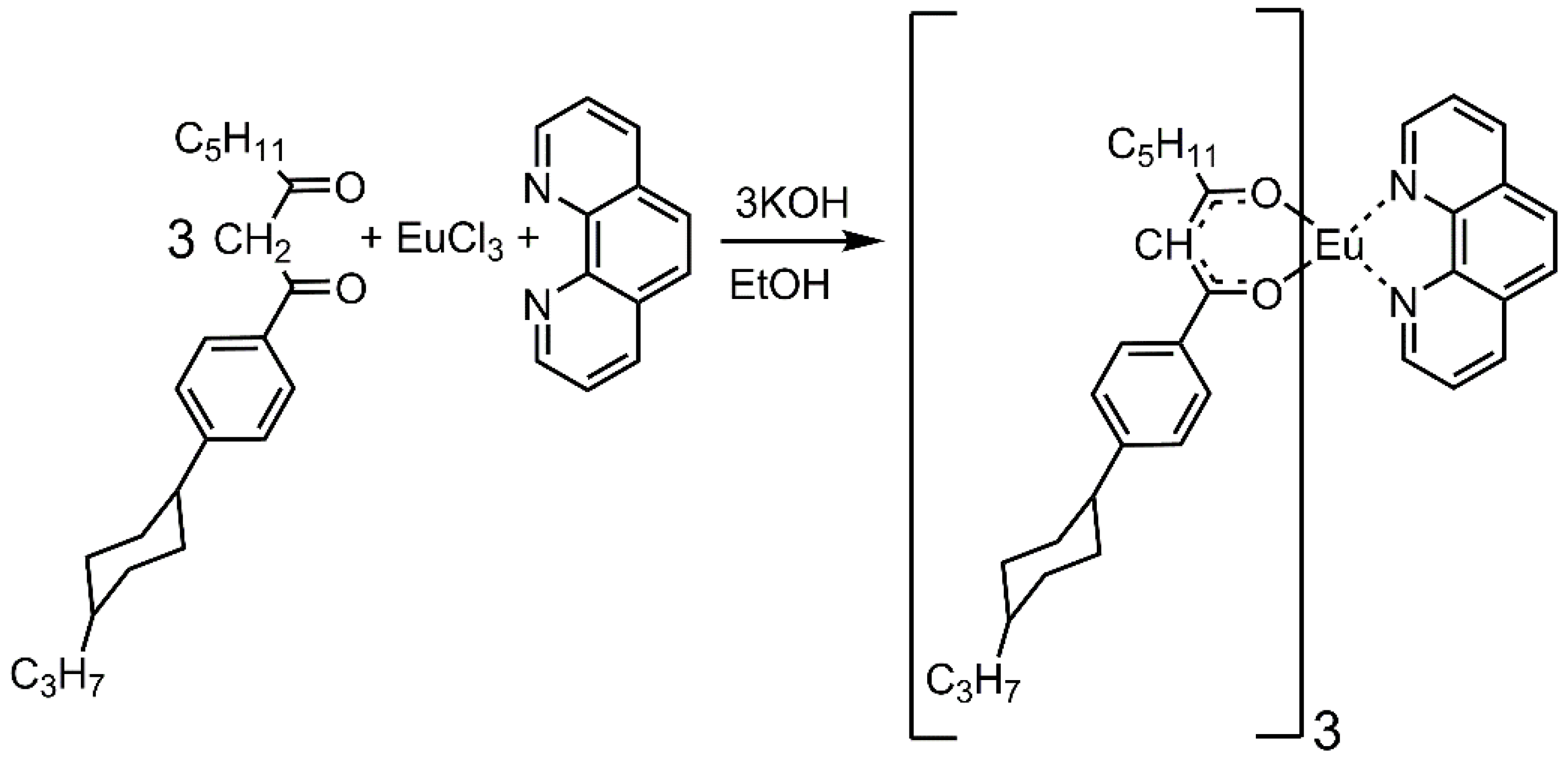
3.3. Synthesis
3.4. Preparation of PLA—Eu(III) Complex Hybrid Films
3.5. Calculation of the Quantum Efficiency
3.6. Calculation of the Thermal Sensitivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sabbatini, N.; Guardigli, M.; Lehn, J.M. Luminescent lanthanide complexes as photochemical supramolecular devices. Coord. Chem. Rev. 1993, 123, 201–228. [Google Scholar] [CrossRef]
- De Oliveira, E.; Neri, C.R.; Serra, O.A.; Prado, A.G.S. Antenna Effect in Highly Luminescent Eu3+ Anchored in Hexagonal Mesoporous Silica. Chem. Mater. 2007, 19, 5437–5442. [Google Scholar] [CrossRef]
- Katkova, M.A.; Vitukhnovsky, A.; Bochkarev, M.N. Coordination compounds of rare-earth metals with organic ligands for electroluminescent diodes. Russ. Chem. Rev. Engl. Transl. 2005, 74, 1089–1109. [Google Scholar] [CrossRef]
- Bünzli, J.C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, S.V.; Bünzli, J.C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Bünzli, J.C.G. Lanthanide luminescence for biomedical analyses and imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef]
- De Bettencourt-Dias, A. Lanthanide-based emitting materials in light-emitting diodes. J. Chem. Soc. Dalt. Trans. 2007, 2007, 2229–2241. [Google Scholar] [CrossRef]
- Comby, S.; Bünzli, J.C.G. Chapter 235 Lanthanide Near-Infrared Luminescence in Molecular Probes and Devices. Handb. Phys. Chem. Rare Earths 2007, 37, 217–470. [Google Scholar]
- Yang, X.P.; Jones, R.A.; Oye, M.M.; Holmes, A.L.; Wong, W.K. Near infrared luminescence and supramolecular structure of a helical triple-decker Yb (III) schiff base cluster. Cryst. Growth Des. 2006, 6, 2122–2125. [Google Scholar] [CrossRef]
- Tang, Y.; Tang, K.-Z.; Zhang, J.; Su, C.-Y.; Liu, W.-S.; Tan, M.-Y. Crystal structures and luminescent properties of the lanthanide picrate complexes with an amide-type tripodal ligand. Inorg. Chem. Commun. 2005, 8, 1018–1021. [Google Scholar] [CrossRef]
- Hein, H.; Koeppel, C.; Vetter, U.; Warkentin, E. Sc, Y, La-Lu, Rare Earth Elements; Springer Science & Business Media: Berlin/Heidelber, Germany, 1989. [Google Scholar]
- de Sá, G.; Malta, O.; de Mello Donegá, C.; Simas, A.; Longo, R.; Santa-Cruz, P.; da Silva, E. Spectroscopic properties and design of highly luminescent lanthanide coordination complexes. Coord. Chem. Rev. 2000, 196, 165–195. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Wada, Y.; Yanagida, S. Strategies for the design of luminescent lanthanide (III) complexes and their photonic applications. J. Photochem. Photobiol. C Photochem. Rev. 2004, 5, 183–202. [Google Scholar] [CrossRef]
- Yanagida, S.; Hasegawa, Y.; Murakoshi, K.; Wada, Y.; Nakashima, N.; Yamanaka, T. Strategies for enhancing photoluminescence of Nd3+ in liquid media. Coord. Chem. Rev. 1998, 171, 461–480. [Google Scholar] [CrossRef]
- Melby, L.R.; Rose, N.J.; Abramson, E.; Caris, J.C. Synthesis and Fluorescence of Some Trivalent Lanthanide Complexes. J. Am. Chem. Soc. 1964, 86, 5117–5125. [Google Scholar] [CrossRef]
- Bauer, H.; Blanc, J.; Ross, D.L. Octacoordinate Chelates of Lanthanides. Two Series of Compounds. J. Am. Chem. Soc. 1964, 86, 5125–5131. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, L.; Zhang, H.; Zheng, Y.; Li, H.; Deng, R.; Peng, Z.; Li, Z. Efficient electroluminescence from new lanthanide (Eu3+, Sm3+) complexes. Inorg. Chem. 2005, 44, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, P.; Yan, H.; Gong, M. Synthesis, characteristics and luminescent properties of a new europium(III) organic complex applied in near UV LED. Sens. Actuators B Chem. 2011, 156, 6–11. [Google Scholar] [CrossRef]
- Di Piazza, E.; Norel, L.; Costuas, K.; Bourdolle, A.; Maury, O.; Rigaut, S. D-f heterobimetallic association between ytterbium and ruthenium carbon-rich complexes: Redox commutation of near-IR luminescence. J. Am. Chem. Soc. 2011, 133, 6174–6176. [Google Scholar] [CrossRef]
- Jang, H.; Shin, C.H.; Jung, B.J.; Kim, D.H.; Shim, H.K.; Do, Y. Synthesis and characterization of dinuclear europium complexes showing pure red electroluminescence. Eur. J. Inorg. Chem. 2006, 2006, 718–725. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, J.; Shoji, S.; Kitagawa, Y.; Hasegawa, Y. Amorphous lanthanide complexes for organic luminescent materials. Coord. Chem. Rev. 2022, 467, 214607. [Google Scholar] [CrossRef]
- Tong, H.; Sengupta, S.; Tanaka, H. Emergent solidity of amorphous materials as a consequence of mechanical self-organisation. Nat. Commun. 2020, 11, 4863. [Google Scholar] [CrossRef] [PubMed]
- Shah, A. Amorphous Silicon Solar Cells. In Springer Series in Materials Science; Springer: Cham, Switzerland, 2020; Volume 301, pp. 139–161. [Google Scholar]
- Ma, X.; Wang, J.; Tian, H. Assembling-Induced Emission: An Efficient Approach for Amorphous Metal-Free Organic Emitting Materials with Room-Temperature Phosphorescence. Acc. Chem. Res. 2019, 52, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Brites, C.D.S.; Millán, A.; Carlos, L.D. Lanthanides in Luminescent Thermometry. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2016; Volume 49, pp. 339–427. [Google Scholar]
- Brites, C.D.S.; Lima, P.P.; Silva, N.J.O.; Millán, A.; Amaral, V.S.; Palacio, F.; Carlos, L.D. Thermometry at the nanoscale. Nanoscale 2012, 4, 4799–4829. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.D.; Wolfbeis, O.S.; Meier, R.J. Luminescent probes and sensors for temperature. Chem. Soc. Rev. 2013, 42, 7834–7869. [Google Scholar] [CrossRef]
- Moßhammer, M.; Brodersen, K.E.; Kühl, M.; Koren, K. Nanoparticle- and microparticle-based luminescence imaging of chemical species and temperature in aquatic systems: A review. Microchim. Acta 2019, 186, 126. [Google Scholar] [CrossRef]
- Hemmer, E.; Acosta-Mora, P.; Méndez-Ramos, J.; Fischer, S. Optical nanoprobes for biomedical applications: Shining a light on upconverting and near-infrared emitting nanoparticles for imaging, thermal sensing, and photodynamic therapy. J. Mater. Chem. B 2017, 5, 4365–4392. [Google Scholar] [CrossRef]
- Qin, T.; Liu, B.; Zhu, K.; Luo, Z.; Huang, Y.; Pan, C.; Wang, L. Organic fluorescent thermometers: Highlights from 2013 to 2017. TrAC-Trends Anal. Chem. 2018, 102, 259–271. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Kitagawa, Y. Thermo-sensitive luminescence of lanthanide complexes, clusters, coordination polymers and metal-organic frameworks with organic photosensitizers. J. Mater. Chem. C 2019, 7, 7494–7511. [Google Scholar] [CrossRef]
- Dramićanin, M.D. Sensing temperature via downshifting emissions of lanthanide-doped metal oxides and salts. A review. Methods Appl. Fluoresc. 2016, 4, 042001. [Google Scholar] [CrossRef] [Green Version]
- Dai, Z.; Tian, L.; Song, B.; Ye, Z.; Liu, X.; Yuan, J. Ratiometric Time-Gated Luminescence Probe for Hydrogen Sulfide Based on Lanthanide Complexes. Anal. Chem. 2014, 86, 11883–11889. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bian, Y.; Yan, M.; Thapaliya, P.S.; Johns, D.; Yan, X.; Galipeau, D.; Jiang, J. Mixed (porphyrinato)(phthalocyaninato) rare-earth (III) double-decker complexes for broadband light harvesting organic solar cells. J. Mater. Chem. 2011, 21, 11131–11141. [Google Scholar] [CrossRef]
- Lenaerts, P.; Storms, A.; Mullens, J.; D’Haen, J.; Görller-Walrand, C.; Binnemans, K.; Driesen, K. Thin Films of Highly Luminescent Lanthanide Complexes Covalently Linked to an Organic−Inorganic Hybrid Material via 2-Substituted Imidazo [4,5-f]-1,10-phenanthroline Groups. Chem. Mater. 2005, 17, 5194–5201. [Google Scholar] [CrossRef]
- Yan, B.; Lu, H.F. Lanthanide-centered covalently bonded hybrids through sulfide linkage: Molecular assembly, physical characterization, and photoluminescence. Inorg. Chem. 2008, 47, 5601–5611. [Google Scholar] [CrossRef] [PubMed]
- Biju, S.; Ambili Raj, D.B.; Reddy, M.L.P.; Jayasankar, C.K.; Cowley, A.H.; Findlater, M. Dual emission from stoichiometrically mixed lanthanide complexes of 3-phenyl-4-benzoyl-5-isoxazolonate and 2,2′-bipyridine. J. Mater. Chem. 2009, 19, 1425–1432. [Google Scholar] [CrossRef]
- Lima, P.P.; Almeida Paz, F.A.; Ferreira, R.A.S.; De Zea Bermudez, V.; Carlos, L.D. Ligand-assisted rational design and supramolecular tectonics toward highly luminescent Eu3+-containing organic-inorganic hybrids. Chem. Mater. 2009, 21, 5099–5111. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug deliveryand imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef]
- Peng, H.; Stich, M.I.J.; Yu, J.; Sun, L.N.; Fischer, L.H.; Wolfbeis, O.S. Luminescent europium (III) nanoparticles for sensing and imaging of temperature in the physiological range. Adv. Mater. 2010, 22, 716–719. [Google Scholar] [CrossRef]
- Li, H.R.; Lin, J.; Zhang, H.J.; Li, H.C.; Fu, L.S.; Meng, Q.G. Novel, covalently bonded hybrid materials of europium (terbium) complexes with silica. Chem. Commun. 2001, 1, 1212–1213. [Google Scholar] [CrossRef]
- Mondragón, M.; Trujillo, G.; Moggio, I.; Arias, E. Luminescent polylactic acid and polysulfone electrospun fibers containing europium (III) complexes. Eur. Polym. J. 2016, 80, 126–133. [Google Scholar] [CrossRef]
- Bender, J.L.; Corbin, P.S.; Fraser, C.L.; Metcalf, D.H.; Richardson, F.S.; Thomas, E.L.; Urbas, A.M. Site-isolated luminescent europium complexes with polyester macroligands: Metal-centered heteroarm stars and nanoscale assemblies with labile block junctions. J. Am. Chem. Soc. 2002, 124, 8526–8527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, Y.; Wang, Z.; Zheng, Y.; Zheng, X.; Gao, L.; Wang, C.; Yang, C.; Tang, H.; Li, Y. Biodegradable film enabling visible light excitation of Hexanuclear Europium (Ⅲ) complex for various applications. J. Lumin. 2021, 229, 117706. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Krupin, A.S.; Romanova, K.A.; Galyametdinov, Y.G. Luminescence and energy transfer in poly (N-vinylcarbazole) blends doped by a highly anisometric Eu (III) complex. J. Coord. Chem. 2016, 69, 1473–1483. [Google Scholar] [CrossRef]
- Knyazev, A.; Krupin, A.; Gubaidullin, A.; Galyametdinov, Y. Optical and structural characteristics of PMMA films doped with a new anisometric Eu III complex. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2019, 75, 570–577. [Google Scholar] [CrossRef]
- Lapaev, D.V.; Nikiforov, V.G.; Lobkov, V.S.; Knyazev, A.A.; Krupin, A.S.; Galyametdinov, Y.G. New insights into UV laser irradiation effect on luminescent behavior of vitrified films based on mesogenic lanthanide (III) β-diketonate complexes. J. Photochem. Photobiol. A Chem. 2019, 382, 111962. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Karyakin, M.E.; Krupin, A.S.; Romanova, K.A.; Galyametdinov, Y.G. Influence of Eu (III) Complexes Structural Anisotropy on Luminescence of Doped Conjugated Polymer Blends. Inorg. Chem. 2017, 56, 6067–6075. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Krupin, A.S.; Galyametdinov, Y.G. Luminescence behavior of PMMA films doped with Tb (III) and Eu (III) complexes. J. Lumin. 2022, 242, 118609. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Krupin, A.S.; Molostova, E.Y.; Romanova, K.A.; Galyametdinov, Y.G. Influence of Structural Anisotropy on Mesogenity of Eu (III) Adducts and Optical Properties of Vitrified Films Formed on their Base. Inorg. Chem. 2015, 54, 8987–8993. [Google Scholar] [CrossRef]
- Puthiyottil, R.; Varghese, S.; Gopalakrishnapanicker, U.; Guthrie, J.T. Characterization of Europium Complex-doped PMMA and PMMA-EVA Polymer Networks. Polym. Plast. Technol. Eng. 2014, 53, 1111–1118. [Google Scholar] [CrossRef]
- Liu, H.G.; Park, S.; Jang, K.; Feng, X.S.; Kim, C.; Seo, H.J.; Lee, Y.I. Influence of ligands on the photoluminescent properties of Eu3+in europium β-diketonate/PMMA-doped systems. J. Lumin. 2004, 106, 47–55. [Google Scholar] [CrossRef]
- Kai, J.; Felinto, M.C.F.C.; Nunes, L.A.O.; Malta, O.L.; Brito, H.F. Intermolecular energy transfer and photostability of luminescence-tuneable multicolour PMMA films doped with lanthanide-β-diketonate complexes. J. Mater. Chem. 2011, 21, 3796–3802. [Google Scholar] [CrossRef]
- Malta, O.L.; Brito, H.F.; Menezes, J.F.S.; Gonçalves E Silva, F.R.; Alves, S.; Farias, F.S.; De Andrade, A.V.M. Spectroscopic properties of a new light-converting device Eu(thenoyltrifluoroacetonate)3 2(dibenzyl sulfoxide). A theoretical analysis based on structural data obtained from a sparkle model. J. Lumin. 1997, 75, 255–268. [Google Scholar] [CrossRef]
- Faustino, W.M.; Junior, S.A.; Thompson, L.C.; De Sá, G.F.; Malta, O.L.; Simas, A.M. Theoretical and experimental luminescence quantum yields of coordination compounds of trivalent europium. Int. J. Quantum. Chem. 2005, 103, 572–579. [Google Scholar] [CrossRef]
- Lahoud, M.G.; Frem, R.C.G.; Gálico, D.A.; Bannach, G.; Nolasco, M.M.; Ferreira, R.A.S.; Carlos, L.D. Intriguing light-emission features of ketoprofen-based Eu (III) adduct due to a strong electron-phonon coupling. J. Lumin. 2016, 170, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Gálico, D.A.; Mazali, Í.O.; Sigoli, F.A. A highly sensitive luminescent ratiometric thermometer based on europium(iii) and terbium(iii) benzoylacetonate complexes chemically bonded to ethyldiphenylphosphine oxide functionalized polydimethylsiloxane. New J. Chem. 2018, 42, 18541–18549. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Fuertes, M.C.; Angelomé, P.C.; Martínez, E.D.; Lima, P.P.; Soler-Illia, G.J.A.A.; Carlos, L.D. Tethering Luminescent Thermometry and Plasmonics: Light Manipulation to Assess Real-Time Thermal Flow in Nanoarchitectures. Nano Lett. 2017, 17, 4746–4752. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Piñol, R.; Antorrena, G.; Brites, C.D.S.; Silva, N.J.O.; Murillo, J.L.; Cases, R.; Díez, I.; Palacio, F.; Torras, N.; et al. Luminescent Thermometers: Implementing Thermometry on Silicon Surfaces Functionalized by Lanthanide-Doped Self-Assembled Polymer Monolayers (Adv. Funct. Mater. 2/2016). Adv. Funct. Mater. 2016, 26, 312. [Google Scholar] [CrossRef]
- Gálico, D.A.; Mazali, I.O.; Sigoli, F.A. Nanothermometer based on intensity variation and emission lifetime of europium (III) benzoylacetonate complex. J. Lumin. 2017, 192, 224–230. [Google Scholar] [CrossRef]
- Piñol, R.; Brites, C.D.S.; Bustamante, R.; Martínez, A.; Silva, N.J.O.; Murillo, J.L.; Cases, R.; Carrey, J.; Estepa, C.; Sosa, C.; et al. Joining time-resolved thermometry and magnetic-induced heating in a single nanoparticle unveils intriguing thermal properties. ACS Nano 2015, 9, 3134–3142. [Google Scholar] [CrossRef]
- Miyagawa, T.; Fujie, T.; Ferdinandus; Vo Doan, T.T.; Sato, H.; Takeoka, S. Glue-Free Stacked Luminescent Nanosheets Enable High-Resolution Ratiometric Temperature Mapping in Living Small Animals. ACS Appl. Mater. Interfaces 2016, 8, 33377–33385. [Google Scholar] [CrossRef]
- Ferdinandus; Arai, S.; Takeoka, S.; Ishiwata, S.; Suzuki, M.; Sato, H. Facilely fabricated luminescent nanoparticle thermosensor for real-time microthermography in living animals. ACS Sens. 2016, 1, 1222–1227. [Google Scholar] [CrossRef]
- Cabral, F.M.; Gálico, D.A.; Mazali, I.O.; Sigoli, F.A. Crystal structure and temperature dependence of the photophysical properties of the [Eu(tta)3(pyphen)] complex. Inorg. Chem. Commun. 2018, 98, 29–33. [Google Scholar] [CrossRef]
- Lapaev, D.V.; Nikiforov, V.G.; Lobkov, V.S.; Knyazev, A.A.; Galyametdinov, Y.G. Reusable temperature-sensitive luminescent material based on vitrified film of europium (III) β-diketonate complex. Opt. Mater. 2018, 75, 787–795. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Karyakin, M.E.; Krupin, A.S.; Galyametdinov, Y.G. Influence of β-diketone structure on optical properties of formed by Eu (III) adducts photostable transparent films with effective luminescence. Dye. Pigment. 2022, 201, 110233. [Google Scholar] [CrossRef]
- Borisov, S.M.; Klimant, I. New luminescent oxygen-sensing and temperature-sensing materials based on gadolinium (III) and europium(III) complexes embedded in an acridone-polystyrene conjugate. Anal. Bioanal. Chem. 2012, 404, 2797–2806. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Wolfbeis, O.S. Temperature-sensitive europium (III) probes and their use for simultaneous luminescent sensing of temperature and oxygen. Anal. Chem. 2006, 78, 5094–5101. [Google Scholar] [CrossRef]
- Borisov, S.M.; Klimant, I. Blue LED excitable temperature sensors based on a new europium (III) chelate. J. Fluoresc. 2008, 18, 581–589. [Google Scholar] [CrossRef]
- Miyata, K.; Konno, Y.; Nakanishi, T.; Kobayashi, A.; Kato, M.; Fushimi, K.; Hasegawa, Y. Chameleon luminophore for sensing temperatures: Control of metal-to-metal and energy back transfer in lanthanide coordination polymers. Angew. Chemie-Int. Ed. 2013, 52, 6413–6416. [Google Scholar] [CrossRef]
- Buterbaugh, J.S.; Toscano, J.P.; Weaver, W.L.; Gord, J.R.; Hadad, C.M.; Gustafson, T.L.; Platz, M.S. Fluorescence lifetime measurements and spectral analysis of adamantyldiazirine. J. Am. Chem. Soc. 1997, 119, 3580–3591. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kitagawa, Y.; Nakanishi, T.; Fushimi, K.; Hasegawa, Y. Ligand-Assisted Back Energy Transfer in Luminescent TbIII Complexes for Thermosensing Properties. Chem.-A Eur. J. 2018, 24, 17719–17726. [Google Scholar] [CrossRef] [Green Version]
- Kitagawa, Y.; Kumagai, M.; Nakanishi, T.; Fushimi, K.; Hasegawa, Y. The Role of π-f Orbital Interactions in Eu(III) Complexes for an Effective Molecular Luminescent Thermometer. Inorg. Chem. 2020, 59, 5865–5871. [Google Scholar] [CrossRef] [PubMed]
- Venchikov, V.Y.; Tsvirko, M.P. Processes of electron excitation energy conversion in europium tris-naphthoyltrifluoroacetonate. J. Appl. Spectrosc. 2001, 68, 473–479. [Google Scholar] [CrossRef]
- Berry, M.T.; Stanley May, P.; Xu, H. Temperature dependence of the Eu3+5D0 lifetime in europium tris (2,2,6,6-tetramethyl-3,5-heptanedionato). J. Phys. Chem. 1996, 100, 9216–9222. [Google Scholar] [CrossRef]
- Kozak, M.; Kalota, B.; Tkaczyk, S.; Tsvirko, M. Luminescent Temperature Sensor Material Based on an Eu (III) β-Diketonate Complex Incorporated into Cellulose Triacetate. J. Appl. Spectrosc. 2014, 81, 678–683. [Google Scholar] [CrossRef]
- Mutti, A.M.G.; Canisares, F.S.M.; Machini, W.B.S.; Pires, A.M.; Teixeira, M.F.S.; Lima, S.A.M. A spectroscopic experimental and semi-empirical study of [Eu(salen)2] as a red-emitter for phosphor-converted UV LED. Optik 2021, 243, 167454. [Google Scholar] [CrossRef]
- Khalil, G.E.; Lau, K.; Phelan, G.D.; Carlson, B.; Gouterman, M.; Callis, J.B.; Dalton, L.R. Europium beta-diketonate temperature sensors: Effects of ligands, matrix, and concentration. Rev. Sci. Instrum. 2004, 75, 192–206. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, S.; Wu, L. Stability, UV shielding properties, and light conversion behavior of Eu (BMDM)3@polysiloxane nanoparticles in water and polyurethane films. Mater. Chem. Phys. 2012, 137, 644–651. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Dzhabarov, V.I.; Lapaev, D.V.; Lobkov, V.S.; Haase, W.; Galyametdinov, Y.G. New nematogenic β-diketones for synthesis of lanthanidomesogens. Russ. J. Gen. Chem. 2010, 80, 756–760. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Karyakin, M.E.; Heinrich, B.; Donnio, B.; Galyametdinov, Y.G. A facile approach for the creation of heteroionic lanthanidomesogens-containing uniform films with enhanced luminescence efficiency. Dye. Pigment. 2021, 187, 109050. [Google Scholar] [CrossRef]
- Lapaev, D.V.; Nikiforov, V.G.; Lobkov, V.S.; Knyazev, A.A.; Ziyatdinova, R.M.; Galyametdinov, Y.G.; Galyametdinov, Y.G. A vitrified film of an anisometric europium(iii) β-diketonate complex with a low melting point as a reusable luminescent temperature probe with excellent sensitivity in the range of 270-370 K. J. Mater. Chem. C 2020, 8, 6273–6280. [Google Scholar] [CrossRef]
- Werts, M.H.V.; Jukes, R.T.F.; Verhoeven, J.W. The emission spectrum and the radiative lifetime of Eu3+ in luminescent lanthanide complexes. Phys. Chem. Chem. Phys. 2002, 4, 1542–1548. [Google Scholar] [CrossRef]
- Bhaumik, M.L. Quenching and temperature dependence of fluorescence in rare-earth chelates. J. Chem. Phys. 1964, 40, 3711–3715. [Google Scholar] [CrossRef]
- Sabbatini, N.; Guardigli, M.; Manet, I.; Ungaro, R.; Casnati, A.; Ziessel, R.; Ulrich, G.; Asfari, Z.; Lehn, J.M. Lanthanide complexes of encapsulating ligands: Luminescent devices at the molecular level. Pure Appl. Chem. 1995, 67, 135–140. [Google Scholar] [CrossRef]
- Prodi, L.; Maestri, M.; Balzani, V.; Lehn, J.M.; Roth, C. Luminescence properties of cryptate europium (III) complexes incorporating heterocyclic N-oxide groups. Chem. Phys. Lett. 1991, 180, 45–50. [Google Scholar] [CrossRef]
- Sabbatini, N.; Perathoner, S.; Lattanzi, G.; Dellonte, S.; Balzani, V. Influence of fluoride ions on the absorption and luminescence properties of the [Eu⊂2.2.1]3+ and [Tb⊂2.2.1]3+ cryptates. J. Phys. Chem. 1987, 91, 6136–6139. [Google Scholar] [CrossRef]
- Keopp, J.L.; Windsor, M.W. Luminescence and energy transfer in solutions of rare-earth complexes. I. Enhancement of fluorescence by deuterium substitution. J. Chem. Phys. 1965, 42, 1599–1608. [Google Scholar] [CrossRef]
- William De, W.H.; Sudnick, D.R. Lanthanide Ion Probes of Structure in Biology. Laser-Induced Luminescence Decay Constants Provide a Direct Measure of the Number of Metal-Coordinated Water Molecules. J. Am. Chem. Soc. 1979, 101, 334–340. [Google Scholar] [CrossRef]
- De Horrocks, W.; Sudnick, D.R. Lanthanide Ion Luminescence Probes of the Structure of Biological Macromolecules. Acc. Chem. Res. 1981, 14, 384–392. [Google Scholar] [CrossRef]
- Beeby, A.; Clarkson, I.M.; Dickins, R.S.; Faulkner, S.; Parker, D.; Royle, L.; De Sousa, A.S.; Williams, J.A.G.; Woods, M. Non-radiative deactivation of the excited states of europium, terbium and ytterbium complexes by proximate energy-matched OH, NH and CH oscillators: An improved luminescence method for establishing solution hydration states. J. Chem. Soc. Perkin Trans. 2 1999, 1999, 493–503. [Google Scholar] [CrossRef]
- Collins, S.F.; Baxter, G.W.; Wade, S.A.; Sun, T.; Grattan, K.T.V.; Zhang, Z.Y.; Palmer, A.W. Comparison of fluorescence-based temperature sensor schemes: Theoretical analysis and experimental validation. J. Appl. Phys. 1998, 84, 4649–4654. [Google Scholar] [CrossRef]

| # | Material | Maximum Relative Sensitivity Sm, %×K−1 | Range, K | Tm, K | Optical Parameter | Reference |
|---|---|---|---|---|---|---|
| 1 | Eu(keto)3(H2O) | 7.0 × 10−2 | 12–300 | 50 | Bandwidth | [57] |
| 2 | PDMS-eddpo-Ln(bzac)3 (Ln = Eu,Tb) | 11 | 158–248 | 203 | Two intensities | [58] |
| 3 | Ln(tfac)3·2H2O (Ln = Eu, Tb) | 7.1 | 293–343 | 293 | Two intensities | [59] |
| 4 | Ln-DPA (Ln = Eu, Tb) | 1.5 | 293–333 | 293 | Two intensities | [60] |
| 5 | Eu(bzac)3(H2O)2 | 1.4 | 188–303 | 293 | Lifetime | [61] |
| 6 | Ln(btfa)3(H2O)2 (Ln = Eu, Tb) | 5.8 | 295–315 | 296 | Two intensities | [62] |
| 7 | Eu3+/RhB-based polymer | 3.6 | 300–310 | 302 | Two intensities | [63] |
| 8 | Eu3+/RhB-based polymer | 3.8 | 300–310 | 302 | Two intensities | [64] |
| 9 | Eu(bzac)3(H2O)2 | 5.3 | 188–303 | 303 | Single intensity | [61] |
| 10 | Eu(tta)3(pyphen) | 1.7 | 283–323 | 323 | Lifetime | [65] |
| 11 | Eu(CPDK3-5)3phen | 2.2 | 298–348 | 298 | Lifetime | [66] |
| 12 | Eu(CPDk3-C3F7)3Phen | 0.87 | 298–363 | 343 | Single intensity | [67] |
| Sample | The Frequency Factor k0, s−1 | Activation Energy Ea, cm−1 | Reference |
|---|---|---|---|
| 5 wt% Eu(III) | 8.78 × 1015 | 6661 | This paper |
| 10 wt% Eu(III) | 4.65 × 1016 | 6982 | This paper |
| 15 wt% Eu(III) | 1.45 × 1017 | 7197 | This paper |
| 20 wt% Eu(III) | 1.96 × 1017 | 7228 | This paper |
| Eu(NTA)3·H2O in PMMA | 1.8 × 1014 | 6300 | [75] |
| Eu(thd)3 | 1.2 × 1013 | 4120 | [76] |
| Eu(tta)3dapm in cellulose triacetate matrix | 7 × 1016 | 7070 | [77] |
| Eu(hfa)3(DPCO)2 | 1.1 × 107 | 3120 | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knyazev, A.A.; Krupin, A.S.; Galyametdinov, Y.G. Composites Based on Polylactide Doped with Amorphous Europium(III) Complex as Perspective Thermosensitive Luminescent Materials. Inorganics 2022, 10, 232. https://doi.org/10.3390/inorganics10120232
Knyazev AA, Krupin AS, Galyametdinov YG. Composites Based on Polylactide Doped with Amorphous Europium(III) Complex as Perspective Thermosensitive Luminescent Materials. Inorganics. 2022; 10(12):232. https://doi.org/10.3390/inorganics10120232
Chicago/Turabian StyleKnyazev, Andrey A., Aleksandr S. Krupin, and Yuriy G. Galyametdinov. 2022. "Composites Based on Polylactide Doped with Amorphous Europium(III) Complex as Perspective Thermosensitive Luminescent Materials" Inorganics 10, no. 12: 232. https://doi.org/10.3390/inorganics10120232






