Cobalt-Based Perovskite Electrodes for Solid Oxide Electrolysis Cells
Abstract
:1. Introduction
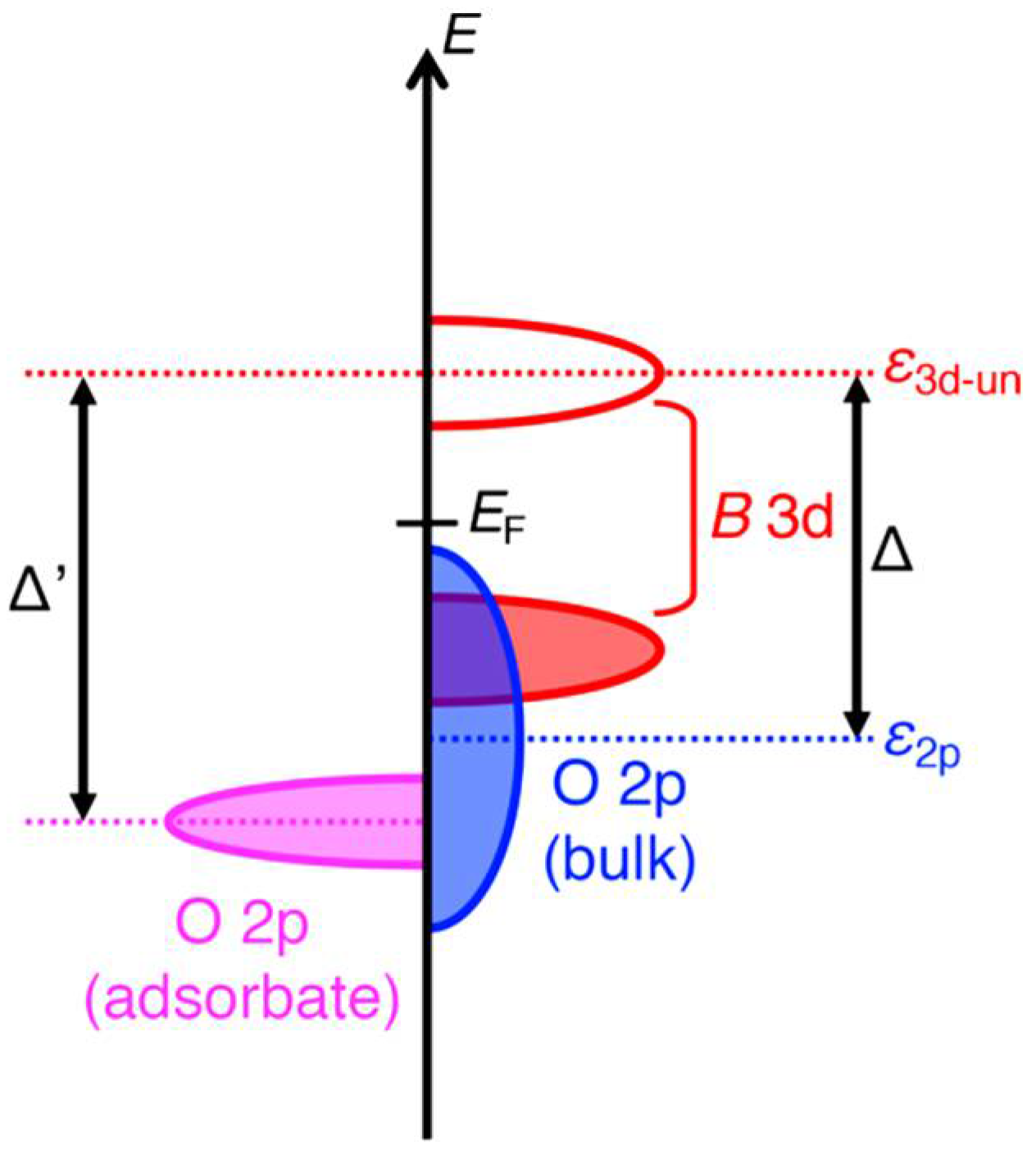
2. Crystal and Electronic Structure of Perovskite Oxides
3. Co-Based Perovskite for SOECs
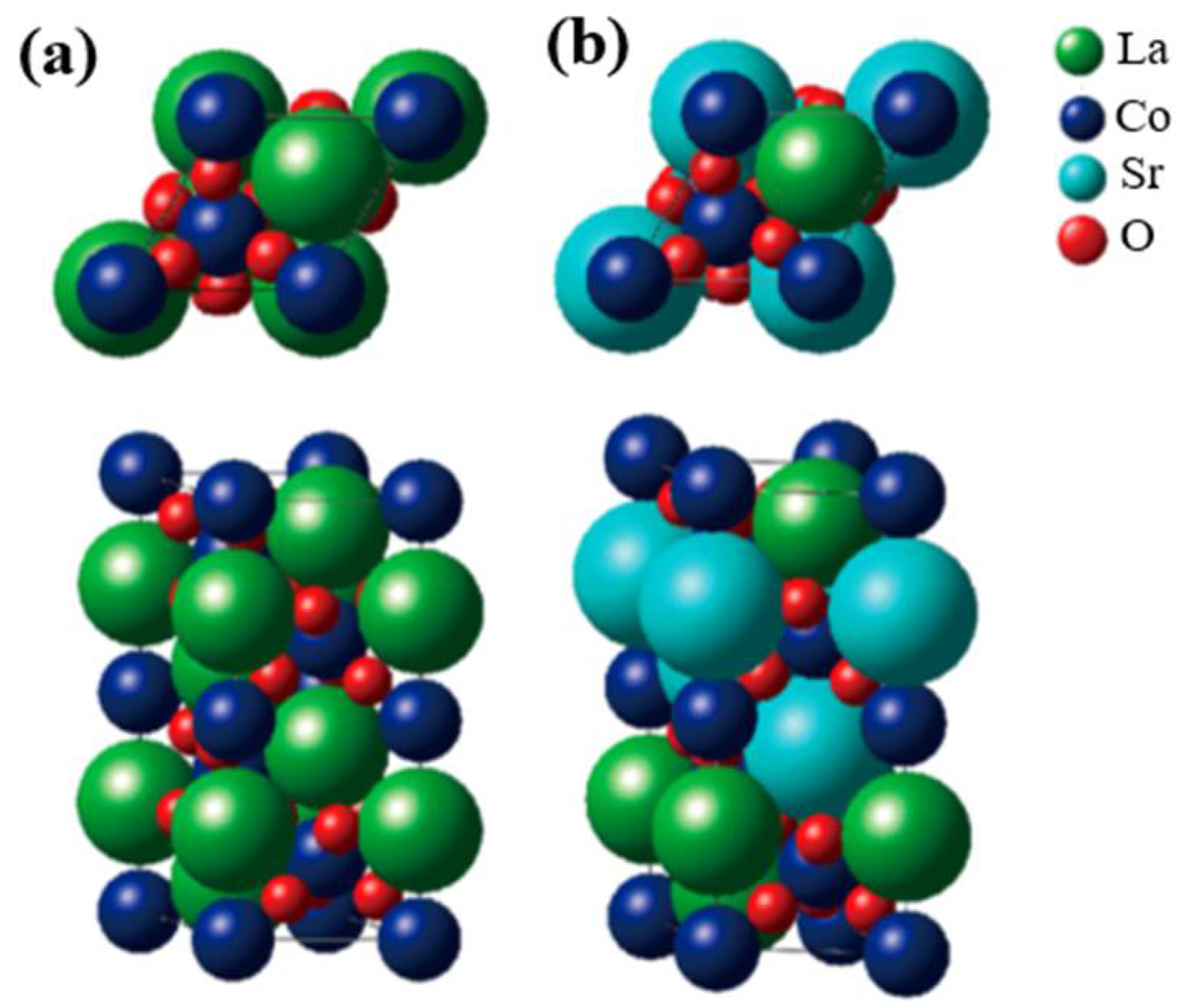
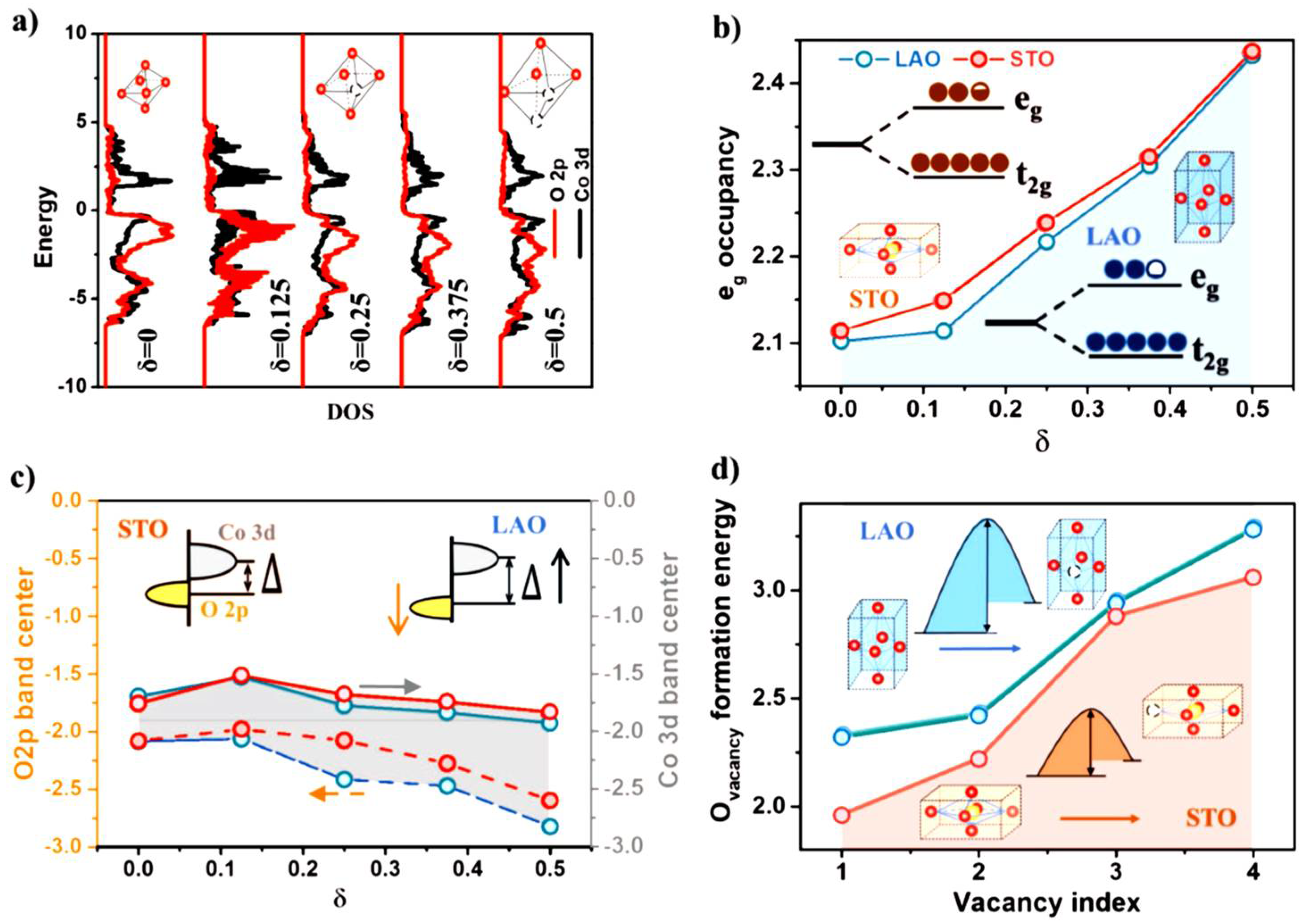
3.1. Pure or Doped Perovskite
3.2. Composite Perovskite
4. Conclusions & Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Tucker, M.C. Progress in metal-supported solid oxide electrolysis cells: A review. Int. J. Hydrogen Energy 2020, 45, 24203–24218. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C. Technological development of hydrogen production by solid oxide electrolyzer cell (SOEC). Int. J. Hydrogen Energy 2008, 33, 2337–2354. [Google Scholar] [CrossRef]
- Gaudillere, C.; Navarrete, L.; Serra, J.M. Syngas production at intermediate temperature through H2O and CO2 electrolysis with a Cu-based solid oxide electrolyzer cell. Int. J. Hydrogen Energy 2014, 39, 3047–3054. [Google Scholar] [CrossRef]
- Buttler, A.; Hartmut, S. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Yu, B.; Zhang, W.; Chen, J.; Qiao, J.; Zhang, J. A review of high temperature co-electrolysis of H2O and CO2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): Advanced materials and technology. Chem. Soc. Rev. 2017, 46, 1427–1463. [Google Scholar] [CrossRef] [PubMed]
- Ritucci, H.; Agersted, K.; Zielke, P.; Wulff, A.C.; Khajavi, P.; Smeacetto, F.; Sabato, A.G.; Kiebach, R. A Ba-free sealing glass with a high coefficient of thermal expansion and excellent interface stability optimized for SOFC/SOEC stack applications. Int. J. Appl. Ceram. Technol. 2018, 15, 1011–1022. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Wang, L.; Bian, L.; Wang, Y.; Chou, K.C. Excellent electrochemical performance of La0.3Sr0.7Fe0.9Ti0.1O3-δ as a symmetric electrode for solid oxide cells. ACS Appl. Mater. Interfaces 2021, 13, 22381–22390. [Google Scholar]
- Li, P.; Xuan, Y.; Jiang, B.; Zhang, S.; Xia, C. Hollow La0.6Sr0.4Ni0.2Fe0.75Mo0.05O3-δ electrodes with exsolved FeNi3 in quasi-symmetrical solid oxide electrolysis cells for direct CO2 electrolysis. Electrochem. Commun. 2022, 134, 107188. [Google Scholar] [CrossRef]
- Trasatti, S. Electrocatalysis by oxides-Attempt at a unifying approach. Electroanal. Chem. Interfacial Electrochem. 1980, 111, 125–131. [Google Scholar] [CrossRef]
- Otagawa, T.; Bockris, J.O. Oxygen evolution on perovskites. J. Phys. Chem. 1983, 87, 2960–2971. [Google Scholar]
- Gupta, S.; Kellogg, W.; Xu, H.; Liu, X.; Cho, J.; Wu, G. Bifunctional perovskite oxide catalysts for oxygen reduction and evolution in alkaline media. Chem. Asian J. 2016, 11, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Jin, K.J.; Lu, H.B.; Jia, J.F.; Qiu, J.; Hu, C.L.; Zhen, G.Z. Influence of oxygen vacancy on transport property of perovskite oxide heterostructures. Chin. Phys. Lett. 2009, 26, 027301. [Google Scholar]
- Lu, C.H.; Biesold-McGee, G.V.; Liu, Y.; Kang, Z.; Lin, Z. Doping and ion substitution in colloidal metal halide perovskite nanoscrystals. Chem. Soc. Rev. 2020, 49, 4953–5007. [Google Scholar] [CrossRef] [PubMed]
- Laguna-Bercero, M.A.; Monzon, H.; Larrea, A.; Orera, V.M. Improved stability of reversible solid oxide cells with a nickelate-based oxygen electrode. J. Mater. Chem. 2016, 4, 1446–1453. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Kim, H. Electrochemical performance of La2NiO4-δ cathode for intermediate-temperature solid oxide fuel cells. Ceram. Int. 2015, 41, 5984–5991. [Google Scholar] [CrossRef]
- Tong, X.; Zhou, F.; Yang, S.; Zhong, S.; Wei, M.; Liu, Y. Performance and stability of Ruddlesden-Popper La2NiO4-δ oxygen electrodes under solid oxide electrolysis cell operation conditions. Ceram. Int. 2017, 43, 10927–10933. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, K.J.; Dayaghi, A.M.; Choi, G.M. Polarization and stability of La2NiO4-δ in comparison with La0.6Sr0.4Co0.2Fe0.8O3-δ as air electrode of solid oxide electrolysis cell. Int. J. Hydrogen Energy 2016, 41, 14498–14506. [Google Scholar] [CrossRef]
- Spiridigliozzi, L.; Di Bartolomeo, E.; Dell’Agli, G. Zurlo, F. GDC-based infiltrated electrodes for solid oxide electrolyzer cells (SOECs). Appl. Sci. 2020, 10, 3882. [Google Scholar] [CrossRef]
- Flura, A.; Nicollet, C.; Vibhu, V.; Zeimetz, B.; Rougier, A.; Bassat, J.-M.; Grenier, J.-C. Application of the Adler-Lane-Steele model to porous La2NiO4-δ SOFCcathode: Influence of interfaces with gadolinia doped ceria. J. Electrochem. Soc. 2016, 163, F523–F532. [Google Scholar] [CrossRef]
- Liu, H.; Qi, J.; Feng, M.; Xu, H.; Liu, R.; Li, N.; Wang, C.; Zhang, Y.; Zhang, Y.; Lu, W. The tunability of oxygen evolution reaction in flexible van der Waals manganite membrane. Adv. Sustain. Sys. 2021, 5, 2100073. [Google Scholar] [CrossRef]
- Huang, M.; Jiang, H.; Liu, X.; Xiao, Y.; Kong, J.; Zhou, T. LSCM-GDC as composite cathodes for high temperature steam electrolysis: Performance optimization by composition and microstructure tailoring. Int. J. Hydrogen Energy 2022, 47, 34784–34793. [Google Scholar] [CrossRef]
- Busse, P.; Yin, Z.; Mierwaldt, D.; Scholz, J.; Kressdorf, B.; Glaser, L.; Miedema, P.S.; Rothkirch, A.; Viefhaus, J.; Jooss, C.; et al. Probing the surface of La0.6Sr0.4MnO3 in water vapor by in situ photo-in/photon-out spectroscopy. J. Phys. Chem. C 2020, 124, 7893–7902. [Google Scholar] [CrossRef]
- Li, J.; Qiu, P.; Xia, M.; Jia, L.; Chi, B.; Pu, J.; Li, J. Microstructure optimization for high performance PrBa0.5Sr0.5Co1.5Fe0.5O5-δ-La2NiO4-δ core-shell cathode of solid oxide fuel cells. J. Power Sources 2018, 379, 206–211. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Qiu, P.; Jia, L.; Chi, B.; Pu, J.; Li, J. A CO2-tolerant La2NiO4-δ-coated PrBa0.5Sr0.5Co1.5Fe0.5O5-δ cathode for intermediate temperature solid oxide fuel cells. J. Power Sources 2017, 342, 623–628. [Google Scholar] [CrossRef]
- Hjalmarsson, P.; Sogaard, M.; Mogensen, M. Electrochemical behaviour of (La1-xSrx)sCo1-yNiyO3-δ as porous SOFC cathodes. Solid State Ionics 2009, 180, 1395–1405. [Google Scholar] [CrossRef]
- Chen, J.; Liang, F.L.; Liu, L.; Jiang, S.P.; Li, J. Characterization and evaluation of La0.8Sr0.2Co0.8Ni0.2O3-δ prepared by a polymer-assisted combustion synthesis as a cathode material for intermediate temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2009, 34, 6845–6851. [Google Scholar] [CrossRef]
- Ai, N.; He, S.; Li, N.; Zhang, Q.; Rickard, W.D.A.; Chen, K.; Zhang, T.; Jiang, S.P. Suppressed Sr segregation and performance of directly assembled La0.6Sr0.4Co0.2Fe0.8O3-δ oxygen electrode on Y2O3-ZrO2 electrolyte of solid oxide electrolysis cells. J. Power Sources 2018, 384, 125–135. [Google Scholar] [CrossRef]
- Qiu, P.; Wang, A.; Li, J.; Li, Z.; Jia, L.; Chi, B.; Pu, J.; Li, J. Promoted CO2-poisoningresistance of La0.8Sr0.2MnO3-δ-coated Ba0.5Sr0.5Co0.8Fe0.2O3-δ cathode for intermediate temperature solid oxide fuel cells. J. Power Sources 2016, 327, 408–413. [Google Scholar] [CrossRef]
- Tan, Y.; Duan, N.; Wang, A.; Yan, D.; Chi, B.; Wang, N.; Pu, J.; Li, J. Performance enhancement of solution impregnated nanostructured La0.8Sr0.2Co0.8Ni0.2O3-δ oxygen electrode for intermediate temperature solid oxide electrolysis cells. J. Power Sources 2016, 305, 168–174. [Google Scholar] [CrossRef]
- Zheng, H.; Tian, Y.; Zhang, L.; Chi, B.; Pu, J.; Jian, L. La0.8Sr0.2Co0.8Ni0.2O3-δ impregnated oxygen electrode for H2O/CO2 co-electrolysis in solid oxide electrolysis cells. J. Power Sources 2018, 383, 93–101. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Liang, F.; Pu, J.; Chi, B.; Jian, L. Thermochemical compatibility and polarization behaviors of La0.8Sr0.2Co0.8Ni0.2O3-δ as a cathode material for solid oxide fuel cell. Int. J. Hydrogen Energy 2013, 38, 6802–6808. [Google Scholar] [CrossRef]
- Sun, C.; Alonso, J.A.; Bian, J. Recent advances in perovskite-type oxides for energy conversion and storage applications. Adv. Energy Mater. 2020, 11, 2000459. [Google Scholar] [CrossRef]
- van Doorn, R.H.E.; Bouwmeester, H.J.M.; Burggraaf, A.J. Kinetic decomposition of La0.3Sr0.7CoO3-δ perovskite membranes during oxygen permeation. Solid State Ionics 1998, 111, 263–272. [Google Scholar] [CrossRef]
- Simböck, J.; Ghiasi, M.; Schönebaum, S.; Simon, U.; de Groot, F.M.F.; Palkovits, R. Electronic parameters in cobalt-based perovskite-type oxides as descriptors for chemocatalytic reactions. Nat. Commun. 2020, 11, 652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.; Rao, R.R.; Giordano, L.; Katayama, Y.; Yu, Y.; Shao-Horn, Y. Perovskites in catalysis and electrocatalysis. Science 2017, 358, 751–756. [Google Scholar] [CrossRef] [Green Version]
- Seo, M.H.; Park, H.W.; Lee, D.U.; Park, M.G.; Chen, Z. Design of highly active perovskite oxides for oxygen evolution reaction by combining experimental and ab initio studies. ACS Catal. 2015, 5, 4337–4344. [Google Scholar] [CrossRef]
- Cheng, Y.; Raman, A.S.; Paige, J.; Zhang, L.; Sun, D.; Chen, M.U.; Vojvodic, A.; Gorte, R.J.; Vohs, J.M. Enhancing oxygen exchange activity by tailoring perovskite surfaces. J. Phys. Chem. Lett. 2019, 10, 4082–4088. [Google Scholar] [CrossRef]
- Zhang, D.; Song, Y.; Du, Z.; Wang, L.; Li, Y.; Goodenough, J.B. Active LaNi1-xFexO3 bifunctional catalysts for cathodes in alkaline media. J. Mater. Chem. A 2015, 3, 9421–9426. [Google Scholar] [CrossRef]
- Kim, J.; Chen, X.; Pan, Y.; Shih, P.; Yang, H. W-doped CaMnO2.5 and CaMnO3 electrocatalysts for enhanced performance in oxygen evolution and reduction reactions. J. Electrochem. Soc. 2017, 164, F1074–F1080. [Google Scholar] [CrossRef]
- Dickens, C.F.; Montoya, J.H.; Kulkarni, A.R.; Bajdich, M.; Norskov, J.K. An electronic structure descriptor for oxygen reactivity at metal and metal-oxide surfaces. Surf. Sci. 2019, 681, 122–129. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Yamada, I.; Takamatsu, A.; Asai, K.; Shirakawa, T.; Ohzuku, H.; Seno, A.; Uchimura, T.; Fujii, H.; Kawaguchi, S.; Wada, K.; et al. Systematic study of descriptors for oxygen evolution reaction catalysis in perovskite oxides. J. Phys. Chem. C 2018, 122, 27885–27892. [Google Scholar] [CrossRef] [Green Version]
- Tong, Y.; Guo, Y.; Chen, P.; Liu, H.; Zhang, M.; Zhang, L.; Yan, W.; Chu, W.; Wu, C.; Xie, Y. Spin-state regulation of perovskite cobaltite to realize enhanced oxygen evolution activity. Chem 2017, 3, 812–821. [Google Scholar] [CrossRef]
- Stoerzinger, K.A.; Wang, X.R.; Hwang, J.; Rao, R.R.; Hong, W.T.; Rouleau, C.M.; Lee, D.; Yu, Y.; Crumlin, E.J.; Shao-Horn, Y. Speciation and electronic structure of La1-xSrxCoO3-δ during oxygen electrolysis. Top. Catal. 2018, 61, 2161–2174. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Zheng, Y.; Guo, Z.; Zhu, Y.; Chen, H.; Li, F.; Liu, P.; Yu, B.; Wang, X.; et al. Uncovering the effect of lattice strain and oxygen deficiency on electrocatalytic activity of perovskite cobaltite thin films. Adv. Sci. 2019, 6, 1801898. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A.; Caicedo, J.M.; Ballesteros, B.; Santiso, J. GdBaCo2O5.5 electrodes in a solid-state electrochemical cell by time-resolved X-ray diffraction. J. Mater. Chem. A 2018, 6, 12430–12439. [Google Scholar] [CrossRef]
- Pramana, S.S.; Cavallaro, A.; Li, C.; Handoko, A.D.; Chan, K.W.; Walker, R.J.; Regoutz, A.; Herrin, J.S.; Yeo, B.S.; Payne, D.J.; et al. Crystal structure and surface characteristic of Sr-doped GdBaCo2O6-δ double perovskites: Oxygen evolution reaction and conductivity. J. Mater. Chem. A 2018, 6, 5335–5345. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Chen, G.; Zhu, Y.; Liu, B.; Zhou, W.; Shao, Z. B-site cation ordered double perovskites as efficient and stable electrocatalysts for oxygen evolution reaction. Chem. Eur. J. 2017, 23, 5722–5728. [Google Scholar] [CrossRef]
- He, D.; He, G.; Jiang, H.; Chen, Z.; Huang, M. Enhanced durability and activity of the perovskite electrocatalyst Pr0.5Ba0.5CoO3-δ by Ca doping for the oxygen evolution reaction at room temperature. Chem. Commun. 2017, 53, 5132–5135. [Google Scholar] [CrossRef]
- Yoon, B.Y.; Bae, J. Characteristics of nano La0.6Sr0.4Co0.2Fe0.8O3-δ-infiltrated La0.8Sr0.2Ga0.8Mg0.2O3-δ scaffold cathode for enhanced oxygen reduction. Int. J. Hydrogen Energy 2013, 38, 13399–13407. [Google Scholar] [CrossRef]
- Risck, M.; Stoerzinger, K.A.; Maruyama, S.; Hong, W.T.; Takeuchi, I.; Shao-Horn, Y. La0.8Sr0.2MnO3−δ decorated with Ba0.5Sr0.5Co0.3Fe0.2O3−δ: A bifunctional surface for oxygen electrocatalysis with enhanced stability and activity. J. Am. Chem. Soc. 2014, 136, 5229–5232. [Google Scholar]
- Li, M.; Hua, B.; Chen, J.; Zhong, Y.; Luo, J.-L. Charge transfer dynamics in RuO2/perovskite nanohybrid for enhanced electrocatalysis in solid oxide electrolyzers. Nano Energy 2019, 57, 186–194. [Google Scholar] [CrossRef]
- Sharma, V.I.; Yildiz, B. Degradation mechanism in La0.8Sr0.2CoO3 as contact layer on the solid oxide electrolysis cell anode. J. Electrochem. Soc. 2010, 157, B441–B448. [Google Scholar] [CrossRef]
- Wei, B.; Chen, K.F.; Zhao, L.; Lu, Z.; Jiang, S.P. Chromium deposition and poisoning at La0.6Sr0.4Co0.2Fe0.8O3-δ oxygen electrodes of solid oxide electrolysis cells. Phys. Chem. Chem. Phys. 2015, 17, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Nam, G.; Kim, S.; Choi, K.; Zhong, Q.; Lee, J.; Qin, Y.; Cho, J.; Kim, G. A tailored bifunctional electrocatalyst: Boosting oxygen reduction/evolution catalysis via electron transfer between N-doped graphene and perovskite oxides. Small 2018, 14, 1802767. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Lu, B.; Xiong, H.; Lin, C.; Fang, L.; Fu, J.; Deng, D.; Fan, X.; Li, Y.; Wu, Q.-H. Cobalt-Based Perovskite Electrodes for Solid Oxide Electrolysis Cells. Inorganics 2022, 10, 187. https://doi.org/10.3390/inorganics10110187
Zhang C, Lu B, Xiong H, Lin C, Fang L, Fu J, Deng D, Fan X, Li Y, Wu Q-H. Cobalt-Based Perovskite Electrodes for Solid Oxide Electrolysis Cells. Inorganics. 2022; 10(11):187. https://doi.org/10.3390/inorganics10110187
Chicago/Turabian StyleZhang, Chi, Bin Lu, Haiji Xiong, Chengjun Lin, Lin Fang, Jile Fu, Dingrong Deng, Xiaohong Fan, Yi Li, and Qi-Hui Wu. 2022. "Cobalt-Based Perovskite Electrodes for Solid Oxide Electrolysis Cells" Inorganics 10, no. 11: 187. https://doi.org/10.3390/inorganics10110187




