Impregnation of Synthetic Saponites with Aldehydes: A Green Approach in the Intercalation of Bioactive Principles
Abstract
:1. Introduction
2. Results and Discussion
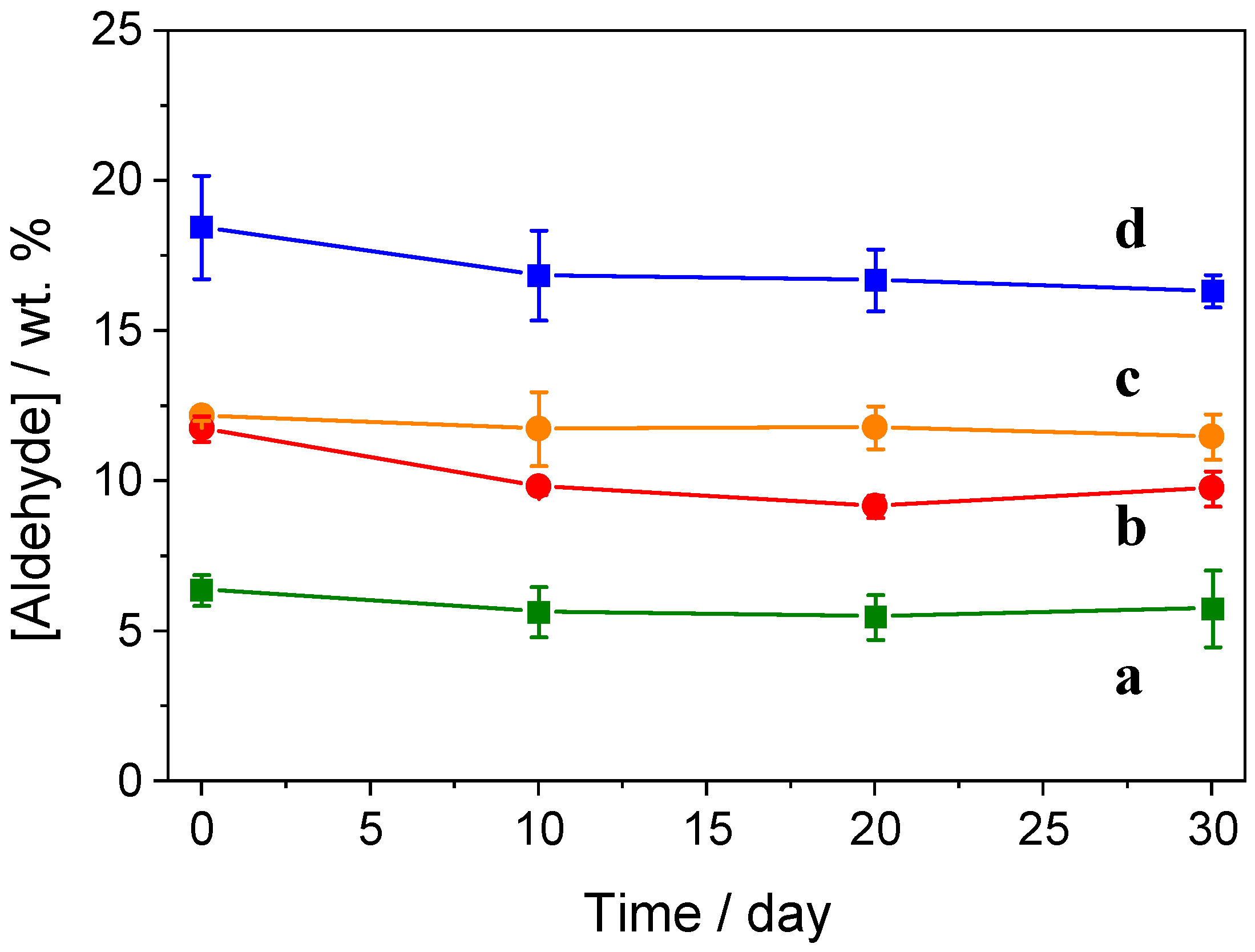
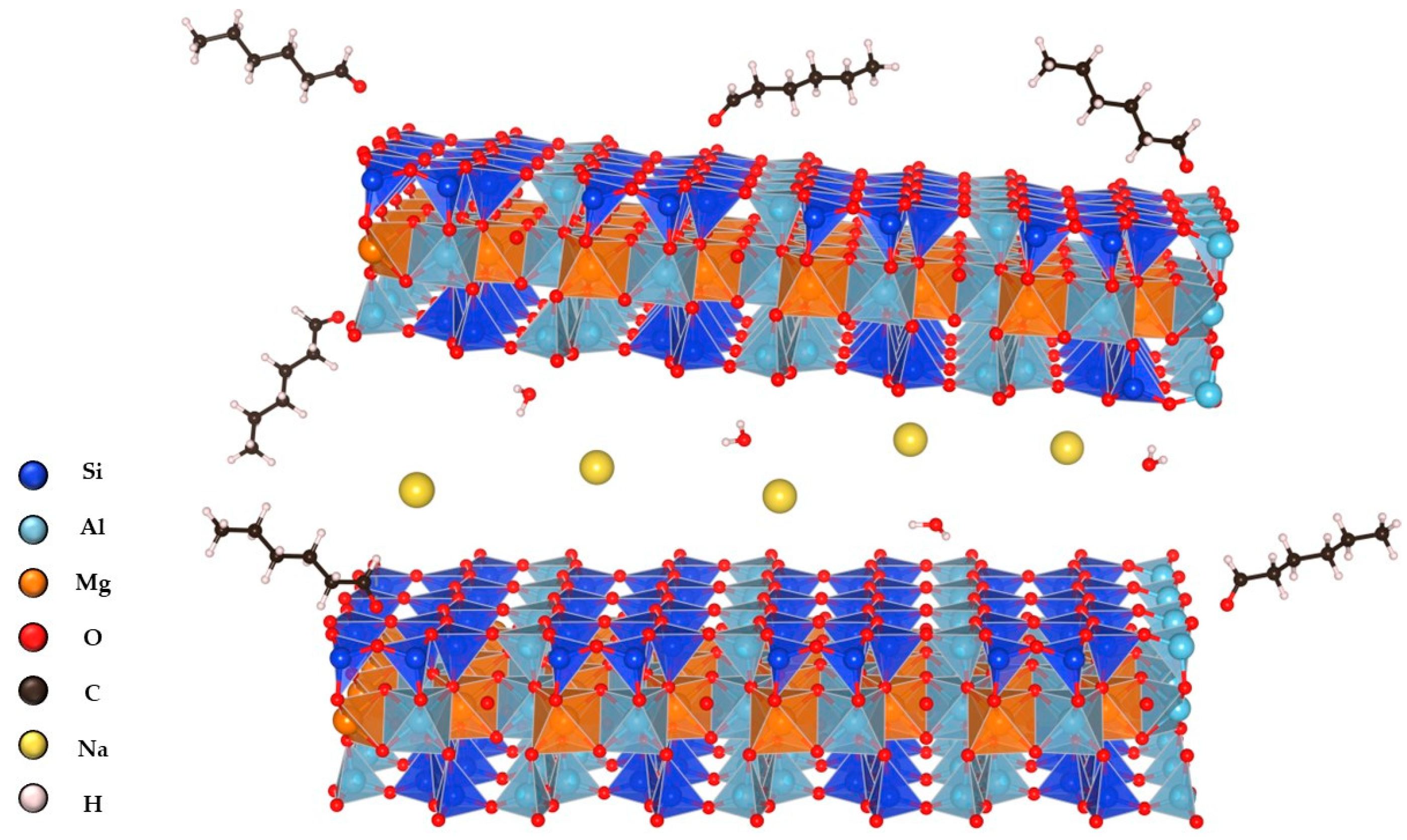
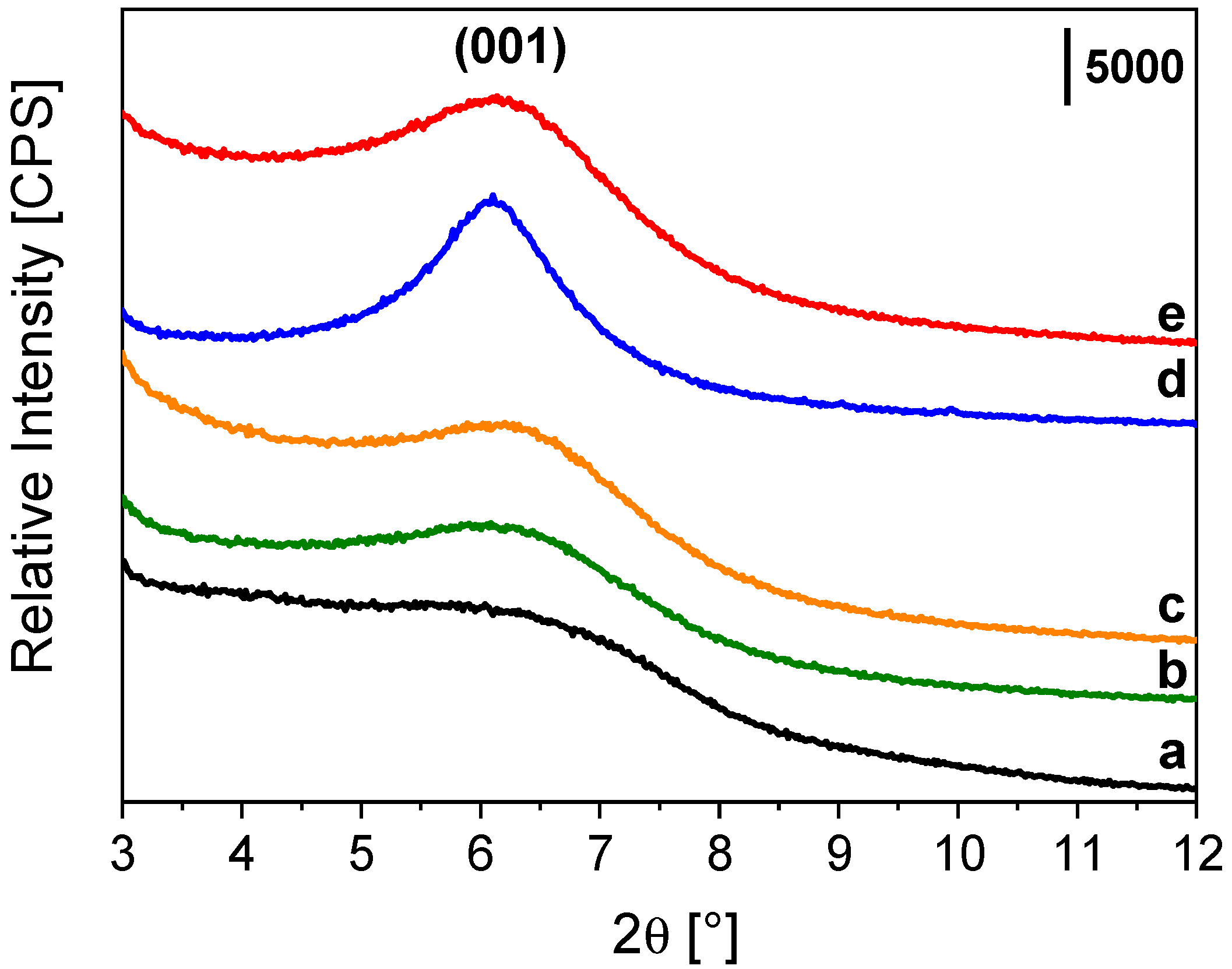
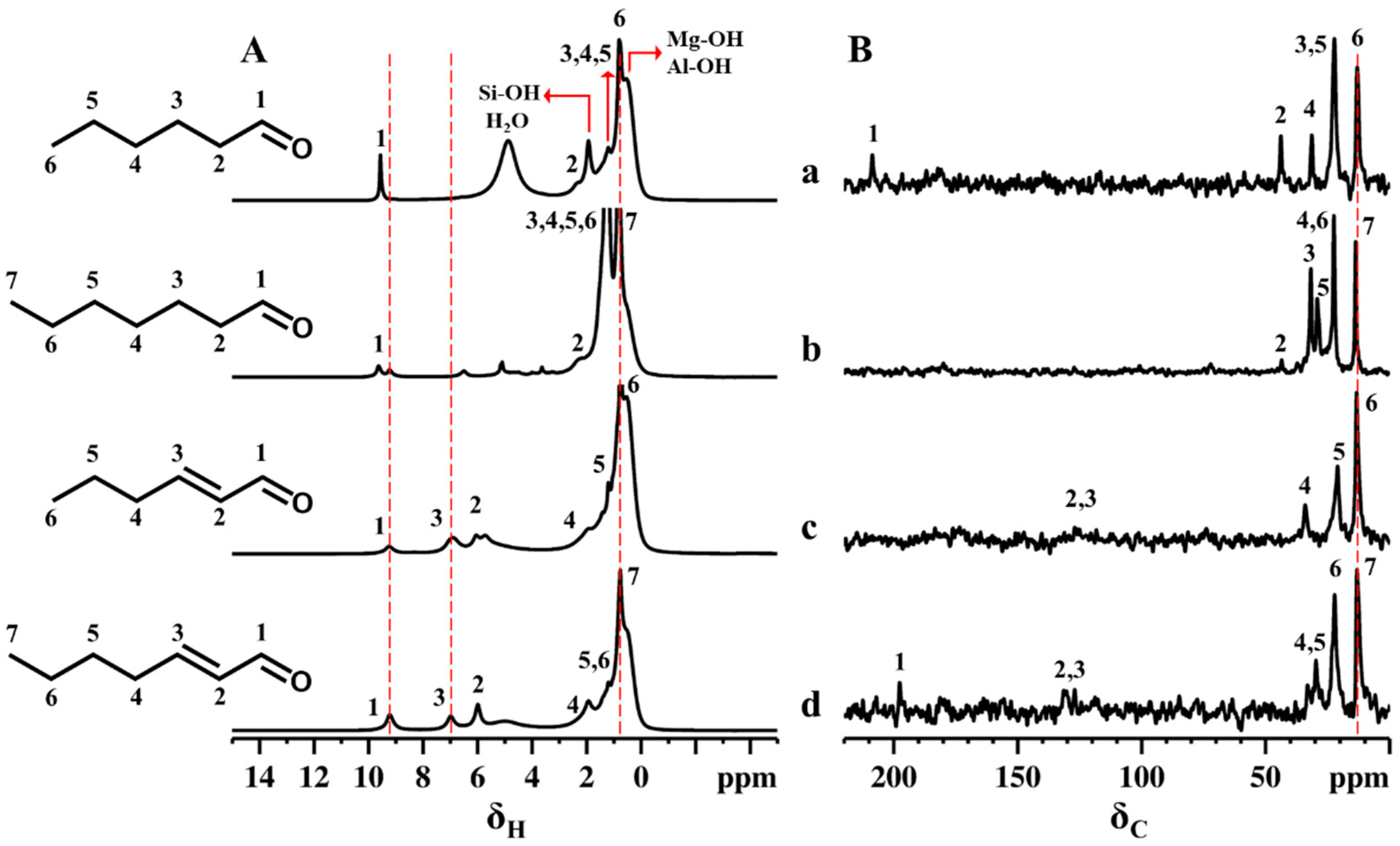
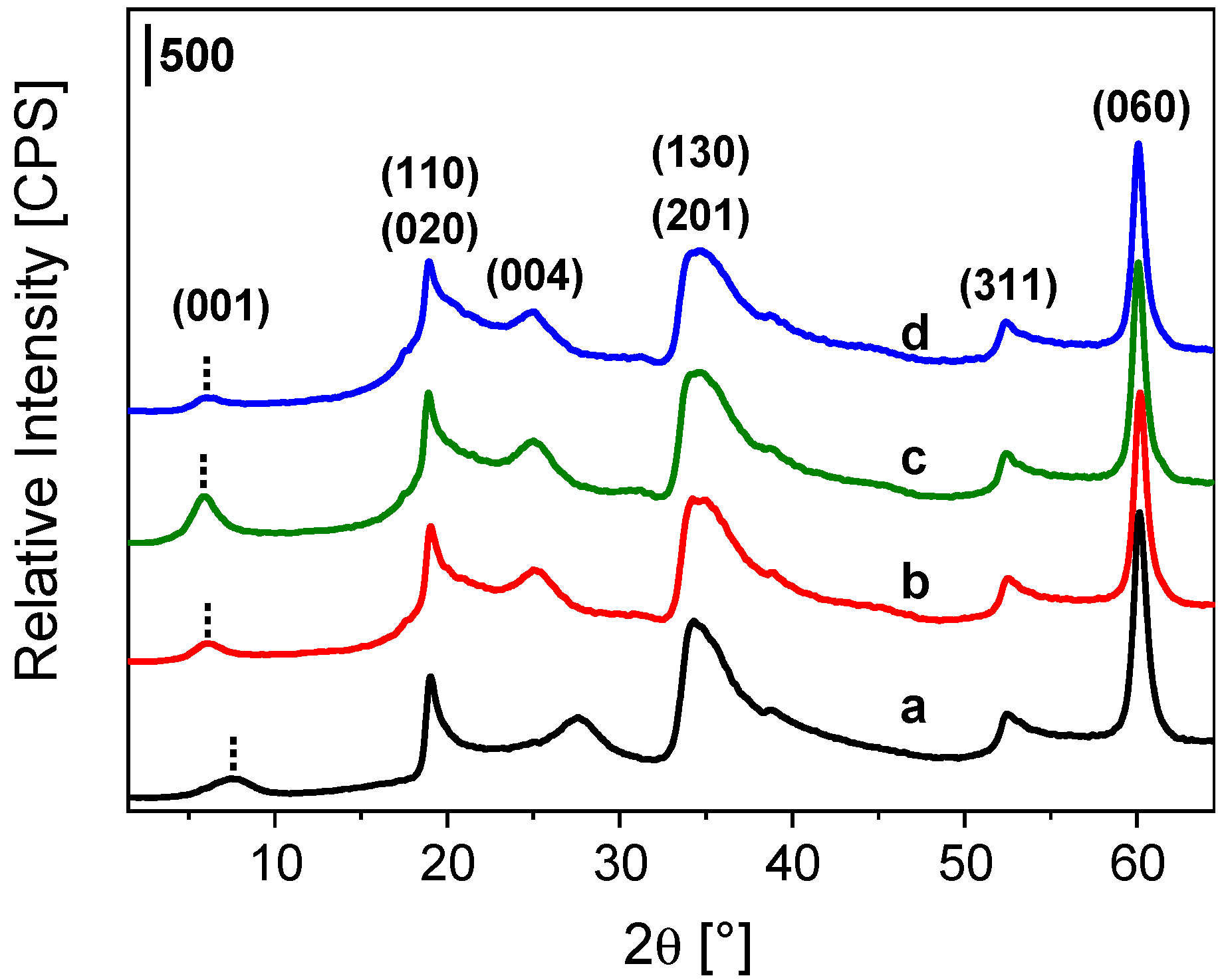
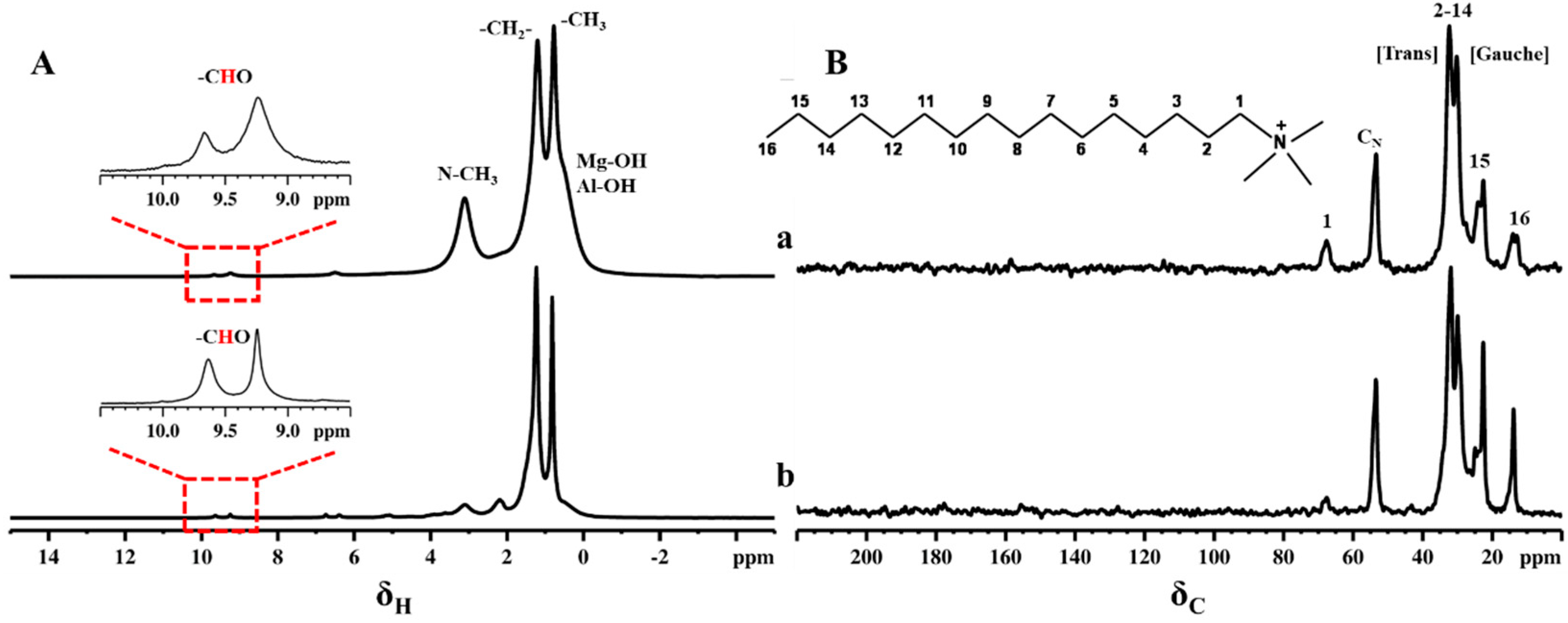
2.1. Impregnation of Synthetic Saponite with Saturated and Unsaturated Aldehydes
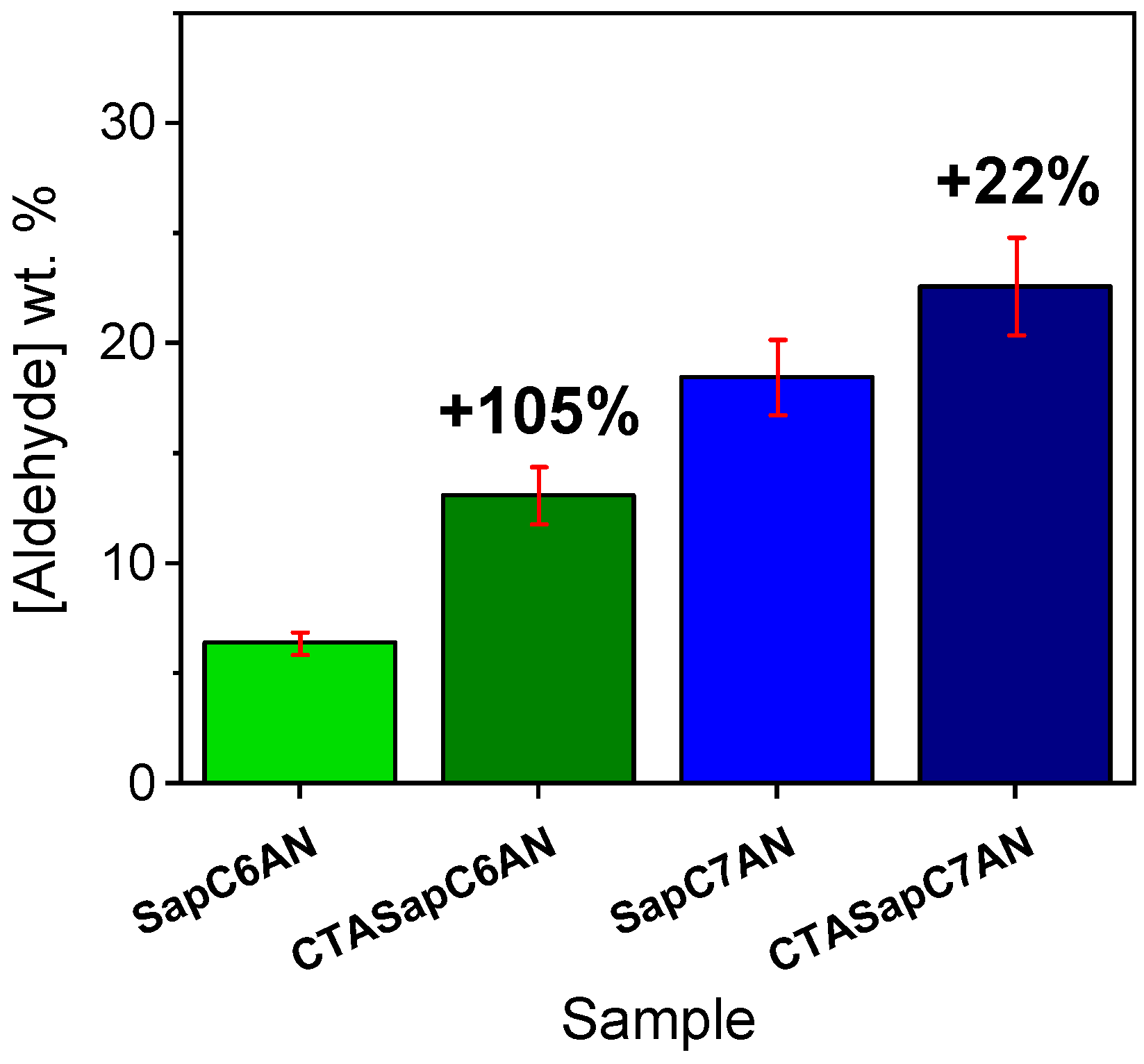
2.2. CTA-Intercalated Synthetic Saponite Clays Impregnated with Saturated Aldehydes
3. Materials and Methods
3.1. Materials
3.1.1. Synthesis of Sap and NaSap Clays
3.1.2. Impregnation of Saturated and Unsaturated Aldehydes on Saponite Clay
3.1.3. Intercalation of CTA+ Groups in Na-Saponite Clay
3.1.4. Impregnation of Saturated Aldehydes on CTASap Clay
3.2. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kloprogge, J.T.; Ponce, C.P. Spectroscopic Studies of Synthetic and Natural Saponites: A Review. Minerals 2021, 11, 112. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Komarneni, S.; Amonette, J.E. Synthesis of Smectite Clay Minerals: A Critical Review. Clays Clay Miner. 1999, 47, 529–554. [Google Scholar] [CrossRef]
- Zhou, C.H.; Zhou, Q.; Wu, Q.Q.; Petit, S.; Jiang, X.C.; Xia, S.T.; Li, C.S.; Yu, W.H. Modification, Hybridization and Applications of Saponite: An Overview. Appl. Clay Sci. 2019, 168, 136–154. [Google Scholar] [CrossRef]
- Carniato, F.; Gatti, G.; Bisio, C. An Overview of the Recent Synthesis and Functionalization Methods of Saponite Clay. New J. Chem. 2020, 44, 9969–9980. [Google Scholar] [CrossRef]
- Bisio, C.; Gatti, G.; Boccaleri, E.; Marchese, L.; Superti, G.B.; Pastore, H.O.; Thommes, M. Understanding Physico–Chemical Properties of Saponite Synthetic Clays. Microporous Mesoporous Mater. 2008, 107, 90–101. [Google Scholar] [CrossRef]
- Bisio, C.; Gatti, G.; Boccaleri, E.; Marchese, L.; Bertinetti, L.; Coluccia, S. On the Acidity of Saponite Materials: A Combined HRTEM, FTIR, and Solid-State NMR Study. Langmuir 2008, 24, 2808–2819. [Google Scholar] [CrossRef]
- Blukis, R.; Schindler, M.; Couasnon, T.; Benning, L.G. Mechanism and Control of Saponite Synthesis from a Self-Assembling Nanocrystalline Precursor. Langmuir 2022, 38, 7678–7688. [Google Scholar] [CrossRef]
- Meyer, S.; Bennici, S.; Vaulot, C.; Rigolet, S.; Dzene, L. Influence of the Precursor and the Temperature of Synthesis on the Structure of Saponite. Clays Clay Miner. 2020, 68, 544–552. [Google Scholar] [CrossRef]
- Vogels, R.J.M.J.; Kloprogge, J.T.; Geus, J.W. Synthesis and Characterization of Saponite Clays. Am. Mineral. 2005, 90, 931–944. [Google Scholar] [CrossRef]
- Corbin, G.; Vulliet, E.; Lanson, B.; Rimola, A.; Mignon, P. Adsorption of Pharmaceuticals onto Smectite Clay Minerals: A Combined Experimental and Theoretical Study. Minerals 2021, 11, 62. [Google Scholar] [CrossRef]
- Nisticò, R. A Comprehensive Study on the Applications of Clays into Advanced Technologies, with a Particular Attention on Biomedicine and Environmental Remediation. Inorganics 2022, 10, 40. [Google Scholar] [CrossRef]
- Zhou, C.H. An Overview on Strategies towards Clay-Based Designer Catalysts for Green and Sustainable Catalysis. Appl. Clay Sci. 2011, 53, 87–96. [Google Scholar] [CrossRef]
- Nagendrappa, G. Organic Synthesis Using Clay and Clay-Supported Catalysts. Appl. Clay Sci. 2011, 53, 106–138. [Google Scholar] [CrossRef]
- Tiozzo, C.; Bisio, C.; Carniato, F.; Marchese, L.; Gallo, A.; Ravasio, N.; Psaro, R.; Guidotti, M. Epoxidation with Hydrogen Peroxide of Unsaturated Fatty Acid Methyl Esters over Nb(V)-Silica Catalysts. Eur. J. Lipid Sci. Technol. 2013, 115, 86–93. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Zhao, Y.; Bai, H.; Wen, T.; Kang, S.; Song, G.; Song, S.; Komarneni, S. Removal of Heavy Metals and Dyes by Clay-Based Adsorbents: From Natural Clays to 1D and 2D Nano-Composites. Chem. Eng. J. 2021, 420, 127574. [Google Scholar] [CrossRef]
- Marchesi, S.; Carniato, F.; Guidotti, M.; Botta, M.; Marchese, L.; Bisio, C. Synthetic Saponite Clays as Promising Solids for Lanthanide Ion Recovery. New J. Chem. 2020, 44, 10033–10041. [Google Scholar] [CrossRef]
- Marchesi, S.; Nascimbene, S.; Guidotti, M.; Bisio, C.; Carniato, F. Application of NMR Relaxometry for Real-Time Monitoring of the Removal of Metal Ions from Water by Synthetic Clays. Dalton Trans. 2022, 51, 4502–4509. [Google Scholar] [CrossRef] [PubMed]
- Novikau, R.; Lujaniene, G. Adsorption Behaviour of Pollutants: Heavy Metals, Radionuclides, Organic Pollutants, on Clays and Their Minerals (Raw, Modified and Treated): A Review. J. Environ. Manag. 2022, 309, 114685. [Google Scholar] [CrossRef]
- Trujillano, R.; González, B.; Rives, V. Phase Change Materials (PCMs) Based in Paraffin/Synthetic Saponite Used as Heat Storage Composites. Energies 2021, 14, 7414. [Google Scholar] [CrossRef]
- Costenaro, D.; Bisio, C.; Carniato, F.; Gatti, G.; Oswald, F.; Meyer, T.B.; Marchese, L. Size Effect of Synthetic Saponite-Clay in Quasi-Solid Electrolyte for Dye-Sensititized Solar Cells. Sol. Energy Mater. Sol. Cells 2013, 117, 9–14. [Google Scholar] [CrossRef]
- Costenaro, D.; Gatti, G.; Carniato, F.; Paul, G.; Bisio, C.; Marchese, L. The Effect of Synthesis Gel Dilution on the Physico-Chemical Properties of Acid Saponite Clays. Microporous Mesoporous Mater. 2012, 162, 159–167. [Google Scholar] [CrossRef]
- Vicente, I.; Salagre, P.; Cesteros, Y.; Medina, F.; Sueiras, J.E. Microwave-Assisted Synthesis of Saponite. Appl. Clay Sci. 2010, 48, 26–31. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, C.-H.; Lin, C.-X.; Tong, D.-S.; Yu, W.-H. Synthesis of Clay Minerals. Appl. Clay Sci. 2010, 50, 1–11. [Google Scholar] [CrossRef]
- Trujillano, R.; Rico, E.; Vicente, M.A.; Herrero, M.; Rives, V. Microwave Radiation and Mechanical Grinding as New Ways for Preparation of Saponite-like Materials. Appl. Clay Sci. 2010, 48, 32–38. [Google Scholar] [CrossRef]
- Marchesi, S.; Guidotti, M.; Marchese, L.; Evangelisti, C.; Carniato, F.; Bisio, C. Bifunctional Europium(III) and Niobium(V)-Containing Saponite Clays for the Simultaneous Optical Detection and Catalytic Oxidative Abatement of Blister Chemical Warfare Agents. Chem. A Eur. J. 2021, 27, 4723–4730. [Google Scholar] [CrossRef]
- Marchesi, S.; Bisio, C.; Carniato, F. Enhancement of the Luminescence Properties of Eu (III) Containing Paramagnetic Saponite Clays. Appl. Sci. 2021, 11, 8903. [Google Scholar] [CrossRef]
- Marchesi, S.; Bisio, C.; Carniato, F. Novel Light-Emitting Clays with Structural Tb 3+ and Eu 3+ for Chromate Anion Detection. RSC Adv. 2020, 10, 29765–29771. [Google Scholar] [CrossRef]
- Carniato, F.; Bisio, C.; Psaro, R.; Marchese, L.; Guidotti, M. Niobium(V) Saponite Clay for the Catalytic Oxidative Abatement of Chemical Warfare Agents. Angew. Chem. Int. Ed. 2014, 53, 10095–10098. [Google Scholar] [CrossRef]
- Costenaro, D.; Bisio, C.; Carniato, F.; Safronyuk, S.L.; Kramar, T.V.; Taran, M.V.; Starodub, M.F.; Katsev, A.M.; Guidotti, M. Physico–Chemical Properties, Biological and Environmental Impact of Nb–Saponites Catalysts for the Oxidative Degradation of Chemical Warfare Agents. ChemistrySelect 2017, 2, 1812–1819. [Google Scholar] [CrossRef]
- Marchesi, S.; Bisio, C.; Lalli, D.; Marchese, L.; Platas-Iglesias, C.; Carniato, F. Bifunctional Paramagnetic and Luminescent Clays Obtained by Incorporation of Gd3+ and Eu3+ Ions in the Saponite Framework. Inorg. Chem. 2021, 60, 10749–10756. [Google Scholar] [CrossRef]
- Ostinelli, L.; Recchia, S.; Bisio, C.; Carniato, F.; Guidotti, M.; Marchese, L.; Psaro, R. Acid/Vanadium-Containing Saponite for the Conversion of Propene into Coke: Potential Flame-Retardant Filler for Nanocomposite Materials. Chem. Asian J. 2012, 7, 2394–2402. [Google Scholar] [CrossRef] [PubMed]
- Carniato, F.; Bisio, C.; Gatti, G.; Roncoroni, S.; Recchia, S.; Marchese, L. On the Properties of a Novel V-Containing Saponite Catalyst for Propene Oxidative Dehydrogenation. Catal. Lett. 2009, 131, 42–48. [Google Scholar] [CrossRef]
- Gil, A.; Santamaría, L.; Korili, S.A.; Vicente, M.A.; Barbosa, L.V.; de Souza, S.D.; Marçal, L.; de Faria, E.H.; Ciuffi, K.J. A Review of Organic-Inorganic Hybrid Clay Based Adsorbents for Contaminants Removal: Synthesis, Perspectives and Applications. J. Environ. Chem. Eng. 2021, 9, 105808. [Google Scholar] [CrossRef]
- Liao, L.; Lv, G.; Cai, D.; Wu, L. The Sequential Intercalation of Three Types of Surfactants into Sodium Montmorillonite. Appl. Clay Sci. 2016, 119, 82–86. [Google Scholar] [CrossRef]
- Bisio, C.; Carniato, F.; Paul, G.; Gatti, G.; Boccaleri, E.; Marchese, L. One-Pot Synthesis and Physicochemical Properties of an Organo-Modified Saponite Clay. Langmuir 2011, 27, 7250–7257. [Google Scholar] [CrossRef]
- Lalli, D.; Marchesi, S.; Carniato, F.; Bisio, C.; Tei, L.; Marchese, L.; Botta, M. Combination of Solid-State NMR and 1H NMR Relaxometry for the Study of Intercalated Saponite Clays with the Macrocyclic Derivatives of Gd(III) and Y(III). Dalton Trans. 2020, 49, 6566–6571. [Google Scholar] [CrossRef]
- Marchesi, S.; Carniato, F.; Bisio, C.; Tei, L.; Marchese, L.; Botta, M. Novel Paramagnetic Clays Obtained through Intercalation of Gd3+-Complexes. Dalton Trans. 2018, 47, 7896–7904. [Google Scholar] [CrossRef]
- Malheiro, R.; Casal, S.; Baptista, P.; Pereira, J.A. A Review of Bactrocera Oleae (Rossi) Impact in Olive Products: From the Tree to the Table. Trends Food Sci. Technol. 2015, 44, 226–242. [Google Scholar] [CrossRef]
- Marchini, D.; Petacchi, R.; Marchi, S. Bactrocera Oleae Reproductive Biology: New Evidence on Wintering Wild Populations in Olive Groves of Tuscany (Italy). Bull. Insectol. 2017, 70, 121–128. [Google Scholar]
- Malheiro, R.; Casal, S.; Cunha, S.C.; Baptista, P.; Pereira, J.A. Olive Volatiles from Portuguese Cultivars Cobrançosa, Madural and Verdeal Transmontana: Role in Oviposition Preference of Bactrocera Oleae (Rossi) (Diptera: Tephritidae). PLoS ONE 2015, 10, e0125070. [Google Scholar] [CrossRef]
- Malheiro, R.; Ortiz, A.; Casal, S.; Baptista, P.; Pereira, J.A. Electrophysiological Response of Bactrocera Oleae (Rossi) (Diptera: Tephritidae) Adults to Olive Leaves Essential Oils from Different Cultivars and Olive Tree Volatiles. Ind. Crops Prod. 2015, 77, 81–88. [Google Scholar] [CrossRef]
- Prieto, O.; Vicente, M.A.; Bañares-Muñoz, M.A. Study of the Porous Solids Obtained by Acid Treatment of a High Surface Area Saponite. J. Porous Mater. 1999, 6, 335–344. [Google Scholar] [CrossRef]
- PubChem Hexanal. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6184 (accessed on 23 August 2022).
- PubChem 2-Hexenal. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5281168 (accessed on 23 August 2022).
- PubChem Heptanal. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/8130 (accessed on 23 August 2022).
- PubChem 2-Heptenal. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5283316 (accessed on 23 August 2022).
- Smith, B.C. Infrared Spectral Interpretation: A Systematic Approach; CRC Press: Boca Raton, FL, USA, 1999; ISBN 978-0-8493-2463-5. [Google Scholar]
- Osman, M.A.; Ernst, M.; Meier, B.H.; Suter, U.W. Structure and Molecular Dynamics of Alkane Monolayers Self-Assembled on Mica Platelets. J. Phys. Chem. B 2002, 106, 653–662. [Google Scholar] [CrossRef]
- Takahashi, C.; Shirai, T.; Hayashi, Y.; Fuji, M. Study of Intercalation Compounds Using Ionic Liquids into Montmorillonite and Their Thermal Stability. Solid State Ion. 2013, 241, 53–61. [Google Scholar] [CrossRef]
- Hlavatý, V.; Fajnor, V.Š. Thermal Stability of Clay/Organic Intercalation Complexes. J. Therm. Anal. Calorim. 2002, 67, 113–118. [Google Scholar] [CrossRef]
- Paul, G.; Bisio, C.; Braschi, I.; Cossi, M.; Gatti, G.; Gianotti, E.; Marchese, L. Combined Solid-State NMR, FT-IR and Computational Studies on Layered and Porous Materials. Chem. Soc. Rev. 2018, 47, 5684–5739. [Google Scholar] [CrossRef]
- Sacchetto, V.; Gatti, G.; Paul, G.; Braschi, I.; Berlier, G.; Cossi, M.; Marchese, L.; Bagatin, R.; Bisio, C. The Interactions of Methyl Tert-Butyl Ether on High Silica Zeolites: A Combined Experimental and Computational Study. Phys. Chem. Chem. Phys. 2013, 15, 13275–13287. [Google Scholar] [CrossRef]
- Braschi, I.; Paul, G.; Gatti, G.; Cossi, M.; Marchese, L. Embedding Monomers and Dimers of Sulfonamide Antibiotics into High Silica Zeolite Y: An Experimental and Computational Study of the Tautomeric Forms Involved. RSC Adv. 2013, 3, 7427–7437. [Google Scholar] [CrossRef]
- Ziyat, H.; Bennani, M.N.; Hajjaj, H.; Qabaqous, O.; Arhzaf, S.; Mekdad, S.; Allaoui, S. Adsorption of Thymol onto Natural Clays of Morocco: Kinetic and Isotherm Studies. J. Chem. 2020, 2020, 4926809. [Google Scholar] [CrossRef]
- Nguemtchouin, M.G.M.; Ngassoum, M.B.; Chalier, P.; Kamga, R.; Ngamo, L.S.T.; Cretin, M. Ocimum Gratissimum Essential Oil and Modified Montmorillonite Clay, a Means of Controlling Insect Pests in Stored Products. J. Stored Prod. Res. 2013, 52, 57–62. [Google Scholar] [CrossRef]
- Nguemtchouin, M.G.M.; Ngassoum, M.B.; Kamga, R.; Deabate, S.; Lagerge, S.; Gastaldi, E.; Chalier, P.; Cretin, M. Characterization of Inorganic and Organic Clay Modified Materials: An Approach for Adsorption of an Insecticidal Terpenic Compound. Appl. Clay Sci. 2015, 104, 110–118. [Google Scholar] [CrossRef]
- Gueu, S.; Tia, V.E.; Bartier, D.; Barres, O.; Soro, F.D. Adsorption of Lippia Multiflora Essential Oil on Two Surfactant-Modified Clays: Qualitative Approach. Clay Miner. 2020, 55, 219–228. [Google Scholar] [CrossRef]
- Giannakas, A.; Tsagkalias, I.; Achilias, D.S.; Ladavos, A. A Novel Method for the Preparation of Inorganic and Organo-Modified Montmorillonite Essential Oil Hybrids. Appl. Clay Sci. 2017, 146, 362–370. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Leontiou, A.; Moschovas, D.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Karakassides, A.; Avgeropoulos, A.; Proestos, C. Performance of Thyme Oil@Na-Montmorillonite and Thyme Oil@Organo-Modified Montmorillonite Nanostructures on the Development of Melt-Extruded Poly-L-Lactic Acid Antioxidant Active Packaging Films. Molecules 2022, 27, 1231. [Google Scholar] [CrossRef]
- Fernández, M.A.; Barberia Roque, L.; Gámez Espinosa, E.; Deyá, C.; Bellotti, N. Organo-Montmorillonite with Biogenic Compounds to Be Applied in Antifungal Coatings. Appl. Clay Sci. 2020, 184, 105369. [Google Scholar] [CrossRef]
- Marçal, L.; de Faria, E.H.; Nassar, E.J.; Trujillano, R.; Martín, N.; Vicente, M.A.; Rives, V.; Gil, A.; Korili, S.A.; Ciuffi, K.J. Organically Modified Saponites: SAXS Study of Swelling and Application in Caffeine Removal. ACS Appl. Mater. Interfaces 2015, 7, 10853–10862. [Google Scholar] [CrossRef]
- Hendry, P.; Ludi, A. Structure, Reactivity, Spectra, and Redox Properties of Cobalt(III) Hexaamines. In Advances in Inorganic Chemistry; Sykes, A.G., Ed.; Academic Press: Cambridge, MA, USA, 1990; Volume 35, pp. 117–198. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchesi, S.; Paul, G.; Guidotti, M.; Econdi, S.; Bisio, C.; Carniato, F. Impregnation of Synthetic Saponites with Aldehydes: A Green Approach in the Intercalation of Bioactive Principles. Inorganics 2022, 10, 159. https://doi.org/10.3390/inorganics10100159
Marchesi S, Paul G, Guidotti M, Econdi S, Bisio C, Carniato F. Impregnation of Synthetic Saponites with Aldehydes: A Green Approach in the Intercalation of Bioactive Principles. Inorganics. 2022; 10(10):159. https://doi.org/10.3390/inorganics10100159
Chicago/Turabian StyleMarchesi, Stefano, Geo Paul, Matteo Guidotti, Stefano Econdi, Chiara Bisio, and Fabio Carniato. 2022. "Impregnation of Synthetic Saponites with Aldehydes: A Green Approach in the Intercalation of Bioactive Principles" Inorganics 10, no. 10: 159. https://doi.org/10.3390/inorganics10100159




