Effects of Cardiorespiratory Fitness and Obesity on Salivary Secretory IgA and Alpha-Amylase in South African Children
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Saliva Sample Collection
2.3. Analyses of Salivary Markers
2.4. Body Composition, Cardiovascular Measurements, and Cardio-Respiratory Fitness
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alwan, A. Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014, 311, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Goedecke, J.; Jennings, C.; Lambert, E. Obesity in South Africa. In Chronic Diseases of Lifestyle in South Africa Since 1995–2005; South African Medical Research Council: Cape Town, South Africa, 2006; pp. 65–79. [Google Scholar]
- Steyn, K. Conceptual framework for chronic diseases of lifestyle in South Africa. In Chronic Diseases of Lifestyle in South Africa: 1995–2005; South African Medical Research Council: Cape Town, South Africa, 2006; pp. 1–8. [Google Scholar]
- Reddy, S.P.; Resnicow, K.; James, S.; Funani, I.N.; Kambaran, N.S.; Omardien, R.G.; Masuka, P.; Sewpaul, R.; Vaughan, R.D.; Mbewu, A. Rapid increases in overweight and obesity among South African adolescents: Comparison of data from the South African National Youth Risk Behaviour Survey in 2002 and 2008. Am. J. Public Health 2012, 102, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Puoane, T.; Steyn, K.; Bradshaw, D.; Laubscher, R.; Fourie, J.; Lambert, V. Obesity in South Africa: The South African demographic and health survey. Obes. Res. 2002, 10, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Cieslak, T.; Frost, G.; Klentron, P. Effect of physical activity, body fat and salivary cortisol on mucosal immunity in children. J. Appl. Physiol. 2003, 95, 2315–2320. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; Woods, J.A.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goetz, L.; et al. Position statement part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar] [PubMed]
- Jedrychowski, W.; Maugeri, U.; Flak, E.; Mroz, E.; Bianchi, I. Predisposition to acute respiratory infections among overweight preadolescent children: An epidemiologic study in Poland. Public Health 1998, 112, 189–195. [Google Scholar] [CrossRef]
- Jedrychowski, W.; Maugeri, U.; Flak, E.; Mroz, E.; Bianchi, I. Cohort study on low physical activity level and recurrent acute respiratory infections in schoolchildren. Cent. Eur. J. Public Health 2001, 9, 126–129. [Google Scholar] [PubMed]
- Teeuw, W.; Bosch, J.A.; Veerman, E.C.; Nieuw Amerongen, A.V. Neuroendocrine regulation of salivary IgA synthesis and secretion: Implications for oral health. Biol. Chem. 2004, 385, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Pallaro, A.; Barbeito, S.; Taberner, P.; Marino, P.; Franchello, A.; Strasnoy, I.; Ramos, O.; Slobodianik, N. Total salivary IgA, serum C3c and IgA in obese school children. J. Nutr. Biochem. 2002. [Google Scholar] [CrossRef]
- Segerstrom, S.C.; Miller, G.E. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol. Bull. 2004, 130, 601–630. [Google Scholar] [CrossRef] [PubMed]
- Shirtcliff, E.A.; Coe, C.L.; Pollak, S.D. Early childhood stress is associated with elevated antibody levels to herpes simplex virus type 1. Proc. Natl. Acad. Sci. USA 2009, 106, 2963–2967. [Google Scholar] [CrossRef] [PubMed]
- Wallerius, S.; Rosmond, R.; Ljung, T.; Holm, G.; Björntorp, P. Rise in morning saliva cortisol is associated with abdominal obesity in men: A preliminary report. J. Endocrinol. Investig. 2003, 26, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Björntorp, P.; Rosmond, R. Obesity and cortisol. Nutrition 2000, 16, 924–936. [Google Scholar] [CrossRef]
- Champaneri, S.; Xu, X.; Carnethon, M.R.; Bertoni, A.G.; Seeman, T.; DeSantis, A.S.; Diez Roux, A.; Shrager, S.; Golden, S.H. Diurnal salivary cortisol is associated with body mass index and waist circumference: The multiethnic study of atherosclerosis. Obesity 2013, 21, E56–E63. [Google Scholar] [CrossRef] [PubMed]
- Nater, U.M.; Rohleder, N.; Scholotz, W.; Ehlert, U.; Kirschbaum, C. Determinants of the diurnal couse of salivary alpha-amylase. Psychoneuroendrocrinology 2007, 32, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Strahler, J.; Mueller, A.; Rosenloecher, F.; Kirschbaum, C.; Rohleder, N. Salivary a-amylase stress reactivity across different age groups. Psychophysiology 2010, 47, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Nater, U.; Rohleder, N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology 2009, 34, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Chatterton, R.; Vogelsong, K.; Lu, Y.; Ellman, A.; Hudgens, G. Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clin. Physiol. 1996, 16, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, G.; Ericson, T.; Ekblom, B.; Birkhed, D. Saliva and marathon running. Scand. J. Med. Sci. Sports 1997, 7, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Steerenberg, P.; van Asperen, I.; van Nieuw Amerongen, A.; Biewenga, J.; Mol, D.; Medema, G. Salivary levels of immunoglobulin A in triathletes. Eur. J. Oral. Sci. 1997, 105, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.; Kivlighan, K.; Blair, C.; El-Sheikh, M.; Mize, J.; Lisonbee, J.A.; Buckhalt, J.A.; Stroud, L.R.; Handwerger, K.; Handwerger, K. Integrating the measurement of salivary alpha amylase into studies of child health, development, and social relationships. J. Soc. Pers. Relat. 2006, 23, 267–290. [Google Scholar] [CrossRef]
- Walsh, N.; Blannin, A.; Clark, A.; Cook, L.; Robson, P.; Gleeson, M. The effects of high-intensity intermittent exercise on saliva IgA, total protein and alpha-amylase. J. Sports Sci. 1999, 17, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Capranica, L.; Lupo, C.; Cortis, C.; Chiodo, S.; Cibelli, G.; Tessitore, A. Salivary cortisol and alpha-amylase reactivity to taekwondo competition in children. Eur. J. Appl. Physiol. 2012, 112, 647–652. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World medical association declaration of helsinki: ethical principles for medical research involving human subjects. JAMA 2013. [Google Scholar] [CrossRef]
- Salimetrics. Improving Saliva Study Results. Available online: https://www.salimetrics.com/newsletter-v1/november-2010 (accessed on 10 May 2016).
- Barrett, B.; Locken, K.; Maberry, R.; Schwamman, J.; Brown, R.; Bobula, J.; Stauffacher, E.A. The Wisconsin upper respiratory symptom survey (WURSS). J. Fam. Pract. 2002, 51, 265–265. [Google Scholar] [PubMed]
- Thomas, S.; Reading, J.; Shephard, R.J. Revision of the physical activity readiness questionnaire (PAR-Q). Can. J. Sport Sci. 1992, 17, 338–345. [Google Scholar] [PubMed]
- Starzak, D.E.; Konkol, K.F.; McKune, A.J. Twelve weeks of soccer-specific training: Effects on mucosal immunity, salivary alpha-amylase and body composition in male African youths. Sport Sci. Health 2016, 12, 1–8. [Google Scholar] [CrossRef]
- Novas, A.; Rowbottom, D.; Jenkins, D. Tennis, incidence of URTI and Salivary IgA. Int. J. Sports Med. 2003, 24, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Brook, C. Determination of body composition of children from skinfold measurements. Arch. Dis. Child. 1971, 46, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Ledger, L.; Lambert, J. A maximal multistage 20 m shuttle run test to predict VO2max. Eur. J. Appl. Physiol. 1982, 49, 1–12. [Google Scholar] [CrossRef]
- Tomkinson, G.R.; Leger, L.A.; Olds, T.S.; Cazorla, G. Secular trends in the performance of children and adolescents (1980–2000): An analysis of 55 studies of the 20 m shuttle run test in 11 countries. Sports Med. 2003, 33, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Daniel, W.W. Applied Nonparametric Statistics, 2nd ed.; PWS-Kent: Boston, MA, USA, 1990; pp. 319–330. [Google Scholar]
- Ogden, C.L.; Flegal, K.M. Changes in terminology for childhood overweight and obesity. Natl. Health Stat. Rep. 2010. [Google Scholar]
- Granger, D.; Kivlighan, K.; El-Sheikh, M.; Gordis, E.; Stroud, L. Salivary alpha-amylase in biobehavioral research. Ann. N. Y. Acad. Sci. 2007, 1098, 122–144. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Ding, Z.Y.; Fong, D.Y.-T.; Karlberg, J. Blood pressure is associated with body mass index in both normal and obese children. Hypertension 2000, 36, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Colon, R.; Franklin, F.A.; Lee, J.Y.; Aldridge, R.; Alexander, L. Prevalence of obesity with increased blood pressure in elementary school-aged children. South. Med. J. 1997, 90, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Farpour-Lambert, N.J.; Aggoun, Y.; Marchand, L.M.; Martin, X.E.; Herrmann, F.R.; Beghetti, M. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J. Am. Coll. Cardiol. 2009, 54, 2396–2406. [Google Scholar] [CrossRef] [PubMed]
- Lurbe, E.; Alvarez, V.; Liao, Y. The impact of obesity and body fat distribution on ambulatory blood pressure in children and adolescents. Am. J. Hypertens. 1998, 11, 418–424. [Google Scholar] [CrossRef]
- Berndtsson, G.; Mattsson, E.; Marcus, C.; Larsson, U.E. Age and gender differences in VO2max in Swedish obese children and adolescents. Acta Paediatr. 2007, 96, 567–571. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.; Collares, E.; Barbieri, M.; Fernandes, M. Production and concentration of saliva and salivary amylase in obese children. Arq. Gastroenterol. 1996, 34, 105–111. [Google Scholar]
- Beltzer, E.K.; Fortunato, C.K.; Guaderrama, M.M.; Peckins, M.K.; Garramone, B.M.; Granger, D.A. Salivary flow and alpha-amylase: Collection technique, duration, and oral fluid type. Physiol. Behav. 2010, 101, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Julius, S. Autonomic nervous system dysregulation in human hypertension. Am. J. Cardiol. 1991, 67, 3B–7B. [Google Scholar] [CrossRef]
- Takai, N.; Yamaguchi, M.; Aragaki, T.; Eto, K.; Uchihashi, K.; Nishikawa, Y. Effect of psychological stress on the salivary cortisol and amylase levels in healthy young adults. Arch. Oral. Biol. 2004, 49, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Pallaroa, A.; Barbeitob, S.; Tabernerb, P.; Marinob, P.; Franchellob, A.; Strasnoyb, I. Total salivary IgA, serum C3c and IgA in obese school children. J. Nutr. Biochem. 2002, 13, 539–542. [Google Scholar] [CrossRef]
- Vermeer, H.J.; van IJzendoorn, M.H.; Groeneveld, M.G.; Granger, D.A. Downregulation of the immune system in low-quality child care: The case of secretory immunoglobulin A (SIgA) in toddlers. Physiol. Behav. 2012, 105, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Kyrou, I.; Tsigos, C. Stress hormones: Physiological stress and regulation of metabolism. Curr. Opin. Pharmacol. 2009, 9, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, P.; Lemieux, I.; Després, J.P. Obesity, inflammation, and cardiovascular risk. Clin. Pharmacol. Ther. 2010, 87, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.Z.; Libby, P. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Miletic, I.; Schiffman, S.; Miletic, V.; Sattely-Miller, E. Salivary IgA secretion rate in young and elderly persons. Physiol. Behav. 1996, 60, 243–248. [Google Scholar] [CrossRef]
- Kugler, J.; Hess, M.; Haake, D. Secretion of salivary immunoglobulin A in relation to age, saliva flow, mood states, secretion of albumin, cortisol, and catecholamines in saliva. J. Clin. Immunol. 1992, 12, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.M. Growth at Adolescence: With a General Consideration of the Effects of Hereditary and Environmental Factors upon Growth and Maturation from Birth to Maturity; Oxford, Blackwell Scientific Publications: London, UK, 1962. [Google Scholar]
| n = 132 | Normal (n = 74) (males 36; females 38) | Overweight (n = 22) (males 8; females 14) | Obese (n = 36) (males 14; females 22) |
|---|---|---|---|
| Age (years) | 10.26 (1.74) | 9.59 (1.71) | 9.97 (1.52) |
| Stature (cm) | 140.25 (11.42) | 141.20 (10.74) | 143.53 (9.19) |
| Mass (kg) | 34.10 (7.58) | 40.83 (8.73) * | 58.03 (11.85) # |
| BMI bb (kg/m2) | 17.11 (1.63) | 20.20 (1.49) *** | 28.03 (4.55) # |
| Waist-Hip Ratio | 0.77 (0.04) | 0.79 (0.04) && | 0.84 (0.07) & |
| Body Fat % | 19.56 (5.47) | 28.18 (4.11) *** | 39.38 (5.37) # |
| Resting HR (b/min) | 87.00 (12.57) | 85.09 (11.21) | 89.11 (12.01) |
| SBP (mmHg) | 94.20 (12.20) | 96.27 (9.67) && | 107.28 (11.67) & |
| DBP aaa (mmHg) | 63.22 (8.33) | 64.64 (8.17) | 75.22 (10.00) # |
| VO2max (mL/kg/min) | 29.35 (5.67) | 25.97 (4.01) a | 22.34 (3.02) &,aa |
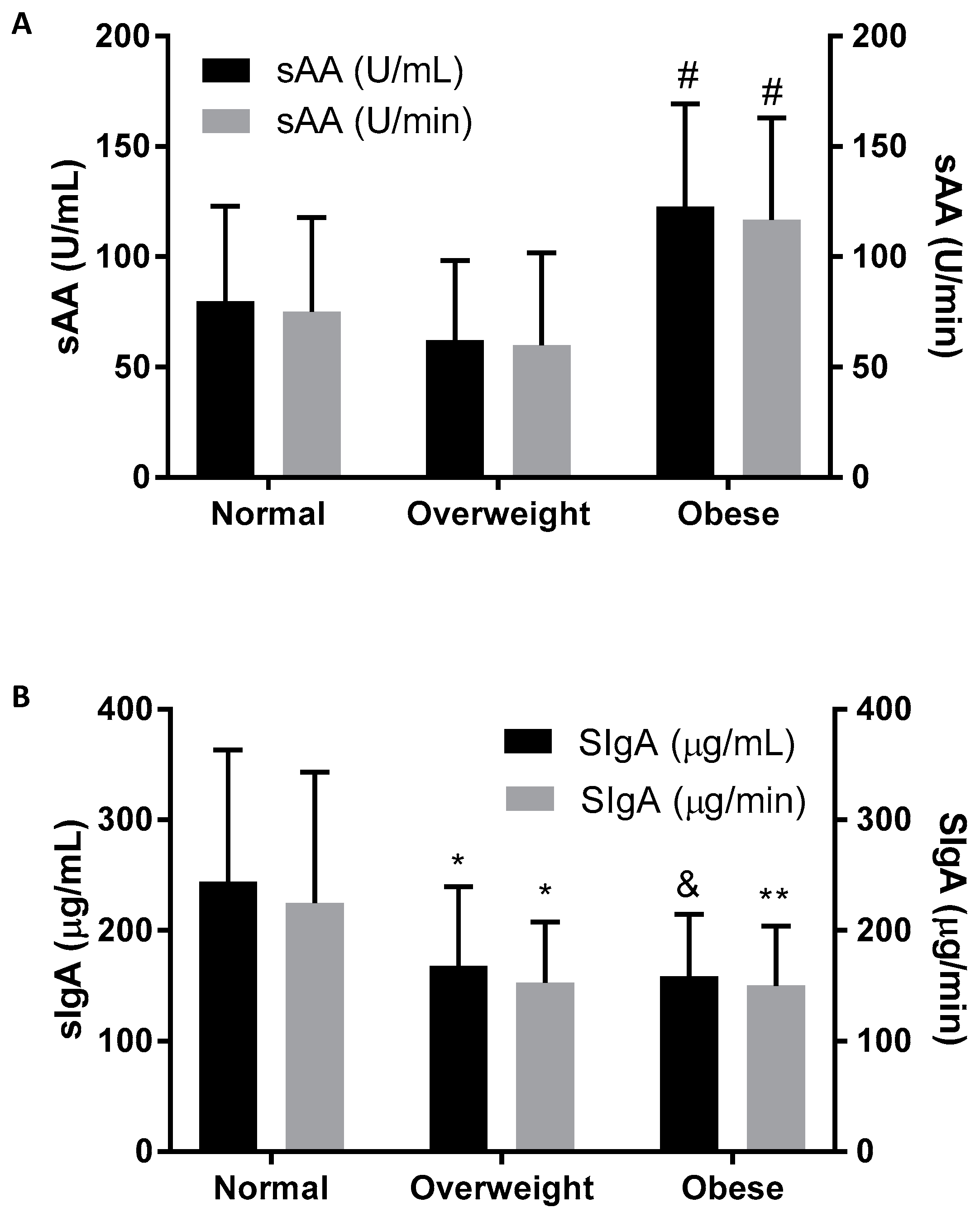
| sAA (U/mL) | 79.83 (43.12) | 62.13 (36.06) | 122.75 (46.50) # |
| sAA (U/min) | 75.07 (42.64) | 59.91 (41.97) | 116.75 (46.21) # |
| SIgA (µg/mL) | 243.95 (119.23) | 167.92 (71.46) * | 158.34 (56.03) & |
| SIgA (µg/min) | 224.88 (118.45) | 152.95 (54.91) * | 150.05 (53.98) ** |
| Models | B * | Std. Error * | t * | p * | |
|---|---|---|---|---|---|
| sAA (U/mL) | (Constant) | 6.964 | 38.75 | 0.18 | 0.86 |
| BMI | 1.934 | 0.92 | 2.09 | 0.04 * | |
| DBP | 0.9208 | 0.46 | 2.00 | 0.04 * | |
| VO2max | −0.7321 | 0.76 | 0.96 | 0.34 | |
| sAA (U/min) | (Constant) | −5.314 | 39.24 | 0.14 | 0.89 |
| BMI | 2.351 | 0.94 | 2.51 | 0.01 * | |
| DBP | 0.7135 | 0.47 | 1.53 | 0.13 | |
| VO2max | −0.2394 | 0.73 | 0.31 | 0.76 | |
| SIgA (µg/mL) | (Constant) | 303.71 | 58.28 | 5.21 | <0.0001 |
| Age | −1.681 | 5.27 | 0.32 | 0.75 | |
| BMI Category (1 = normal, 2 = overweight, 3 = obese) | −45.737 | 10.09 | 4.54 | <0.0001 * | |
| SIgA (µg/min) | (Constant) | 233.04 | 56.97 | 4.09 | <0.0001 |
| Age | 2.210 | 5.15 | 0.43 | 0.67 | |
| BMI Category (1 = normal, 2 = overweight, 3 = obese) | −34.886 | 9.86 | 3.54 | 0.0006 * | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starzak, D.E.; Konkol, K.F.; McKune, A.J. Effects of Cardiorespiratory Fitness and Obesity on Salivary Secretory IgA and Alpha-Amylase in South African Children. Children 2016, 3, 12. https://doi.org/10.3390/children3030012
Starzak DE, Konkol KF, McKune AJ. Effects of Cardiorespiratory Fitness and Obesity on Salivary Secretory IgA and Alpha-Amylase in South African Children. Children. 2016; 3(3):12. https://doi.org/10.3390/children3030012
Chicago/Turabian StyleStarzak, Dorota E., Kristen F. Konkol, and Andrew J. McKune. 2016. "Effects of Cardiorespiratory Fitness and Obesity on Salivary Secretory IgA and Alpha-Amylase in South African Children" Children 3, no. 3: 12. https://doi.org/10.3390/children3030012







