Identifying Preoperative Clinical Characteristics of Unexpected Gastrointestinal Perforation in Infants—A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Extraction
2.2. Outcome Parameters
2.3. Statistical Analysis
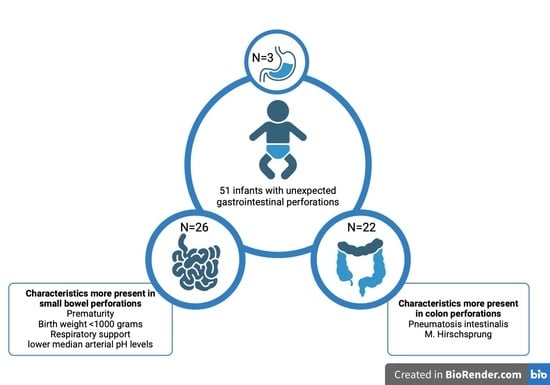
3. Results
3.1. Radiological Findings
3.2. Intraoperative Findings and Postoperative Diagnosis
3.3. Small Bowel versus Colon Perforations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.; Wu, Y.; Jin, D.; Tang, Q.; Yuan, P.; Lu, Q. Development and Validation of a Nomogram for Preoperative Prediction of Localization of Neonatal Gastrointestinal Perforation. Front. Pediatr. 2021, 9, 754623. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.L.; Payne, N.R.; Roback, S.A. Spontaneous, isolated intestinal perforations in neonates with birth weight less than 1000 g not associated with necrotizing enterocolitis. J. Pediatr. Surg. 1991, 26, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Singhal, N.; da Silva, O.; Rouvinez-Bouali, N.; Seshia, M.; Lee, S.K.; Shah, P.S.; Canadian Neonatal Network. Intestinal perforation in very preterm neonates: Risk factors and outcomes. J. Perinatol. 2015, 35, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-M.; Lee, H.-C.; Kao, H.-A.; Hung, H.-Y.; Hsu, C.-H.; Yeung, C.-Y.; Sheu, J.-C.; Wang, N.-L. Neonatal Gastric Perforation: Report of 15 Cases and Review of the Literature. Pediatr. Neonatol. 2008, 49, 65–70. [Google Scholar] [CrossRef] [PubMed]
- St-Vil, D.; LeBouthillier, G.; Luks, F.I.; Bensoussan, A.L.; Blanchard, H.; Youssef, S. Neonatal gastrointestinal perforations. J. Pediatr. Surg. 1992, 27, 1340–1342. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Kiely, E.M.; Agrawal, M.; Brereton, R.J.; Spitz, L. Neonatal gastrointestinal perforation. J. Pediatr. Surg. 1989, 24, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Lagman, E.D.; Alsina-Casanova, M.; Jorge, I.d.H.; Carrasco, C.C.; Teresa-Palacio, M. Neonatal gastric perforation and non invasive mechanical ventilation. J. Pediatr. Surg. 2022, 57, 483–484. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, C.; Sandlas, G.; Jayaswal, S.; Shah, H. Spontaneous Intestinal Perforation in Neonates. J. Neonatal Surg. 2015, 4, 14. [Google Scholar] [CrossRef]
- Saraç, M.; Bakal, Ü.; Aydın, M.; Tartar, T.; Orman, A.; Taşkın, E.; Canpolat, Ş.; Kazez, A. Neonatal Gastrointestinal Perforations: The 10-Year Experience of a Reference Hospital. Indian. J. Surg. 2016, 79, 431–436. [Google Scholar] [CrossRef]
- Beltman, L.; Labib, H.; Oosterlaan, J.; van Heurn, E.; Derikx, J. Risk factors for complications in patients with Hirschsprung disease while awaiting surgery: Beware of bowel perforation. J. Pediatr. Surg. 2022, 57, 561–568. [Google Scholar] [CrossRef]
- Sureka, B.; Bansal, K.; Arora, A. Pneumoperitoneum: What to look for in a radiograph? J. Fam. Med. Prim. Care 2015, 4, 477–478. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Muir, M.T.; Cohn, S.M.; Salhanick, M.A.; Lankford, D.B.; Katabathina, V.S. The etiology of pneumoperitoneum in the 21st century. J. Trauma Inj. Infect. Crit. Care 2012, 73, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.; Giroir, B.; Randolph, A. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med. 2005, 6, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Swanson, J.R.; Hair, A.; Clark, R.H.; Gordon, P.V. Spontaneous intestinal perforation (SIP) will soon become the most common form of surgical bowel disease in the extremely low birth weight (ELBW) infant. J. Perinatol. 2022, 42, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, M.M.; Othman, H.F.; Heis, F.; Qattea, I.; Aly, H. Spontaneous intestinal perforation in premature infants: A national study. J. Perinatol. 2021, 41, 1122–1128. [Google Scholar] [CrossRef]
- Vongbhavit, K.; Underwood, M. Intestinal perforation in the premature infant. J. Neonatal-Perinat. Med. 2017, 10, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Iacusso, C.; Boscarelli, A.; Fusaro, F.; Bagolan, P.; Morini, F. Pathogenetic and Prognostic Factors for Neonatal Gastric Perforation: Personal Experience and Systematic Review of the Literature. Front. Pediatr. 2018, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Prgomet, S.; Lukšić, B.; Pogorelić, Z.; Jurić, I.; Čapkun, V.; Arapović, A.; Boban, N. Perinatal risk factors in newborns with gastrointestinal perforation. World J. Gastrointest. Surg. 2017, 9, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.C.; Carlisle, E.M.; Paxton, J.B.; Gordon, P.V. Insulin-like Growth Factor-I Governs Submucosal Growth and Thickness in the Newborn Mouse Ileum. Pediatr. Res. 2004, 55, 507–513. [Google Scholar] [CrossRef]
- Gordon, P.V.; Marshall, D.D.; Stiles, A.D.; Price, W.A. The clinical, morphologic, and molecular changes in the ileum associated with early postnatal dexamethasone administration: From the baby’s bowel to the researcher’s bench. Mol. Genet. Metab. 2001, 72, 91–103. [Google Scholar] [CrossRef]
- Shah, T.A.; Meinzen-Derr, J.; Gratton, T.; Steichen, J.; Donovan, E.F.; Yolton, K.; Alexander, B.; Narendran, V.; Schibler, K.R. Hospital and neurodevelopmental outcomes of extremely low-birth-weight infants with necrotizing enterocolitis and spontaneous intestinal perforation. J. Perinatol. 2012, 32, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Gordon, P.V.; Attridge, J.T. Understanding Clinical Literature Relevant to Spontaneous Intestinal Perforations. Am. J. Perinatol. 2009, 26, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Eaton, S.; Rees, C.M.; Hall, N.J. Current Research on the Epidemiology, Pathogenesis, and Management of Necrotizing Enterocolitis. Neonatology 2017, 111, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.S.; Gee, A.C.; Cho, S.D.; Limbaugh, K.; Underwood, S.; Ham, B.; Schreiber, M.A. Management and outcome of pneumatosis intestinalis. Am. J. Surg. 2008, 195, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Ambartsumyan, L.; Smith, C.; Kapur, R.P. Diagnosis of Hirschsprung Disease. Pediatr. Dev. Pathol. 2020, 23, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Gözaydin, N.; Kaya, M.; Boleken, M.; Kanmaz, T.; Yücesan, S. Intestinal perforation in Hirschsprung’s disease: A report of three cases. Gazi Med. J. 2005, 16, 136–138. [Google Scholar]
- Newman, B.; Nussbaum, A.; Kirkpatrick, J.A., Jr. Bowel perforation in Hirschsprung’s disease. AJR Am. J. Roentgenol. 1987, 148, 1195–1197. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, K.; Vermeulen, F.; Dupont, L. The diagnosis of cystic fibrosis. Presse Med. 2017, 46 Pt 2, e97–e108. [Google Scholar] [CrossRef] [PubMed]
- Gorter, R.R.; Karimi, A.; Sleeboom, C.; Kneepkens, C.M.; Heij, H.A. Clinical and Genetic Characteristics of Meconium Ileus in Newborns with and without Cystic Fibrosis. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 569–572. [Google Scholar] [CrossRef]
- Siddiqui, M.M.F.; Burge, D.M. Neonatal spontaneous colonic perforation due to cystic fibrosis. Pediatr. Surg. Int. 2008, 24, 863–864. [Google Scholar] [CrossRef]

| Variable | Total N = 51 N (%) |
|---|---|
| Presentation | |
| During hospital admittance | 26 (51.0) |
| Referred to our center | 23 (45.1) |
| Emergency department | 2 (3.9) |
| Male | 36 (70.6) |
| Premature | 40 (78.4) |
| Median gestational age in weeks | 28 (26.0–36.0) |
| Twins | 15 (29.5) |
| Method of delivery | |
| Vaginal | 33 (64.7) |
| C-section | 18 (35.3) |
| Median age at surgery in days (IQR) | 5 (3–10) |
| Birthweight groups | |
| <1000 g | 20 (39.2) |
| 1001–1500 g | 10 (19.6) |
| >1500 g | 21 (41.2) |
| Sepsis | 8 (15.7) |
| Missing | 11 (21.6) |
| Exteral feeding before perforation | 48 (94.1) |
| Meconium > 48 h after birth | 20 (39.2) |
| Abdominal distention | 46 (90.2) |
| Bloody stool | 4 (7.8) |
| Respiratory support | 40 (78.4) |
| Mechanical ventilation | 21 (52.5) |
| Non-invasive ventilation | 17 (42.5) |
| Unclear | 2 (5.0) |
| Respiratory rate | |
| Normal | 17 (33.3) |
| Tachypnea | 6 (11.7) |
| Increased incidences | 19 (37.3) |
| Missing | 9 (17.6) |
| Blood results, median | |
| Arterial pH (IQR) | 7.30 (7.19–7.36) |
| Lactate, in mmol/L (IQR) | 2.6 (1.7–3.2) |
| CRP, in mg/L (IQR) | 20.0 (5.9–33.0) |
| Leucocytes, in 10E9/L (IQR) | 9.5 (5.9–25.1) |
| Platelets, in 10E9/L (IQR) | 169 (87–235) |
| Hb, in mmol/L (IQR) | 8.4 (7.3–10.0) |
| Variable | Total N = 51 N (%) |
|---|---|
| Type of radiology | |
| X-ray | 45 (88.2) |
| Ultrasound | 5 (9.8) |
| Fistulogram | 1 (2.0) |
| Median age at radiological imaging in days (IQR) | 5 (3–9) |
| Amount of abdominal air | |
| Massive | 35 (74.5) |
| Locally | 9 (19.1) |
| Peel of air | 3 (6.4) |
| Dilated bowel | |
| None | 1 (2.1) |
| Some | 15 (31.9) |
| Multiple | 31 (66.0) |
| Pneumatosis intestinalis | 10 (21.3) |
| Ascites | 16 (34.0) |
| Variable | Total N = 51 N (%) |
|---|---|
| Type of perforation | |
| Gastric | 3 (6.0) |
| Small bowel | 26 (50.9) |
| Colon | 22 (43.1) |
| Multiple perforations | 9 (17.3) |
| Resection of bowel | 36 (70.6) |
| Intraoperative diagnostic | |
| Mapping | 5 (9.8) |
| Trans-anal biopsy taken | 25 (49.0) |
| Additional diagnostics | |
| Pathology of resected bowel | 24 (47.0) |
| CF diagnostics 1 | 5 (9.8) |
| Rectal suction biopsy | 1 (2.0) |
| Clinical genetics consultation | 3 (5.9) |
| Mode of treatment | |
| Stoma creation 2 | 16 (31.4) |
| Primary anastomosis | 13 (25.5) |
| Primary closure of bowel | 8 (15.7) |
| Combination | 14 (27.5) |
| Final diagnosis | |
| Focal intestinal perforation | 27 (52.9) |
| Necrotizing enterocolitis | 9 (17.6) |
| Meconium ileus | 5 (9.8) |
| Iatrogenic injury | 4 (7.8) |
| Hirschsprung disease | 4 (7.8) |
| Milk-curd syndrome | 1 (2.0) |
| Appendicitis | 1 (2.0) |
| Associated anomaly | 7 (13.7) |
| Cardiac | 4 (7.8) |
| Cystic fibrosis | 1 (2.0) |
| Trisomy 13 | 1 (2.0) |
| Congenital Disorders of Glycosylation | 1 (2.0) |
| Variable | N = 26 Small Bowel Perforations | N = 22 Colon Perforations | p-Value |
|---|---|---|---|
| Male gender | 17 (65.4) | 18 (81.8) | 0.202 1 |
| Prematurity | 25 (96.2) | 12 (54.5) | 0.001 1 |
| Median gestational age | 26.0 (25.0–27.3) | 36.5 (31.8–39.0) | 0.001 |
| Median age at surgery | 4.5 (3.0–7.0) | 7.0 (2.8–25.3) | 0.164 |
| Birthweight groups | 0.001 1 | ||
| <1000 | 15 (57.7) | 4 (18.1) | |
| 1000–1500 | 8 (30.8) | 1 (4.5) | |
| >1500 | 3 (11.5) | 17 | |
| Mode of delivery | 0.041 1 | ||
| Vaginal | 14 (53.8) | 18 (81.8) | |
| C-section | 12 (46.2) | 4 (18.1) | |
| Associated anomaly | 1 (3.8) | 6 (27.2) | 0.022 1 |
| Respiratory support | 0.001 1 | ||
| Mechanical ventilation | 3 (11.5) | 4 (18.1) | |
| Non-invasive ventilation | 12 (46.2) | 5 (22.7) | |
| Both | 9 (34.6) | 3 (13.6) | |
| Sepsis | 3 (11.5) | 5 (22.7) | 0.255 1 |
| Major cardiac anomaly | 1 (3.8) | 3 (13.6) | 0.221 1 |
| Mortality within 30 days | 5 (19.2) | 6 (27.2) | 0.509 1 |
| Blood results, median | |||
| Arterial pH (IQR) | 7.20 (7.17–7.30) | 7.36 (7.31–7.41) | 0.001 2 |
| Lactate, in mmol/L (IQR) | 2.2 (1.2–2.9) | 2.8 (2.0–3.5) | 0.109 2 |
| CRP, in mg/L (IQR) | 8.0 (1.5–22.9) | 30.5 (17.2–60.2) | 0.012 2 |
| Leucocytes, in 10E9/L (IQR) | 9.5 (7.3–37.0) | 8.8 (5.0–15.5) | 0.234 2 |
| Platelets, in 10E9/L (IQR) | 155 (118–197) | 137 (78–251) | 0.863 2 |
| Hb, in mmol/L (IQR) | 8.5 (7.3–9.3) | 8.3 (7.2–10.5) | 0.678 2 |
| Radiological characteristics | |||
| Abdominal free air | 0.009 1 | ||
| Massive | 23 (88.5) | 10 (45.5) | |
| Locally | 2 (7.7) | 6 (27.2) | |
| Minimally | 0 (0.0) | 3 (13.6) | |
| Dilated bowel | 0.355 1 | ||
| Multiple | 17 (65.4) | 13 (59.1) | |
| Some | 9 (34.6) | 4 (18.1) | |
| None | 0 (0.0) | 1 (4.5) | |
| Pneumatosis intestinalis | 2 (7.7) | 8 (36.4) | 0.004 1 |
| Ascites | 7 (26.9) | 7 (31.8) | 0.402 1 |
| Final diagnosis | |||
| Focal intestinal perforation | 17 (65.4) | 9 (40.9) | |
| Necrotizing enterocolitis | 5 (19.2) | 4 (18.1) | |
| Meconium ileus | 3 (11.5) | 2 (9.1) | |
| Iatrogenic injury | 1 (3.8) | 1(4.5) | |
| Hirschsprung disease | 0 (0.0) | 4 (18.1) | |
| Milk-curd syndrome | 0 (0.0) | 1 (4.5) | |
| Appendicitis | 0 (0.0) | 1 (4.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pijpers, A.G.H.; Gorter, R.R.; Eeftinck Schattenkerk, L.D.; van Schuppen, J.; van den Akker, C.H.P.; Vanhamel, S.; van Heurn, E.L.W.; Musters, G.D.; Derikx, J.P.M. Identifying Preoperative Clinical Characteristics of Unexpected Gastrointestinal Perforation in Infants—A Retrospective Cohort Study. Children 2024, 11, 505. https://doi.org/10.3390/children11050505
Pijpers AGH, Gorter RR, Eeftinck Schattenkerk LD, van Schuppen J, van den Akker CHP, Vanhamel S, van Heurn ELW, Musters GD, Derikx JPM. Identifying Preoperative Clinical Characteristics of Unexpected Gastrointestinal Perforation in Infants—A Retrospective Cohort Study. Children. 2024; 11(5):505. https://doi.org/10.3390/children11050505
Chicago/Turabian StylePijpers, Adinda G. H., Ramon R. Gorter, Laurens D. Eeftinck Schattenkerk, Joost van Schuppen, Chris H. P. van den Akker, Sylvie Vanhamel, Ernest L. W. van Heurn, Gijsbert D. Musters, and Joep P. M. Derikx. 2024. "Identifying Preoperative Clinical Characteristics of Unexpected Gastrointestinal Perforation in Infants—A Retrospective Cohort Study" Children 11, no. 5: 505. https://doi.org/10.3390/children11050505