Pediatric Migraine and Visual Cortical Excitability: A Prospective Observational Study with Sound-Induced Flash Illusions
Abstract
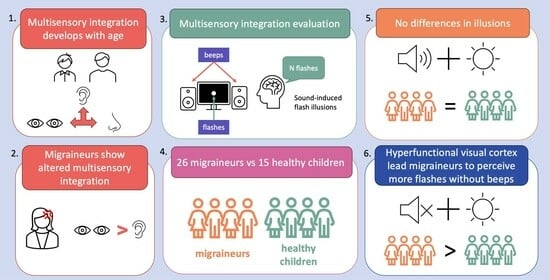
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Stimulation and Task
2.3. Statistical Analysis
3. Results
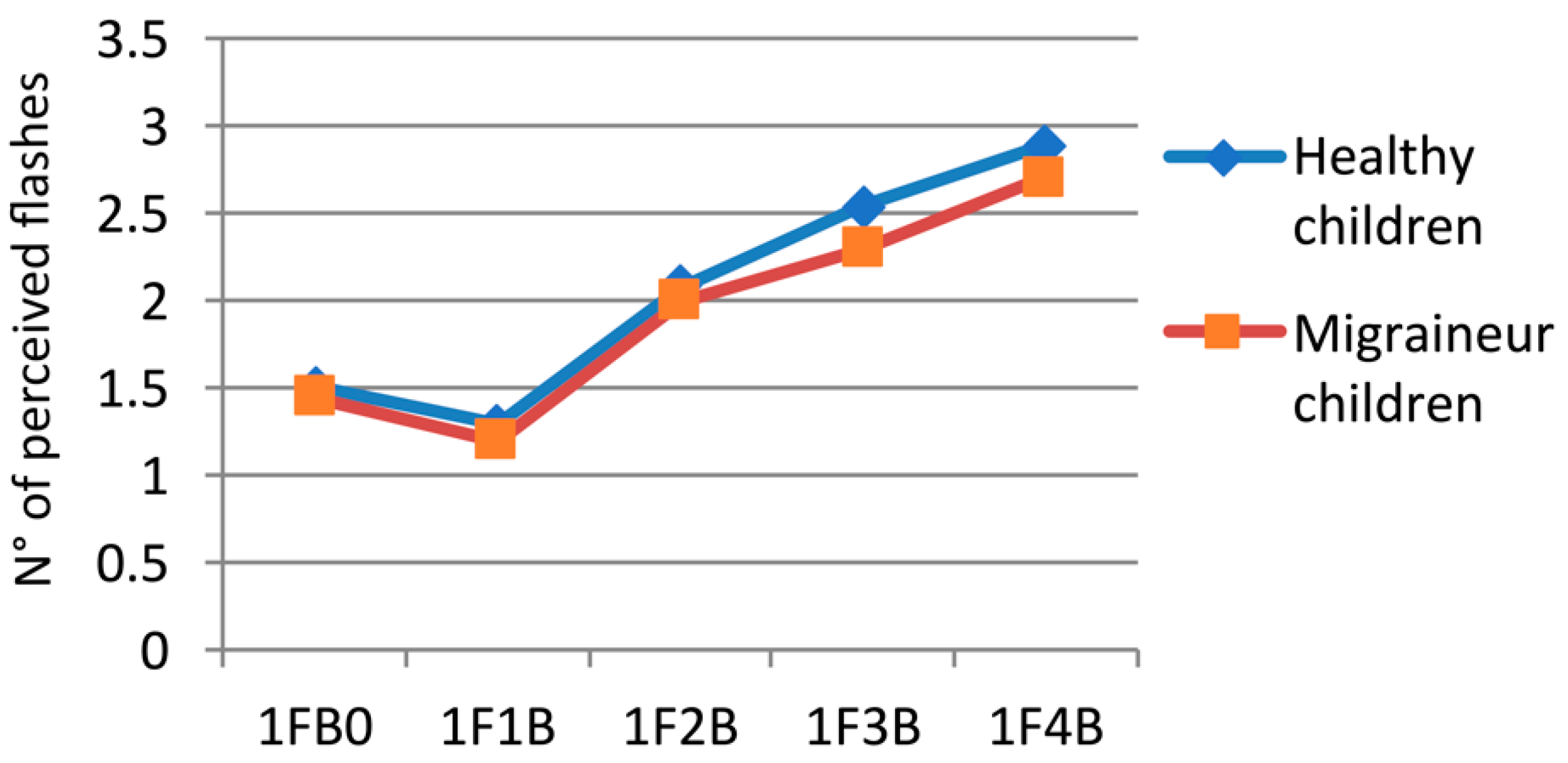
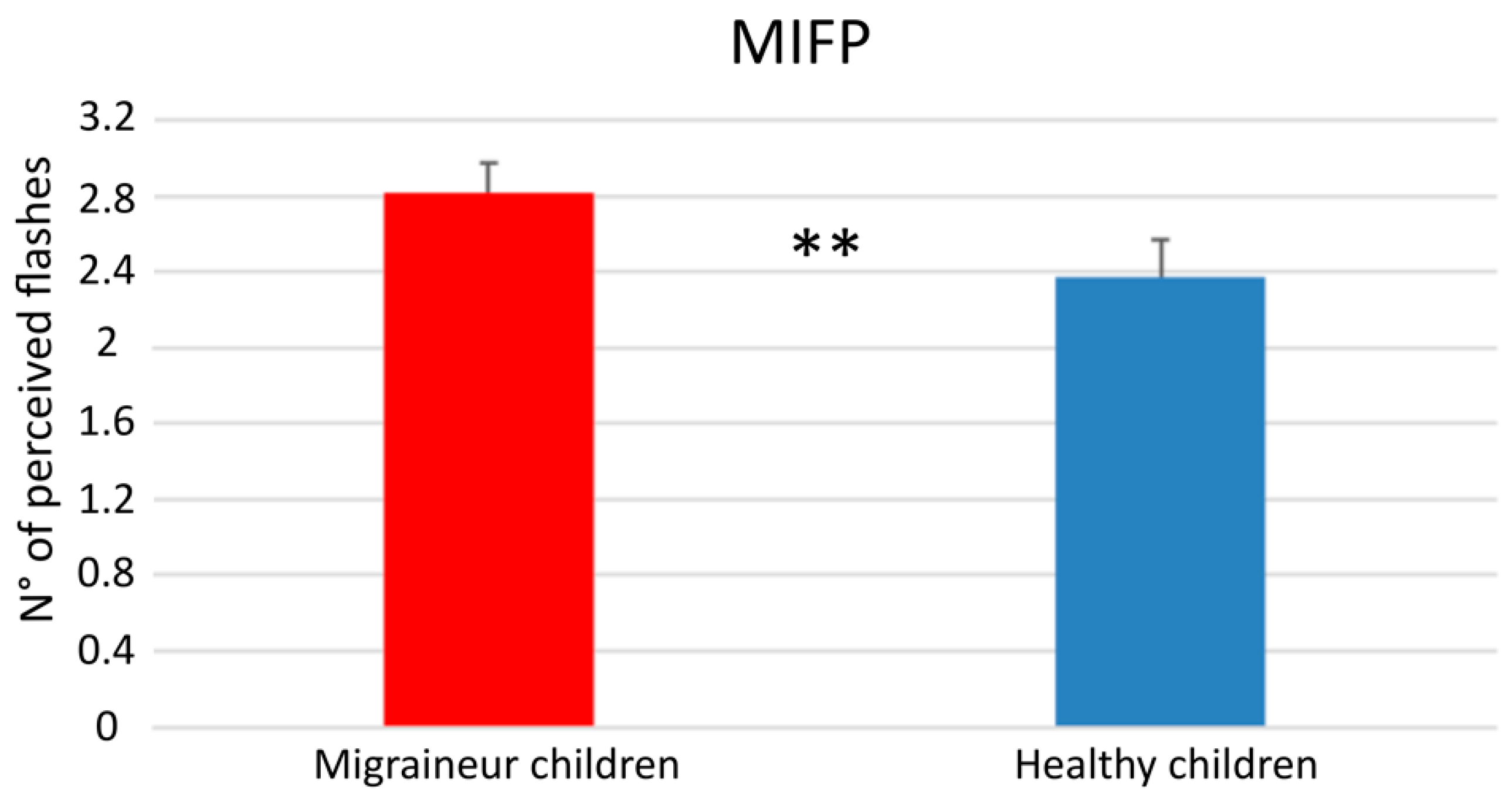
3.1. Singe-Flash Trials
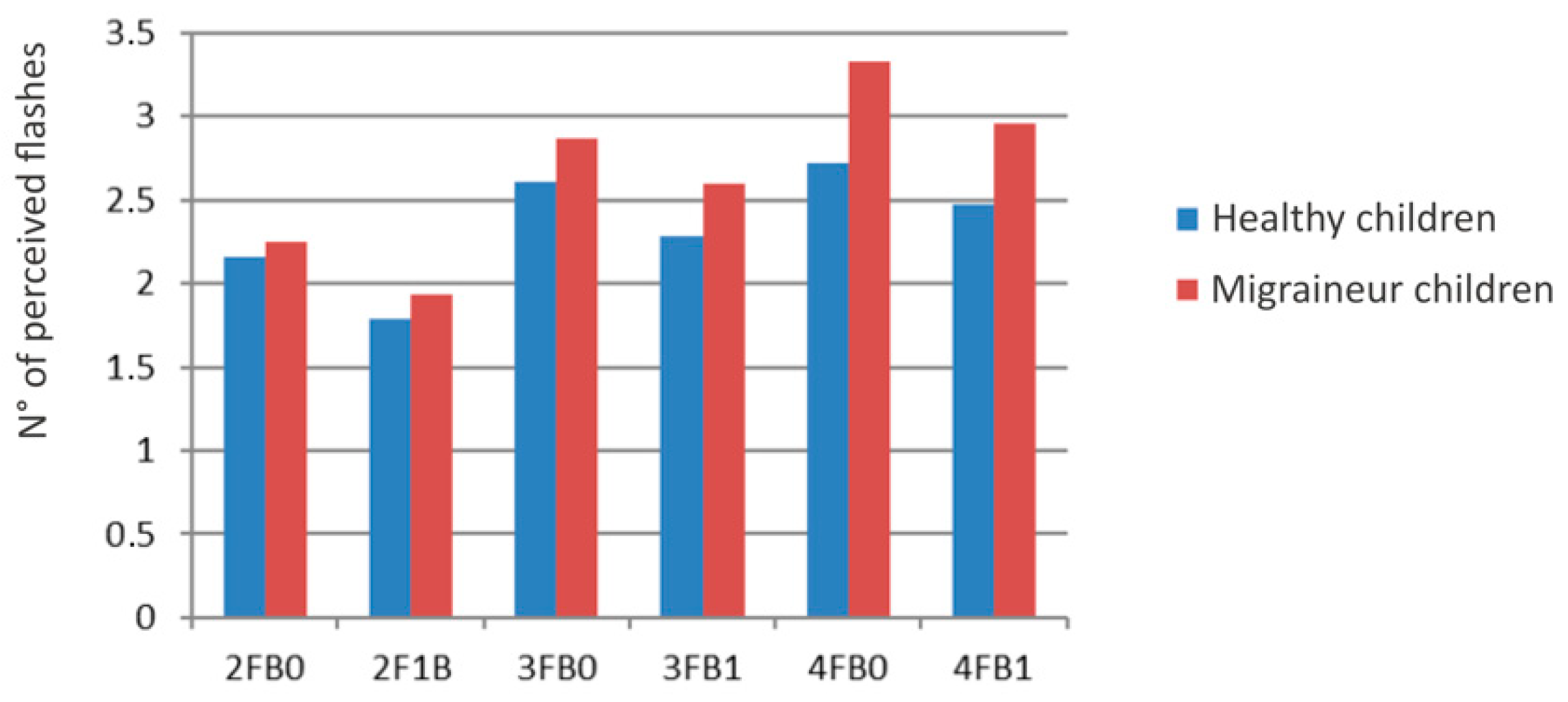
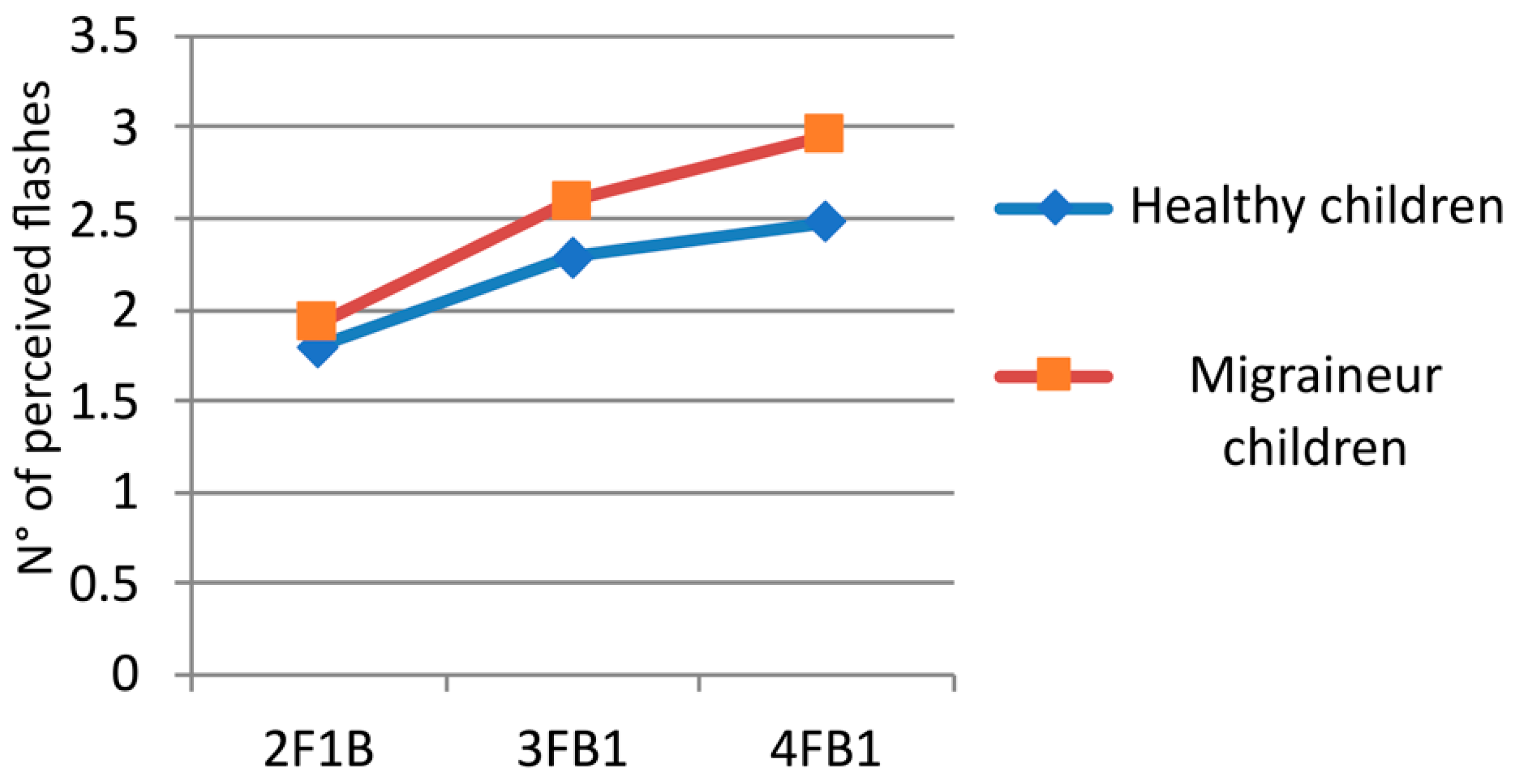
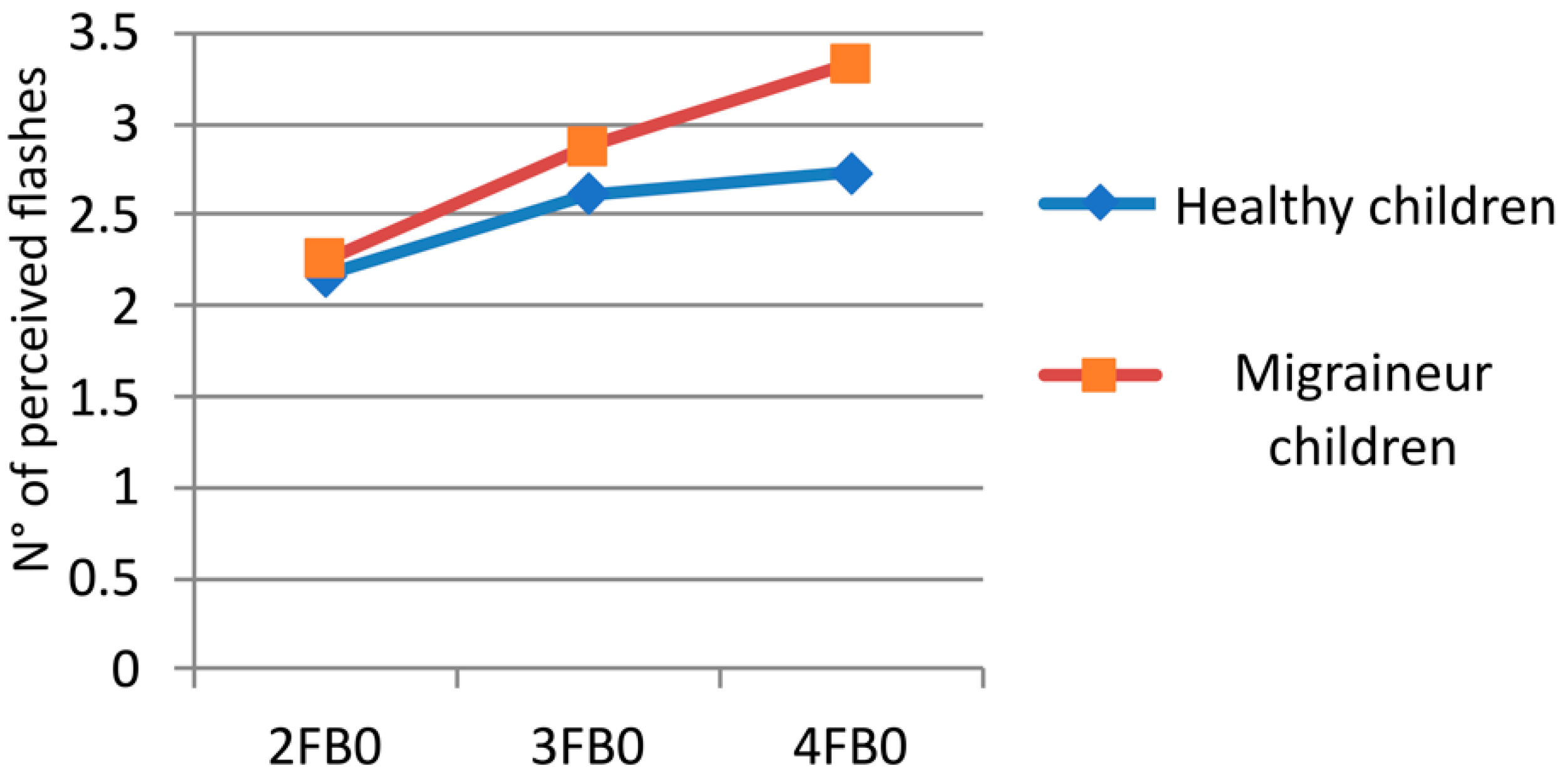
3.2. Multiple Flash Trials
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Onofri, A.; Pensato, U.; Rosignoli, C.; Wells-Gatnik, W.; Stanyer, E.; Ornello, R.; Chen, H.Z.; De Santis, F.; Torrente, A.; Mikulenka, P.; et al. Primary headache epidemiology in children and adolescents: A systematic review and meta-analysis. J. Headache Pain 2023, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.W. Pediatric migraine. Pediatr. Rev. 2007, 28, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Raieli, V.; D’Amico, A.; Piro, E. Migraine in Children Under 7 Years of Age: A Review. Curr. Pain Headache Rep. 2020, 24, 79. [Google Scholar] [CrossRef] [PubMed]
- García-Azorín, D.; Moya-Alarcón, C.; Armada, B.; Sánchez del Río, M. Societal and economic burden of migraine in Spain: Results from the 2020 National Health and Wellness Survey. J. Headache Pain 2024, 25, 38. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, G.; Gallai, B.; Mattioni, A.; Floridi, F.; Foti, F.; Allegretti, M.; D’angelo, R. Cost assessment of headache in childhood and adolescence: Preliminary data. J. Headache Pain 2005, 6, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Finning, K.; Neochoriti Varvarrigou, I.; Ford, T.; Panagi, L.; Ukoumunne, O.C. Mental health and school absenteeism in children with long-term physical conditions: A secondary analysis of the British Child and Adolescent Mental Health Surveys 2004 and 2007. Child Care Health Dev. 2022, 48, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W. A Phase-by-Phase Review of Migraine Pathophysiology. Headache 2018, 58, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar]
- Bose, P.; Karsan, N.; Goadsby, P.J. The Migraine Postdrome. Contin. Lifelong Learn. Neurol. 2018, 24, 1023–1031. [Google Scholar] [CrossRef]
- Gelfand, A.A.; Goadsby, P.J.; Allen, I.E. The relationship between migraine and infant colic: A systematic review and meta-analysis. Cephalalgia 2015, 35, 63–72. [Google Scholar] [CrossRef]
- Özge, A.; Abu-Arafeh, I.; Gelfand, A.A.; Goadsby, P.J.; Cuvellier, J.C.; Valeriani, M.; Sergeev, A.; Barlow, K.; Uludüz, D.; Yalın, O.; et al. Experts’ opinion about the pediatric secondary headaches diagnostic criteria of the ICHD-3 beta. J. Headache Pain 2017, 18, 113. [Google Scholar] [CrossRef] [PubMed]
- Raieli, V.; Capizzi, M.; Marino, A.; Di Nardo, G.; Raucci, U.; Parisi, P. Study on “Atypical” Migraine Auras in the Pediatric Age: The Role of Cortical Spreading Depression and the Physiopathogenetic Hypothesis Arising from Our Clinical Cases. Life 2022, 12, 450. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, A.A.; Reider, A.C.; Goadsby, P.J. Cranial autonomic symptoms in pediatric migraine are the rule, not the exception. Neurology 2013, 81, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Jernigan, T.L.; Baaré, W.F.C.; Stiles, J.; Madsen, K.S. Postnatal brain development: Structural imaging of dynamic neurodevelopmental processes. Prog. Brain Res. 2011, 189, 77–92. [Google Scholar] [PubMed]
- Stiles, J.; Jernigan, T.L. The basics of brain development. Neuropsychol. Rev. 2010, 20, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, V.; Galli, F.; Termine, C. Headache in children. Handb. Clin. Neurol. 2010, 97, 739–754. [Google Scholar] [PubMed]
- Deodato, M.; Granato, A.; Martini, M.; Stella, A.B.; Galmonte, A.; Murena, L.; Manganotti, P. Neurophysiological and Clinical Outcomes in Episodic Migraine without Aura: A Cross-Sectional Study. J. Clin. Neurophysiol. 2024. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef] [PubMed]
- Dichgans, M.; Freilinger, T.; Eckstein, G.; Babini, E.; Lorenz-Depiereux, B.; Biskup, S.; Ferrari, M.D.; Herzog, J.; van den Maagdenberg, A.M.J.M.; Pusch, M.; et al. Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet 2005, 366, 371–377. [Google Scholar] [CrossRef]
- Van den Maagdenberg, A.M.; Pietrobon, D.; Pizzorusso, T.; Kaja, S.; Broos, L.A.; Cesetti, T.; van de Ven, R.C.; Tottene, A.; van der Kaa, J.; Plomp, J.J.; et al. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron 2004, 41, 701–710. [Google Scholar] [CrossRef]
- Ophoff, R.A.; Terwindt, G.M.; Vergouwe, M.N.; van Eijk, R.; Oefner, P.J.; Hoffman, S.M.; Lamerdin, J.E.; Mohrenweiser, H.W.; Bulman, D.E.; Ferrari, M.; et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell 1996, 87, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Creutzfeldt, O.D.; Fromm, G.H.; Kapp, H. Influence of transcortical d-c currents on cortical neuronal activity. Exp. Neurol. 1962, 5, 436–452. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, G.; Di Marco, S.; Ferlisi, S.; Valentino, F.; Capitano, W.M.; Fierro, B.; Brighina, F. Intracortical facilitation within the migraine motor cortex depends on the stimulation intensity. A paired-pulse TMS study. J. Headache Pain 2018, 19, 65. [Google Scholar] [CrossRef] [PubMed]
- Brighina, F.; Piazza, A.; Daniele, O.; Fierro, B. Modulation of visual cortical excitability in migraine with aura: Effects of 1 Hz repetitive transcranial magnetic stimulation. Exp. Brain Res. 2002, 145, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Shams, L.; Kamitani, Y.; Shimojo, S. What you see is what you hear. Nature 2000, 408, 788. [Google Scholar] [CrossRef] [PubMed]
- Bolognini, N.; Rossetti, A.; Casati, C.; Mancini, F.; Vallar, G. Neuromodulation of multisensory perception: A tDCS study of the sound-induced flash illusion. Neuropsychologia 2011, 49, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Brighina, F.; Bolognini, N.; Cosentino, G.; Maccora, S.; Paladino, P.; Baschi, R.; Vallar, G.; Fierro, B. Visual cortex hyperexcitability in migraine in response to sound-induced flash illusions. Neurology 2015, 84, 2057–2061. [Google Scholar] [CrossRef] [PubMed]
- Innes-Brown, H.; Barutchu, A.; Shivdasani, M.N.; Crewther, D.P.; Grayden, D.B.; Paolini, A.G. Susceptibility to the flash-beep illusion is increased in children compared to adults. Dev. Sci. 2011, 14, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Nava, E.; Pavani, F. Changes in sensory dominance during childhood: Converging evidence from the colavita effect and the sound-induced flash illusion. Child. Dev. 2013, 84, 604–616. [Google Scholar] [CrossRef]
- Afra, J.; Mascia, A.; Gérard, P.; Maertens de Noordhout, A.; Schoenen, J. Interictal cortical excitability in migraine: A study using transcranial magnetic stimulation of motor and visual cortices. Ann. Neurol. 1998, 44, 209–215. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013, 33, 629–808. [Google Scholar] [CrossRef]
- Zhang, X.; Levy, D.; Kainz, V.; Noseda, R.; Jakubowski, M.; Burstein, R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann. Neurol. 2011, 69, 855–865. [Google Scholar] [CrossRef]
- Mulleners, W.M.; Chronicle, E.P.; Palmer, J.E.; Koehler, P.J.; Vredeveld, J.W. Visual cortex excitability in migraine with and without aura. Headache 2001, 41, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Battelli, L.; Black, K.R.; Wray, S.H. Transcranial magnetic stimulation of visual area V5 in migraine. Neurology 2002, 58, 1066–1069. [Google Scholar] [CrossRef] [PubMed]
- Custers, A.; Mulleners, W.M.; Chronicle, E.P. Assessing cortical excitability in migraine: Reliability of magnetic suppression of perceptual accuracy technique over time. Headache 2005, 45, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Pro, S.; Tarantino, S.; Capuano, A.; Vigevano, F.; Valeriani, M. Primary headache pathophysiology in children: The contribution of clinical neurophysiology. Clin. Neurophysiol. 2014, 125, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Degrauw, X.; Korostenskaja, M.; Korman, A.M.; O’Brien, H.L.; Kabbouche, M.A.; Powers, S.W.; Hershey, A.D. Altered cortical activation in adolescents with acute migraine: A magnetoencephalography study. J. Pain 2013, 14, 1553–1563. [Google Scholar] [CrossRef]
- Leiken, K.A.; Xiang, J.; Curry, E.; Fujiwara, H.; Rose, D.F.; Allen, J.R.; Kacperski, J.E.; O’brien, H.L.; Kabbouche, M.A.; Powers, S.W.; et al. Quantitative neuromagnetic signatures of aberrant cortical excitability in pediatric chronic migraine. J. Headache Pain 2016, 17, 46. [Google Scholar] [CrossRef] [PubMed]
- Bowyer, S.M.; Aurora, K.S.; Moran, J.E.; Tepley, N.; Welch, K.M. Magnetoencephalographic fields from patients with spontaneous and induced migraine aura. Ann. Neurol. 2001, 50, 582–587. [Google Scholar] [CrossRef]
- Cao, Y.; Welch, K.M.; Aurora, S.; Vikingstad, E.M. Functional MRI-BOLD of visually triggered headache in patients with migraine. Arch. Neurol. 1999, 56, 548–554. [Google Scholar] [CrossRef]
- Akin, O.; Ünay, B.; Arslan, M.; Mazman, S.; Taşçilar, E.; Eker, I. Evaluation of somatosensory evoked potentials (SEPs) in obese children. Gulhane Med. J. 2018, 60, 5–8. [Google Scholar] [CrossRef]
- Ernst, M.O. Multisensory integration: A late bloomer. Curr. Biol. 2008, 18, R519–R521. [Google Scholar] [CrossRef] [PubMed]
- Siniatchkin, M.; Reich, A.L.; Shepherd, A.J.; van Baalen, A.; Siebner, H.R.; Stephani, U. Peri-ictal changes of cortical excitability in children suffering from migraine without aura. Pain 2009, 147, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Vollono, C.; Ferraro, D.; Miliucci, R.; Vigevano, F.; Valeriani, M. The abnormal recovery cycle of somatosensory evoked potential components in children with migraine can be reversed by topiramate. Cephalalgia 2010, 30, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Valeriani, M.; Galli, F.; Tarantino, S.; Graceffa, D.; Pignata, E.; Miliucci, R.; Biondi, G.; Tozzi, A.; Vigevano, F.; Guidetti, V. Correlation between abnormal brain excitability and emotional symptomatology in paediatric migraine. Cephalalgia 2009, 29, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, V.; Orecchio, S.; Esposito, D.; Faedda, N.; Natalucci, G.; Guidetti, V. Tension-Type Headache in Children and Adolescents. Life 2023, 13, 825. [Google Scholar] [CrossRef] [PubMed]
- Onan, D.; Younis, S.; Wellsgatnik, W.D.; Farham, F.; Andruškevičius, S.; Abashidze, A.; Jusupova, A.; Romanenko, Y.; Grosu, O.; Moldokulova, M.Z.; et al. Debate: Differences and similarities between tension-type headache and migraine. J. Headache Pain 2023, 24, 92. [Google Scholar] [CrossRef]
- Puca, F.; de Tommaso, M. Clinical neurophysiology in childhood headache. Cephalalgia 1999, 19, 137–146. [Google Scholar] [CrossRef]

| Variable | Mean | SD |
|---|---|---|
| Migraine frequency (days/month) | 4.31 | 2.33 |
| Months since diagnosis | 34.42 | 23.37 |
| Headache intensity (NRS) | 7.50 | 1.45 |
| Attack duration (hours) | 5.96 | 6.31 |
| Test | Group | Mean | SD |
|---|---|---|---|
| 1FB0 | HC | 1.51 | 0.396 |
| MC | 1.44 | 0.403 | |
| 1F1B | HC | 1.29 | 0.403 |
| MC | 1.20 | 0.251 | |
| 1F2B | HC | 2.08 | 0.650 |
| MC | 1.99 | 0.412 | |
| 1F3B | HC | 2.54 | 0.595 |
| MC | 2.30 | 0.603 | |
| 1F4B | HC | 2.89 | 0.673 |
| MC | 2.71 | 0.725 |
| Test | Group | Mean | SD |
|---|---|---|---|
| 2FB0 | HC | 2.06 | 0.447 |
| MC | 2.25 | 0.380 | |
| 2F1B | HC | 1.57 | 0.562 |
| MC | 1.93 | 0.403 | |
| 3FB0 | HC | 2.38 | 0.470 |
| MC | 2.87 | 0.407 | |
| 3FB1 | HC | 1.95 | 0.670 |
| MC | 2.60 | 0.465 | |
| 4FB0 | HC | 2.67 | 0.575 |
| MC | 3.33 | 0.429 | |
| 4FB1 | HC | 2.23 | 0.662 |
| MC | 2.96 | 0.522 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Marco, S.; Pilati, L.; Torrente, A.; Maccora, S.; Santangelo, A.; Cosentino, G.; Correnti, E.; Raieli, V.; Fierro, B.; Brighina, F. Pediatric Migraine and Visual Cortical Excitability: A Prospective Observational Study with Sound-Induced Flash Illusions. Children 2024, 11, 394. https://doi.org/10.3390/children11040394
Di Marco S, Pilati L, Torrente A, Maccora S, Santangelo A, Cosentino G, Correnti E, Raieli V, Fierro B, Brighina F. Pediatric Migraine and Visual Cortical Excitability: A Prospective Observational Study with Sound-Induced Flash Illusions. Children. 2024; 11(4):394. https://doi.org/10.3390/children11040394
Chicago/Turabian StyleDi Marco, Salvatore, Laura Pilati, Angelo Torrente, Simona Maccora, Andrea Santangelo, Giuseppe Cosentino, Edvige Correnti, Vincenzo Raieli, Brigida Fierro, and Filippo Brighina. 2024. "Pediatric Migraine and Visual Cortical Excitability: A Prospective Observational Study with Sound-Induced Flash Illusions" Children 11, no. 4: 394. https://doi.org/10.3390/children11040394