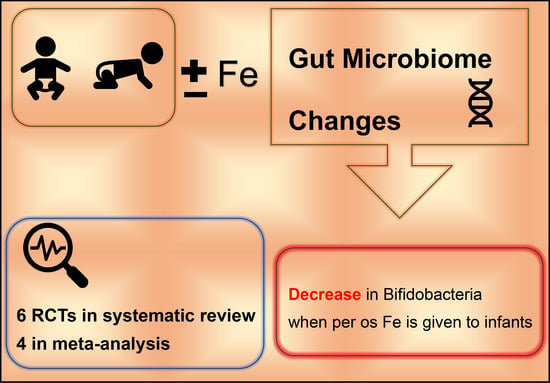
The Effect of Oral Iron Supplementation/Fortification on the Gut Microbiota in Infancy: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
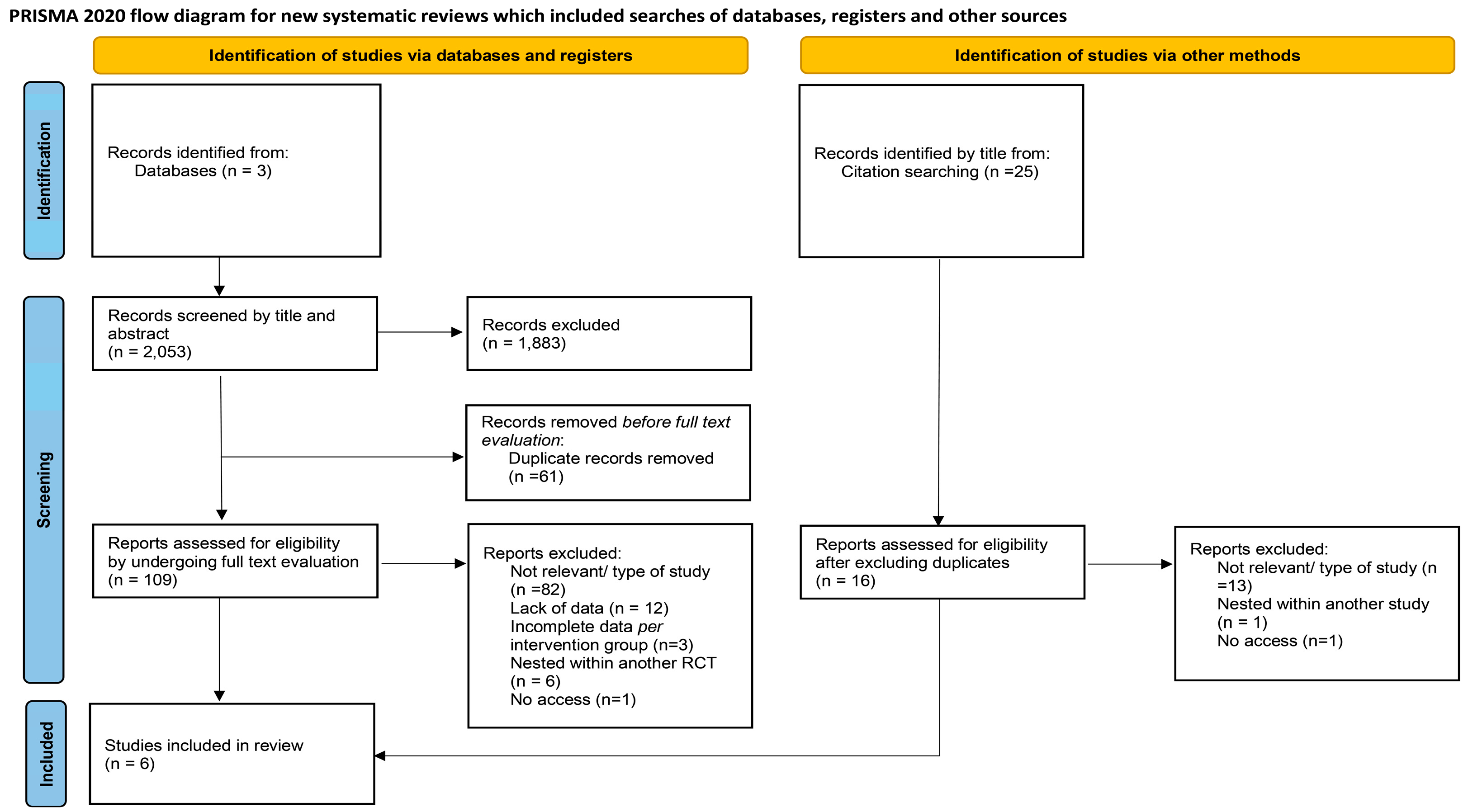
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
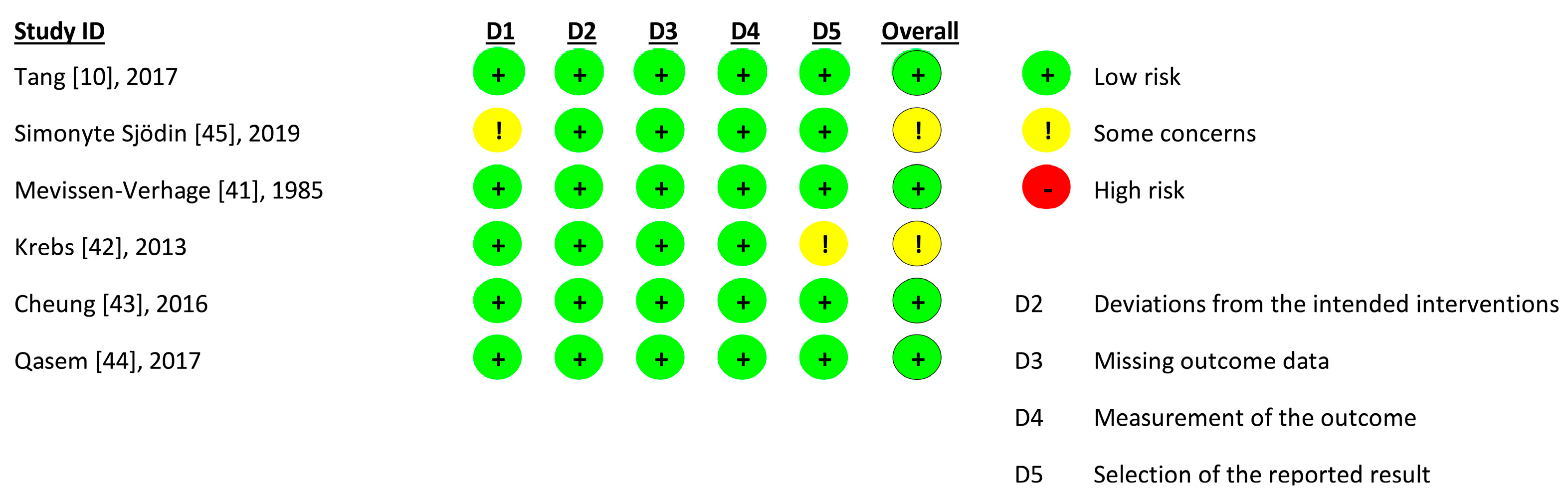
2.4. Risk of Bias and Study Quality Appraisal
2.5. Statistical Analysis
3. Results
3.1. Study Characteristics and Participants
3.2. Age
3.3. Groups
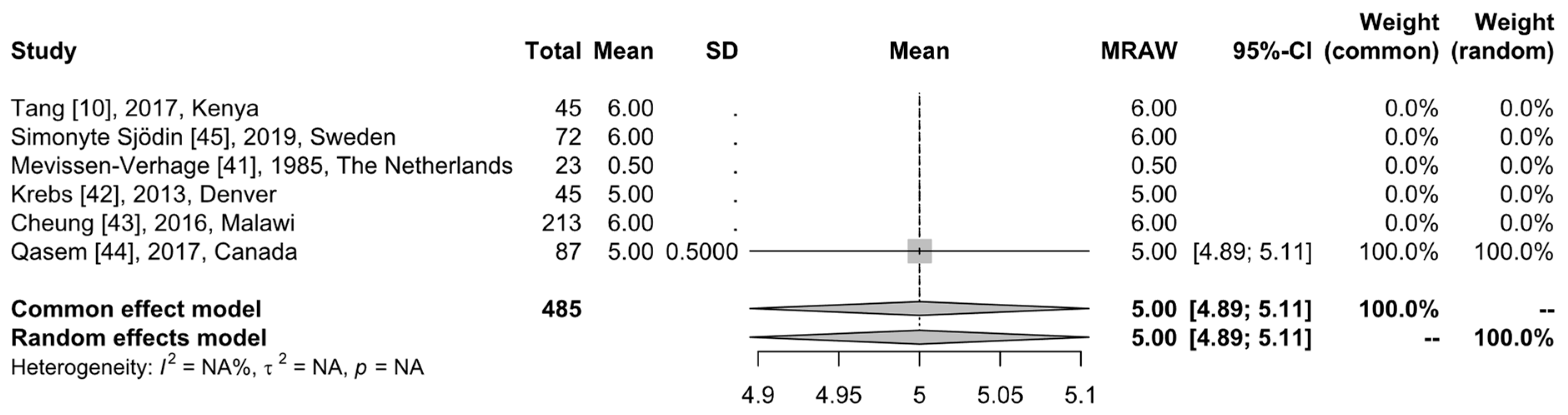
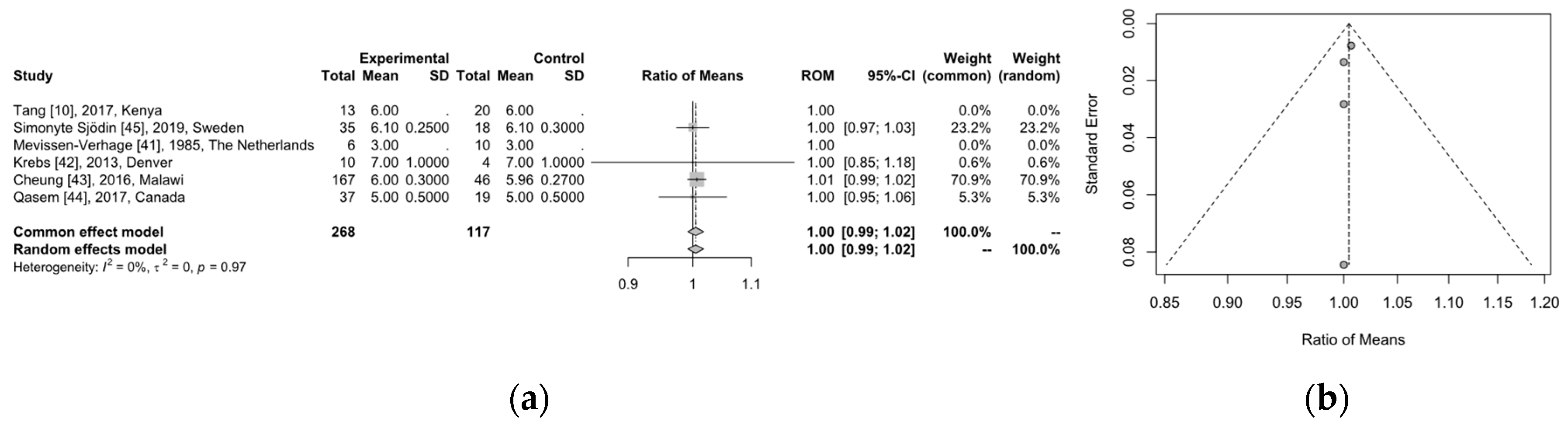
3.4. Microbiome Analysis
3.5. Quality of Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donker, A.E.; van der Staaij, H.; Swinkels, D.W. The critical roles of iron during the journey from fetus to adolescent: Developmental aspects of iron homeostasis. Blood Rev. 2021, 50, 100866. [Google Scholar] [CrossRef]
- Parmanand, B.A.; Kellingray, L.; Le Gall, G.; Basit, A.W.; Fairweather-Tait, S.; Narbad, A. A decrease in iron availability to human gut microbiome reduces the growth of potentially pathogenic gut bacteria; an in vitro colonic fermentation study. J. Nutr. Biochem. 2019, 67, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ghanchi, A.; James, P.T.; Cerami, C. Guts, germs, and iron: A systematic review on iron supplementation, iron fortification, and diarrhea in children aged 4–59 months. Curr. Dev. Nutr. 2019, 3, nzz005. [Google Scholar] [CrossRef] [PubMed]
- Fantozzi, P.; Del Grande, C.; Berloffa, S.; Tolomei, G.; Salluce, C.; Narzisi, A.; Salarpi, G.; Capovani, B.; Masi, G. Neurodevelopmental disorders, schizophrenia spectrum disorders and catatonia: The “iron triangle” rediscovered in a case report. Children 2022, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Finlayson-Trick, E.C.; Fischer, J.A.; Goldfarb, D.M.; Karakochuk, C.D. The effects of iron supplementation and fortification on the gut microbiota: A review. Gastrointest. Disord. 2020, 2, 327–340. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Hepcidin-ferroportin interaction controls systemic iron homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Hayirli, Z.; Drakesmith, H.; Andrews, S.C.; Lewis, M.C. Effects of iron deficiency and iron supplementation at the host-microbiota interface: Could a piglet model unravel complexities of the underlying mechanisms? Front. Nutr. 2022, 9, 927754. [Google Scholar] [CrossRef]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef]
- Puga, A.M.; Samaniego-Vaesken, M.L.; Montero-Bravo, A.; Ruperto, M.; Partearroyo, T.; Varela-Moreiras, G. Iron supplementation at the crossroads of nutrition and gut microbiota: The state of the art. Nutrients 2022, 14, 1926. [Google Scholar] [CrossRef]
- Tang, M.; Frank, D.N.; Hendricks, A.E.; Ir, D.; Esamai, F.; Liechty, E.; Hambidge, K.M.; Krebs, N.F. Iron in micronutrient powder promotes an unfavorable gut microbiota in kenyan infants. Nutrients 2017, 9, 776. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Kortman, G.A.M.; Boekhorst, J.; Lee, P.; Khan, M.R.; Ahmed, F. Effect of low-iron micronutrient powder (mnp) on the composition of gut microbiota of bangladeshi children in a high-iron groundwater setting: A randomized controlled trial. Eur. J. Nutr. 2021, 60, 3423–3436. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Chassard, C.; Rohner, F.; N’Goran, E.K.; Nindjin, C.; Dostal, A.; Utzinger, J.; Ghattas, H.; Lacroix, C.; Hurrell, R.F. The effects of iron fortification on the gut microbiota in african children: A randomized controlled trial in cote d’ivoire. Am. J. Clin. Nutr. 2010, 92, 1406–1415. [Google Scholar] [CrossRef]
- Tang, M.; Frank, D.N.; Sherlock, L.; Ir, D.; Robertson, C.E.; Krebs, N.F. Effect of vitamin e with therapeutic iron supplementation on iron repletion and gut microbiome in us iron deficient infants and toddlers. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 379–385. [Google Scholar] [CrossRef]
- Cerdo, T.; Dieguez, E.; Campoy, C. Infant growth, neurodevelopment and gut microbiota during infancy: Which nutrients are crucial? Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Beard, J. Iron deficiency alters brain development and functioning. J. Nutr. 2003, 133, 1468S–1472S. [Google Scholar] [CrossRef] [PubMed]
- Armitage, A.E.; Moretti, D. The importance of iron status for young children in low- and middle-income countries: A narrative review. Pharmaceuticals 2019, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B.; Beard, J.; Connor, J.; Barbara, F.; Georgieff, M.; Schallert, T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr. Rev. 2006, 64, S34–S43; discussion S72–S91. [Google Scholar] [CrossRef] [PubMed]
- Lonnerdal, B.; Georgieff, M.K.; Hernell, O. Developmental physiology of iron absorption, homeostasis, and metabolism in the healthy term infant. J. Pediatr. 2015, 167, S8–S14. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K. Iron assessment to protect the developing brain. Am. J. Clin. Nutr. 2017, 106, 1588S–1593S. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guideline: Daily Iron Supplementation in Infants and Children: 2016. Executive Summary. Available online: https://www.ncbi.nlm.nih.gov/books/NBK362027/ (accessed on 10 January 2024).
- Paganini, D.; Uyoga, M.A.; Zimmermann, M.B. Iron fortification of foods for infants and children in low-income countries: Effects on the gut microbiome, gut inflammation, and diarrhea. Nutrients 2016, 8, 494. [Google Scholar] [CrossRef] [PubMed]
- Dostal, A.; Baumgartner, J.; Riesen, N.; Chassard, C.; Smuts, C.M.; Zimmermann, M.B.; Lacroix, C. Effects of iron supplementation on dominant bacterial groups in the gut, faecal scfa and gut inflammation: A randomised, placebo-controlled intervention trial in south african children. Br. J. Nutr. 2014, 112, 547–556. [Google Scholar] [CrossRef]
- Khasheii, B.; Mahmoodi, P.; Mohammadzadeh, A. Siderophores: Importance in bacterial pathogenesis and applications in medicine and industry. Microbiol. Res. 2021, 250, 126790. [Google Scholar] [CrossRef]
- Vazquez-Gutierrez, P.; Lacroix, C.; Jaeggi, T.; Zeder, C.; Zimmerman, M.B.; Chassard, C. Bifidobacteria strains isolated from stools of iron deficient infants can efficiently sequester iron. BMC Microbiol. 2015, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhang, Y.; Guo, H.; Hai, Y.; Luo, Y.; Yue, T. Mechanism and intervention measures of iron side effects on the intestine. Crit. Rev. Food Sci. Nutr. 2020, 60, 2113–2125. [Google Scholar] [CrossRef] [PubMed]
- Miniello, V.L.; Verga, M.C.; Miniello, A.; Di Mauro, C.; Diaferio, L.; Francavilla, R. Complementary feeding and iron status: “The unbearable lightness of being” infants. Nutrients 2021, 13, 4201. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Hróbjartsson, A.; Boutron, I.; Reeves, B.; Eldridge, S. A revised tool for assessing risk of bias in randomized trials. In Cochrane Methods. Cochrane Database of Systematic Reviews 2016; Chandler, J., McKenzie, J., Boutron, I., Welch, V., Eds.; Cochrane: Hoboken, NJ, USAP, 2016. [Google Scholar]
- R Foundation. The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 12 September 2023).
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with r: A practical tutorial. Evid.-Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, G. Meta: An r package for meta-analysis. R News 2007, 7, 40–45. [Google Scholar]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Bland, M. Estimating mean and standard deviation from the sample size, three quartiles, minimum, and maximum. Int. J. Stat. Med. Res. 2015, 4, 57–64. [Google Scholar] [CrossRef]
- Deepanshu, S.; Surya, P.U.; Vinay, S.; Sakshi, P.; Ravi, R.K.N. Deep Meta Tool: Gui Tool to Obtain Mean and Standard Deviation (sd) from Median and Interquartile Range (iqr). Available online: https://www.researchsquare.com/article/rs-828102/v1 (accessed on 25 November 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- World Health Organisation. Exclusively Breastfeed for 6 Months. Available online: https://www.emro.who.int/nutrition/breastfeeding/exclusively-breastfeed-for-6-months.html (accessed on 2 February 2024).
- Martin, C.R.; Ling, P.R.; Blackburn, G.L. Review of infant feeding: Key features of breast milk and infant formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef]
- Ma, J.; Li, Z.; Zhang, W.; Zhang, C.; Zhang, Y.; Mei, H.; Zhuo, N.; Wang, H.; Wang, L.; Wu, D. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: A study of 91 term infants. Sci. Rep. 2020, 10, 15792. [Google Scholar] [CrossRef] [PubMed]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast milk, a source of beneficial microbes and associated benefits for infant health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Breast milk microbiota: A review of the factors that influence composition. J. Infect. 2020, 81, 17–47. [Google Scholar] [CrossRef] [PubMed]
- Mevissen-Verhage, E.A.; Marcelis, J.H.; Harmsen-van Amerongen, W.C.; de Vos, N.M.; Berkel, J.; Verhoef, J. Effect of iron on neonatal gut flora during the first week of life. Eur. J. Clin. Microbiol. 1985, 4, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Krebs, N.F.; Sherlock, L.G.; Westcott, J.; Culbertson, D.; Hambidge, K.M.; Feazel, L.M.; Robertson, C.E.; Frank, D.N. Effects of different complementary feeding regimens on iron status and enteric microbiota in breastfed infants. J. Pediatr. 2013, 163, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.B.; Xu, Y.; Mangani, C.; Fan, Y.M.; Dewey, K.G.; Salminen, S.J.; Maleta, K.; Ashorn, P. Gut microbiota in malawian infants in a nutritional supplementation trial. Trop. Med. Int. Health 2016, 21, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Qasem, W.; Azad, M.B.; Hossain, Z.; Azad, E.; Jorgensen, S.; Castillo San Juan, S.; Cai, C.; Khafipour, E.; Beta, T.; Roberts, L.J., 2nd; et al. Assessment of complementary feeding of canadian infants: Effects on microbiome & oxidative stress, a randomized controlled trial. BMC Pediatr. 2017, 17, 54. [Google Scholar] [CrossRef]
- Simonyte Sjodin, K.; Domellof, M.; Lagerqvist, C.; Hernell, O.; Lonnerdal, B.; Szymlek-Gay, E.A.; Sjodin, A.; West, C.E.; Lind, T. Administration of ferrous sulfate drops has significant effects on the gut microbiota of iron-sufficient infants: A randomised controlled study. Gut 2019, 68, 2095–2097. [Google Scholar] [CrossRef]
- Mevissen-Verhage, E.A.; Marcelis, J.H.; Harmsen-Van Amerongen, W.C.; de Vos, N.M.; Verhoef, J. Effect of iron on neonatal gut flora during the first three months of life. Eur. J. Clin. Microbiol. 1985, 4, 273–278. [Google Scholar] [CrossRef]
- Georgieff, M.K.; Krebs, N.F.; Cusick, S.E. The benefits and risks of iron supplementation in pregnancy and childhood. Annu. Rev. Nutr. 2019, 39, 121–146. [Google Scholar] [CrossRef] [PubMed]
- Aakko, J.; Grzeskowiak, L.; Asukas, T.; Paivansade, E.; Lehto, K.M.; Fan, Y.M.; Mangani, C.; Maleta, K.; Ashorn, P.; Salminen, S. Lipid-based nutrient supplements do not affect gut bifidobacterium microbiota in malawian infants: A randomized trial. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Jaeggi, T.; Kortman, G.A.; Moretti, D.; Chassard, C.; Holding, P.; Dostal, A.; Boekhorst, J.; Timmerman, H.M.; Swinkels, D.W.; Tjalsma, H.; et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in kenyan infants. Gut 2015, 64, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Thompson-Chagoyan, O.C.; Maldonado, J.; Gil, A. Colonization and impact of disease and other factors on intestinal microbiota. Dig. Dis. Sci. 2007, 52, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Lievin, V.; Peiffer, I.; Hudault, S.; Rochat, F.; Brassart, D.; Neeser, J.R.; Servin, A.L. Bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity. Gut 2000, 47, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, X.; Ho, C.L. Recent development of probiotic bifidobacteria for treating human diseases. Front. Bioeng. Biotechnol. 2021, 9, 770248. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Diaz-Ropero, M.P.; Martin, R.; Rodriguez, J.M.; Xaus, J. Antimicrobial potential of four lactobacillus strains isolated from breast milk. J. Appl. Microbiol. 2006, 101, 72–79. [Google Scholar] [CrossRef]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. For gastrointestinal health: Current and future perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef]
- Vizzari, G.; Morniroli, D.; Ceroni, F.; Verduci, E.; Consales, A.; Colombo, L.; Cerasani, J.; Mosca, F.; Gianni, M.L. Human milk, more than simple nourishment. Children 2021, 8, 863. [Google Scholar] [CrossRef]
- Owolabi, A.J.; Senbanjo, I.O.; Oshikoya, K.A.; Boekhorst, J.; Eijlander, R.T.; Kortman, G.A.M.; Hageman, J.H.J.; Samuel, F.; Melse-Boonstra, A.; Schaafsma, A. Multi-nutrient fortified dairy-based drink reduces anaemia without observed adverse effects on gut microbiota in anaemic malnourished nigerian toddlers: A randomised dose-response study. Nutrients 2021, 13, 1566. [Google Scholar] [CrossRef]
- Chu, B.C.; Garcia-Herrero, A.; Johanson, T.H.; Krewulak, K.D.; Lau, C.K.; Peacock, R.S.; Slavinskaya, Z.; Vogel, H.J. Siderophore uptake in bacteria and the battle for iron with the host; a bird’s eye view. Biometals 2010, 23, 601–611. [Google Scholar] [CrossRef]
- Paganini, D.; Uyoga, M.A.; Kortman, G.A.M.; Cercamondi, C.I.; Moretti, D.; Barth-Jaeggi, T.; Schwab, C.; Boekhorst, J.; Timmerman, H.M.; Lacroix, C.; et al. Prebiotic galacto-oligosaccharides mitigate the adverse effects of iron fortification on the gut microbiome: A randomised controlled study in kenyan infants. Gut 2017, 66, 1956–1967. [Google Scholar] [CrossRef] [PubMed]
- de Goffau, M.C.; Jallow, A.T.; Sanyang, C.; Prentice, A.M.; Meagher, N.; Price, D.J.; Revill, P.A.; Parkhill, J.; Pereira, D.I.A.; Wagner, J. Gut microbiomes from gambian infants reveal the development of a non-industrialized prevotella-based trophic network. Nat. Microbiol. 2022, 7, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Fallani, M.; Young, D.; Scott, J.; Norin, E.; Amarri, S.; Adam, R.; Aguilera, M.; Khanna, S.; Gil, A.; Edwards, C.A.; et al. Intestinal microbiota of 6-week-old infants across europe: Geographic influence beyond delivery mode, breast-feeding, and antibiotics. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.; Bik, E.M.; DiGiulio, D.B.; Relman, D.A.; Brown, P.O. Development of the human infant intestinal microbiota. PLoS Biol. 2007, 5, e177. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: A systematic review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef]
- Backhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Moran, C.P.; Shanahan, F. Gut microbiota and obesity: Role in aetiology and potential therapeutic target. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Miqdady, M.; Al Mistarihi, J.; Azaz, A.; Rawat, D. Prebiotics in the infant microbiome: The past, present, and future. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; Peano, C.; Pass, D.A.; Foroni, E.; Severgnini, M.; Claesson, M.J.; Kerr, C.; Hourihane, J.; Murray, D.; Fuligni, F.; et al. Diversity of bifidobacteria within the infant gut microbiota. PLoS ONE 2012, 7, e36957. [Google Scholar] [CrossRef]
- Lund, E.K.; Fairweather-Tait, S.J.; Wharf, S.G.; Johnson, I.T. Chronic exposure to high levels of dietary iron fortification increases lipid peroxidation in the mucosa of the rat large intestine. J. Nutr. 2001, 131, 2928–2931. [Google Scholar] [CrossRef] [PubMed]
- Kaplina, A.; Kononova, S.; Zaikova, E.; Pervunina, T.; Petrova, N.; Sitkin, S. Necrotizing enterocolitis: The role of hypoxia, gut microbiome, and microbial metabolites. Int. J. Mol. Sci. 2023, 24, 2471. [Google Scholar] [CrossRef]
- Tarracchini, C.; Milani, C.; Longhi, G.; Fontana, F.; Mancabelli, L.; Pintus, R.; Lugli, G.A.; Alessandri, G.; Anzalone, R.; Viappiani, A.; et al. Unraveling the microbiome of necrotizing enterocolitis: Insights in novel microbial and metabolomic biomarkers. Microbiol. Spectr. 2021, 9, e0117621. [Google Scholar] [CrossRef]
- de la Cochetiere, M.F.; Piloquet, H.; des Robert, C.; Darmaun, D.; Galmiche, J.P.; Roze, J.C. Early intestinal bacterial colonization and necrotizing enterocolitis in premature infants: The putative role of clostridium. Pediatr. Res. 2004, 56, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Olm, M.R.; Bhattacharya, N.; Crits-Christoph, A.; Firek, B.A.; Baker, R.; Song, Y.S.; Morowitz, M.J.; Banfield, J.F. Necrotizing enterocolitis is preceded by increased gut bacterial replication, klebsiella, and fimbriae-encoding bacteria. Sci. Adv. 2019, 5, eaax5727. [Google Scholar] [CrossRef]
- Kamng’ona, A.W.; Young, R.; Arnold, C.D.; Patson, N.; Jorgensen, J.M.; Kortekangas, E.; Chaima, D.; Malamba, C.; Ashorn, U.; Cheung, Y.B.; et al. Provision of lipid-based nutrient supplements to mothers during pregnancy and 6 months postpartum and to their infants from 6 to 18 months promotes infant gut microbiota diversity at 18 months of age but not microbiota maturation in a rural malawian setting: Secondary outcomes of a randomized trial. J. Nutr. 2020, 150, 918–928. [Google Scholar] [CrossRef]
- Soofi, S.; Cousens, S.; Iqbal, S.P.; Akhund, T.; Khan, J.; Ahmed, I.; Zaidi, A.K.; Bhutta, Z.A. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in pakistan: A cluster-randomised trial. Lancet 2013, 382, 29–40. [Google Scholar] [CrossRef]
- Lee, S.H.; Shinde, P.; Choi, J.; Park, M.; Ohh, S.; Kwon, I.K.; Pak, S.I.; Chae, B.J. Effects of dietary iron levels on growth performance, hematological status, liver mineral concentration, fecal microflora, and diarrhea incidence in weanling pigs. Biol. Trace Elem. Res. 2008, 126 (Suppl. 1), S57–S68. [Google Scholar] [CrossRef]
- Kortman, G.A.; Mulder, M.L.; Richters, T.J.; Shanmugam, N.K.; Trebicka, E.; Boekhorst, J.; Timmerman, H.M.; Roelofs, R.; Wiegerinck, E.T.; Laarakkers, C.M.; et al. Low dietary iron intake restrains the intestinal inflammatory response and pathology of enteric infection by food-borne bacterial pathogens. Eur. J. Immunol. 2015, 45, 2553–2567. [Google Scholar] [CrossRef]
- Li, Y.; Hansen, S.L.; Borst, L.B.; Spears, J.W.; Moeser, A.J. Dietary iron deficiency and oversupplementation increase intestinal permeability, ion transport, and inflammation in pigs. J. Nutr. 2016, 146, 1499–1505. [Google Scholar] [CrossRef]
- Ding, H.; Yu, X.; Chen, L.; Han, J.; Zhao, Y.; Feng, J. Tolerable upper intake level of iron damages the intestine and alters the intestinal flora in weaned piglets. Met. Integr. Biometal Sci. 2020, 12, 1356–1369. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, X.; Wang, X.; Shao, Y.; Tu, Q.; Yang, H.; Yin, J.; Yin, Y. Responses of intestinal microbiota and immunity to increasing dietary levels of iron using a piglet model. Front. Cell Dev. Biol. 2020, 8, 603392. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, J.R.; Piccolo, B.D.; Robeson, M.S.; Barney, D.E., Jr.; Ali, J.; Singh, P.; Hennigar, S.R. Iron deficient diets modify the gut microbiome and reduce the severity of enteric infection in a mouse model of s. Typhimurium-induced enterocolitis. J. Nutr. Biochem. 2022, 107, 109065. [Google Scholar] [CrossRef] [PubMed]
- Dewey, K.G.; Domellof, M.; Cohen, R.J.; Landa Rivera, L.; Hernell, O.; Lonnerdal, B. Iron supplementation affects growth and morbidity of breast-fed infants: Results of a randomized trial in sweden and honduras. J. Nutr. 2002, 132, 3249–3255. [Google Scholar] [CrossRef] [PubMed]
- Paganini, D.; Zimmermann, M.B. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: A review. Am. J. Clin. Nutr. 2017, 106, 1688S–1693S. [Google Scholar] [CrossRef] [PubMed]
- Helmyati, S.; Rahayu, S.E.; Kandarina, B.J.I.; Juffrie, M. No difference between iron supplementation only and iron supplementation with synbiotic fermented milk on iron status, growth, and gut microbiota profile in elementary school children with iron deficiency. Curr. Nutr. Food Sci. 2020, 16, 220–227. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Rusu, I.G.; Suharoschi, R.; Vodnar, D.C.; Pop, C.R.; Socaci, S.A.; Vulturar, R.; Istrati, M.; Morosan, I.; Farcas, A.C.; Kerezsi, A.D.; et al. Iron supplementation influence on the gut microbiota and probiotic intake effect in iron deficiency-a literature-based review. Nutrients 2020, 12, 1993. [Google Scholar] [CrossRef] [PubMed]
- Popovic, A.; Bourdon, C.; Wang, P.W.; Guttman, D.S.; Soofi, S.; Bhutta, Z.A.; Bandsma, R.H.J.; Parkinson, J.; Pell, L.G. Micronutrient supplements can promote disruptive protozoan and fungal communities in the developing infant gut. Nat. Commun. 2021, 12, 6729. [Google Scholar] [CrossRef] [PubMed]

| First Author | Year | Country | Study Type | Intervention | Age of Participants | Duration of Intervention | N1 | Changes in Beneficial Bacteria | Changes in Pathogenic Bacteria | N2 | Changes in Beneficial Bacteria | Changes in Pathogenic Bacteria |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mevissen-Verhage [41] | 1985 | The Netherlands | RCT | Iron-fortified milk. | At birth | 3 months | 6 | Mean concentration of counts of lactobacilli: ~107 CFU/g feces. The majority of the anaerobes were bifidobacteria, with mean counts of >109.5 CFU/g feces. | Mean number of Escherichia coli in bottle-fed infants with iron: 109 CFU/g feces. Mean concentration of counts of enterococci in fecal samples of both bottle-fed groups: 108 CFU/g feces. Counts of bacteroides: more or less constant over time; mean concentration: 109 CFU/g feces. Counts of clostridia in both bottle-fed groups: 107 CFU/g feces (most frequently isolated microorganisms among the anaerobes in bottle-fed infants supplemented with iron). | 10 | Mean concentration of counts of lactobacilli: ~107 CFU/g feces. The majority of the anaerobes were bifidobacteria, with mean counts of >109.5 CFU/g feces. | Mean number of Escherichia coli in breastfed infants: 108.1 CFU/g feces. Counts of enterococci in fecal samples of breastfed infants (mean concentration about 106 CFU/g feces). Counts of bacteroides: more or less constant over time; mean concentration: 109 CFU/g feces. Counts of clostridia in breastfed infants: about 106.4 CFU/g feces. Overall isolation frequency of clostridia in breastfed infants: 25%, (significantly lower than in both bottle-fed groups). |
| Krebs [42] | 2013 | Denver | RCT | Iron-fortified cereal. | 5 months | Approximately 3 months | 10 | Bifidobacterium Δ% abudance in Fe + Zn: stable (−10%, 10%), in Fe group: decreased by >10%. Lactobacillales in Fe + Zn: stable (−10%, 10%), in Fe group: decreased by >10%. | Bacteroidetes Δ% abudance in Fe + Zn: decreased by >10%, in Fe group: increased by >25%. Enterobacteriaceae: in Fe + Zn: stable (−10%, 10%), in Fe group: decreased by >10%. Clostridia Δ% abudance in Fe + Zn: increased by >25%, in Fe group: stable (−10%, 10%). | 4 | Bifidobacterium Δ% abudance in meat group: stable (−10%, 10%). Lactobacillales: stable (−10%, 10%). | Bacteroidetes Δ% abudance in meat group: stable (−10%, 10%). Enterobacteriaceae: decreased by >10%. Clostridia Δ% abudance: increased by >25%. |
| Cheung [43] | 2016 | Malawi | Substudy of a four-arm RCT | Iron fortification. | 6 months | 12 months | 167 | Soya LNS seemed to have higher counts of some lactobacillus species. Bifidobacteria: decreased over time (each p < 0.001). Lactobacilli: relatively constant. Bifidobacterium log-transformed normalised read counts CBS: baseline (median: 5.2; IQR: 4.9, 5.3) 18 months (median: 4.5; IQR: 3.9, 5.0). Milk LNS baseline (median: 5.2; IQR: 5.0, 5.4) 18 months (median: 4.7; IQR: 4.3, 4.8). Soya LNS baseline (median: 5.2; IQR: 5.0, 5.4) 18 months (median: 4.7; IQR: 4.2, 5.0). Lactobacillus log-transformed normalised read counts CBS: baseline (median: 3.8; IQR: 2.4, 4.1) 18 months (median: 3.5; IQR: 2.6, 4.1). Milk LNS baseline (median: 3.7; IQR: 2.7, 4.4) 18 months (median: 3.7; IQR: 3.3, 4.0). Soya LNS baseline (median: 3.6; IQR: 2.8, 4.1) 18 months (median: 3.9; IQR: 3.2, 4.3). | A small fraction of samples were found. Salmonella-positive: CSB: 8.1% at baseline, and 3.2% at 18 months. Milk LNS: positive 0% at baseline, and 8.0% at 18 months. Soya LNS: positive: 3.6% at baseline, and 3.6% at 18 months. Shigella log-transformed normalised read counts CBS: baseline (median: 2.0; IQR: 1.5, 2.5) 18 months (median: 0.7; IQR: 0.0, 1.4). Milk LNS baseline (median: 2.1; IQR: 1.6, 2.6) 18 months (median: 1.0; IQR: 0.0, 1.7). Soya LNS baseline (median: 2.0; IQR: 1.4, 2.7) 18 months (median: 0.5; IQR: 0.0, 1.1). Escherichia log-transformed normalised read counts CBS: baseline (median: 1.2; IQR: 0.0, 1.7) 18 months (median: 0.0; IQR: 0.0, 0.8). Milk LNS baseline (median: 1.2; IQR: 0.0, 1.8) 18 months (median: 0.0; IQR: 0.0, 0.8). Soya LNS baseline (median: 1.4; IQR: 0.0, 1.9) 18 months (median: 0.0; IQR: 0.0, 0.0). | 46 | Bifidobacteria decreased over time (each p < 0.001). Lactobacilli: relatively constant. Bifidobacterium log-transformed normalised read counts. Control: baseline (median: 5.1; IQR: 5.0, 5.4) 18 months (median: 4.7; IQR: 4.3, 5.0). Lactobacillus log-transformed normalised read counts. Control: baseline (median: 3.6; IQR: 2.6, 4.4) 18 months (median: 3.7; IQR: 3.3, 4.0). | A small fraction of samples were found. Salmonella-positive: 6.5% controls at baseline, and 10.9% control at 18 months. Shigella log-transformed normalised read counts. Control: baseline (median: 2.5; IQR: 1.9, 3.0) 18 months (median: 0.8; IQR: 0.0, 1.6). Escherichia log-transformed normalised read counts. Control: baseline (median: 1.5; IQR: 1.0, 2.1) 18 months (median: 0.0; IQR: 0.0, 0.8). |
| Tang [10] | 2017 | Kenya | Double-blind RCT | Iron fortification with MNPs. | 6 months | 3 months | 13 | Decrease in the relative abundance of bifidobacterium in MNP + Fe group (−6.38 +/− 2.5%, p = 0.02). | No significant decrease in the relative abundance of Escherichia/Shigella in the MNP + Fe group (−6.0 +/− 9%, p = 0.41). | 20 | No decrease in the relative abundance of bifidobacterium in the MNP-Fe group (−4.3 +/− 5%, p = 0.44). Decrease in the relative abundance of bifidobacterium in control group (−8.05 +/− 1.46%, p = 0.01). | Significant decrease in the relative abundance of Escherichia/Shigella in the MNP-Fe group (−16.05 +/− 6.9%, p = 0.05). Clostridium increased abundance in MNP-Fe only (1.94 +/− 2%, p = 0.007). Significant decrease in the relative abundance of Escherichia/Shigella in the control group (−19.75 +/− 4.5%, p = 0.01). |
| Qasem [44] | 2017 | Canada | RCT | Iron-fortified cereal. | 4–6 months | 2–4 weeks | 37 | Median relative abundance of bifidobacteriaceae: before CF: in Fe-cereal 50.016, in Fe + fruit: 58.638; after CF: in Fe-cereal 37.257, in Fe + fruit: 50.446. Median relative abundance of lactobacillales: before CF: in Fe-cereal 0.008, in Fe + fruit: 0.013; after CF: in Fe-cereal 0.014, in Fe + fruit: 0.033. | Median relative abundance of bacteroidetes: before CF: in Fe-cereal 4.789, in Fe + fruit: 0.112; after CF: in Fe-cereal 13.511, in Fe + fruit: 5.993. Median relative abundance of enterobacteriaceae: before CF: in Fe-cereal 6.544, in Fe + Fruit: 3.697; after CF: in Fe-cereal 4.894, in Fe + fruit: 6.617. Median relative abundance of enterococcaceae: before CF: in Fe-cereal 0.400, in Fe + fruit: 2.079; after CF: in Fe-cereal 1.152, in Fe + fruit: 1.326. Median relative abundance of clostridia: before CF: in Fe-cereal 0.003, in Fe + fruit: 0.006; after CF: in Fe-cereal 0.018, in Fe + fruit: 0.003. | 19 | Median relative abundance of bifidobacteriaceae: before CF: in meat group 41.377; after CF: in meat group 41.725. Median relative abundance of lactobacillales in meat group: before CF: 0.026; after CF: 0.026 | Median relative abundance of bacteroidetes in meat group: before CF: 0.031; after CF: 2.246. Median relative abundance of enterobacteriaceae in meat group: before CF: 7.836; after CF: 10.330. Median relative abundance of enterococcaceae in meat group: before CF: 1.041; after CF: 2.084. Median relative abundance of clostridia: before CF: in meat group 0.003; after CF: 0.026 |
| Simonyte Sjodin [45] | 2019 | Sweden | RCT | Iron-fortified milk and iron supplementation. | 6 months | 45 days | 35 | High iron formula: decreased relative abundance of bifidobacterium (p < 0.001, 60% vs. 78%) after only 45 days of intervention. High iron formula: relative abundance of lactobacillus sp (42%). Iron drops group: relative abundance of lactobacillus sp (8%). | High iron formula: no enhanced growth of pathogenic bacteria. High iron formula: abundance of streptococcus (0.9%), clostridium (9%), and bacteroides (0.9%). Iron drops group: abundance of streptococcus (0.2%), clostridium, (25%) and bacteroides (1.2%). | 18 | Relative abundance of lactobacillus sp (32%); *In this study, all groups received formula with added galacto-oligosaccharides (GOS) at 3.3 g/L. | No data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karamantziani, T.; Pouliakis, A.; Xanthos, T.; Ekmektzoglou, K.; Paliatsiou, S.; Sokou, R.; Iacovidou, N. The Effect of Oral Iron Supplementation/Fortification on the Gut Microbiota in Infancy: A Systematic Review and Meta-Analysis. Children 2024, 11, 231. https://doi.org/10.3390/children11020231
Karamantziani T, Pouliakis A, Xanthos T, Ekmektzoglou K, Paliatsiou S, Sokou R, Iacovidou N. The Effect of Oral Iron Supplementation/Fortification on the Gut Microbiota in Infancy: A Systematic Review and Meta-Analysis. Children. 2024; 11(2):231. https://doi.org/10.3390/children11020231
Chicago/Turabian StyleKaramantziani, Theoni, Abraham Pouliakis, Theodoros Xanthos, Konstantinos Ekmektzoglou, Styliani Paliatsiou, Rozeta Sokou, and Nicoletta Iacovidou. 2024. "The Effect of Oral Iron Supplementation/Fortification on the Gut Microbiota in Infancy: A Systematic Review and Meta-Analysis" Children 11, no. 2: 231. https://doi.org/10.3390/children11020231