Theoretical–Methodological Foundations for the Global Integration Method (Método de Integração Global—MIG) in the Treatment of Autism Spectrum Disorder
Abstract
:1. Introduction
2. Clinical Epidemiology of Autism
3. Biopsychosocial Impacts of Autism
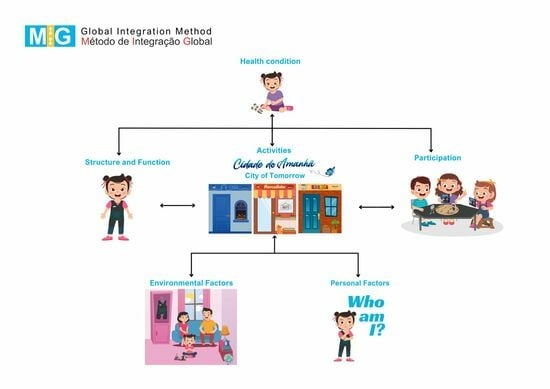
3.1. Body Structure and Function
3.2. Activities
3.3. Participation
3.4. Environmental Factors
3.4.1. Family
3.4.2. Health Services
3.4.3. Educational Services
3.5. Personal Factors
4. Neuropsychological Profile of the Population with Autism
5. Interventions for ASD
6. Global Integration Method (Método de Integração Global–MIG)
6.1. Evidence Rationale for MIG
6.2. Applied Behavior Analysis
6.3. Relevance of Social Learning for Autism
6.4. Relevance of Predictive Coding for Autism
6.5. Relevance of Embodied Cognition for Autism
7. Flexible Therapeutic Suit (MIG Flex) Based on Myofascial Trains
8. Intervention Results Assessment Strategy
9. Strengths and Limitations
10. Final Considerations
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Publishing, Inc.: Arlington VA, USA, 2022. [Google Scholar]
- Hume, K.; Steinbrenner, J.R.; Odom, S.L.; Morin, K.L.; Nowell, S.W.; Tomaszewski, B.; Szendrey, S.; McIntyre, N.S.; Yücesoy-Özkan, S.; Savage, M.N. Evidence-Based Practices for Children, Youth, and Young Adults with Autism: Third Generation Review. J. Autism Dev. Disord. 2021, 51, 4013–4032, Erratum in J. Autism Dev. Disord. 2023, 53, 514. [Google Scholar] [CrossRef]
- Bhat, A.N. Fewer children with autism spectrum disorder with motor challenges receive physical and recreational therapies compared to standard therapies: A SPARK data set analysis. Autism 2023. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.A.L.; Petrulla, V.; Zampella, C.J.; Waller, R.; Schultz, R.T. Gross motor impairment and its relation to social skills in autism spectrum disorder: A systematic review and two meta-analyses. Psychol. Bull. 2022, 148, 273–300. [Google Scholar] [CrossRef] [PubMed]
- Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill. Summ. 2014, 63, 1–21. [Google Scholar]
- Rayan, A.; Ahmad, M. Effectiveness of mindfulness-based interventions on quality of life and positive reappraisal coping among parents of children with autism spectrum disorder. Res. Dev. Disabil. 2016, 55, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Odom, S.L.; Hume, K.A.; Cox, A.W.; Fettig, A.; Kucharczyk, S.; Brock, M.E.; Plavnick, J.B.; Fleury, V.P.; Schultz, T.R. Evidence-Based Practices for Children, Youth, and Young Adults with Autism Spectrum Disorder: A Comprehensive Review. J. Autism Dev. Disord. 2015, 45, 1951–1966. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, Y.E.M.; Minjarez, M.B.; Schreibman, L.; Stahmer, A.C. Naturalistic Developmental Behavioral Interventions for Autism Spectrum Disorder; Brookes Publishing Company: Baltimore, MD, USA, 2020. [Google Scholar]
- Mesibov, G.B.; Shea, V.; Schopler, E. The TEACCH Approach to Autism Spectrum Disorders; Springer Science & Business Media: Berlin, Germany, 2005. [Google Scholar]
- Bhat, A.N. Is Motor Impairment in Autism Spectrum Disorder Distinct from Developmental Coordination Disorder? A Report from the SPARK Study. Phys. Ther. 2020, 100, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Brugha, T.S.; Charman, T.; Cusack, J.; Dumas, G.; Frazier, T.; Jones, E.J.H.; Jones, R.M.; Pickles, A.; State, M.W.; et al. Autism spectrum disorder. Nat. Rev. Dis. Prim. 2020, 6, 5. [Google Scholar] [CrossRef]
- Russell, G. The Rise of Autism: Risk and Resistance in the Age of Diagnosis; Taylor & Francis: Didcot, UK, 2021; Available online: https://library.oapen.org/ (accessed on 25 November 2023).
- Siegel, B. Helping Children with Autism Learn: Treatment Approaches for Parents and Professionals; Oxford University Press: Oxford, UK, 2003; Available online: https://books.google.com.br/books?hl=pt-BR&lr=&id=F-mdBwbAHg0C&oi=fnd&pg=PR7&dq=Siegel (accessed on 24 January 2024).
- Siegel, D.B. The Politics of Autism; Oxford University Press: Oxford, UK, 2018; Available online: https://books.google.com.br/books?hl=pt-BR&lr=&id=iWFmDwAAQBAJ&oi=fnd&pg=PP1&dq=Siegel (accessed on 24 January 2024).
- Vermeulen, P. Autism and The Predictive Brain: Absolute Thinking in a Relative World; Taylor & Francis: Didcot, UK, 2022; Available online: https://books.google.com.br/books?hl=pt-BR&lr=&id=J5eSEAAAQBAJ&oi=fnd&pg=PT8&dq=Vermeulen (accessed on 24 January 2024).
- Waterhouse, L. Heterogeneity thwarts autism explanatory power: A proposal for endophenotypes. Front. Psychiatry 2022, 13, 947653. [Google Scholar] [CrossRef]
- Salari, N.; Rasoulpoor, S.; Shohaimi, S.; Jafarpour, S.; Abdoli, N.; Khaledi-Paveh, B.; Mohammadi, M. The global prevalence of autism spectrum disorder: A comprehensive systematic review and meta-analysis. Ital. J. Pediatr. 2022, 48, 112. [Google Scholar] [CrossRef]
- Maenner, M.J.; Shaw, K.A.; Baio, J.; Washington, A.; Patrick, M.; DiRienzo, M.; Christensen, D.L.; Wiggins, L.D.; Pettygrove, S.; Andrews, J.G.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2020, 69, 1–12, Erratum in MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 503. [Google Scholar] [CrossRef]
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR. Surveill. Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shenouda, J.; Barrett, E.; Davidow, A.L.; Sidwell, K.; Lescott, C.; Halperin, W.; Silenzio, V.M.B.; Zahorodny, W. Prevalence and Disparities in the Detection of Autism without Intellectual Disability. Pediatrics 2023, 151, e2022056594. [Google Scholar] [CrossRef] [PubMed]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Crespi, B.J. Autism as a Disorder of High Intelligence. Front. Neurosci. 2016, 10, 300. [Google Scholar] [CrossRef] [PubMed]
- Lundström, S.; Reichenberg, A.; Anckarsäter, H.; Lichtenstein, P.; Gillberg, C. Autism phenotype versus registered diagnosis in Swedish children: Prevalence trends over 10 years in general population samples. BMJ 2015, 350, h1961. [Google Scholar] [CrossRef] [PubMed]
- Polimanti, R.; Gelernter, J. Widespread signatures of positive selection in common risk alleles associated to autism spectrum disorder. PLoS Genet. 2017, 13, e1006618. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.; Collishaw, S.; Golding, J.; Kelly, S.E.; Ford, T. Changes in diagnosis rates and behavioural traits of autism spectrum disorder over time. BJPsych Open 2015, 1, 110–115. [Google Scholar] [CrossRef]
- Giudice, M.D. Evolutionary Psychopathology: A Unified Approach; Oxford University Press: Oxford, UK, 2018; Available online: https://books.google.com.br/books?hl=pt-BR&lr=&id=aiRjDwAAQBAJ&oi=fnd&pg=PP1&dq=Del+Giudice (accessed on 24 January 2024).
- Toma, C. Genetic Variation across Phenotypic Severity of Autism. Trends Genet. 2020, 36, 228–231. [Google Scholar] [CrossRef]
- Richards, C.; Jones, C.; Groves, L.; Moss, J.; Oliver, C. Prevalence of autism spectrum disorder phenomenology in genetic disorders: A systematic review and meta-analysis. Lancet Psychiatry 2015, 2, 909–916. [Google Scholar] [CrossRef]
- Carpita, B.; Migli, L.; Chiarantini, I.; Battaglini, S.; Montalbano, C.; Carmassi, C.; Cremone, I.M.; Dell’osso, L. Autism Spectrum Disorder and Fetal Alcohol Spectrum Disorder: A Literature Review. Brain Sci. 2022, 12, 792. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, C.; McCarthy, F.P.; Ryan, R.M.; Khashan, A.S. Maternal Alcohol Consumption During Pregnancy and the Risk of Autism Spectrum Disorders in Offspring: A Retrospective Analysis of the Millennium Cohort Study. J. Autism Dev. Disord. 2018, 48, 3773–3782. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Rehm, J.; Anagnostou, E.; Popova, S. Prevalence of externalizing disorders and Autism Spectrum Disorders among children with Fetal Alcohol Spectrum Disorder: Systematic review and meta-analysis. Biochem. Cell Biol. 2018, 96, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Ornoy, A.; Weinstein-Fudim, L.; Ergaz, Z. Prenatal factors associated with autism spectrum disorder (ASD). Reprod. Toxicol. 2015, 56, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.B.; Aylsworth, A.S.; Cordero, C.; Croen, L.A.; DiGuiseppi, C.; Fallin, M.D.; Herring, A.H.; Hooper, S.R.; Pretzel, R.E.; Schieve, L.A.; et al. Prenatal Alcohol Exposure in Relation to Autism Spectrum Disorder: Findings from the Study to Explore Early Development (SEED). Paediatr. Périnat. Epidemiol. 2017, 31, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Auyeung, B.; Baron-Cohen, S. Hormonal Influences in Typical Development: Implications for Autism. In Chapter the Neuroscience of Autism Spectrum Disorders; Buxbaum, J.D., Hof, P.R., Eds.; Academic Press: Oxford, UK, 2013; pp. 215–232. [Google Scholar]
- Auyeung, B.; Ashwin, E.; Knickmeyer, R.; Taylor, K.; Hackett, G.; Baron-Cohen, S. Fetal testosterone and autistic traits. Br. J. Psychol. 2009, 100, 1–22. [Google Scholar] [CrossRef]
- Cesta, C.E.; Månsson, M.; Palm, C.; Lichtenstein, P.; Iliadou, A.N.; Landén, M. Polycystic ovary syndrome and psychiatric disorders: Co-morbidity and heritability in a nationwide Swedish cohort. Psychoneuroendocrinology 2016, 73, 196–203. [Google Scholar] [CrossRef]
- Knickmeyer, R.; Baron-Cohen, S.; Fane, B.A.; Wheelwright, S.; Mathews, G.A.; Conway, G.S.; Brook, C.G.; Hines, M. Androgens and autistic traits: A study of individuals with congenital adrenal hyperplasia. Horm. Behav. 2006, 50, 148–153. [Google Scholar] [CrossRef]
- Kosidou, K.; Dalman, C.; Widman, L.; Arver, S.; Lee, B.K.; Magnusson, C.; Gardner, R.M. Maternal polycystic ovary syndrome and the risk of autism spectrum disorders in the offspring: A population-based nationwide study in Sweden. Mol. Psychiatry 2015, 21, 1441–1448. [Google Scholar] [CrossRef]
- Lilienfeld, S.O.; Marino, L. Mental disorder as a Roschian concept: A critique of Wakefield’s “harmful dysfunction” analysis. J. Abnorm. Psychol. 1995, 104, 411–420. [Google Scholar] [CrossRef]
- Wakefield, J.C. The concept of mental disorder: On the boundary between biological facts and social values. Am. Psychol. 1992, 47, 373–388. [Google Scholar] [CrossRef]
- Wakefield, J.C. The concept of mental disorder: Diagnostic implications of the harmful dysfunction analysis. World Psychiatry 2007, 6, 149–156. [Google Scholar]
- Crespi, B.J. The hallmarks of autism. Front. Psychiatry 2022, 13, 937163. [Google Scholar] [CrossRef]
- Bölte, S.; Mahdi, S.; De Vries, P.J.; Granlund, M.; Robison, J.E.; Shulman, C.; Swedo, S.; Tonge, B.; Wong, V.; Zwaigenbaum, L.; et al. The Gestalt of functioning in autism spectrum disorder: Results of the international conference to develop final consensus International Classification of Functioning, Disability and Health core sets. Autism 2019, 23, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Schiariti, V.; Mahdi, S.; Bölte, S. International Classification of Functioning, Disability and Health Core Sets for cerebral palsy, autism spectrum disorder, and attention-deficit-hyperactivity disorder. Dev. Med. Child Neurol. 2018, 60, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.B.; Filipini, R.; Monteiro, C.B.d.M.E.; Valenti, V.; de Carvalho, S.M.F.; Wajnsztejn, R.; de Farias, M.D.C.A.D.; Macedo, C.C.; de Abreu, L.C. The biopsychosocial processes in autism spectrum disorder. Int. Arch. Med. 2013, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Classification of Functioning, Disability, and Health: Children & Youth Version: ICF-CY; World Health Organization: Geneva, Switzerland, 2007; Available online: https://books.google.com.br/books?hl=pt-BR&lr=&id=SWFQDXyU-rcC&oi=fnd&pg=PR8&dq=+International+Classification+of+Functioning (accessed on 24 January 2024).
- Fombonne, E.; MacFarlane, H.; Salem, A.C.; Zuckerman, K.E. Epidemiological Surveys of ASD: Current Findings and New Directions. Autism and child psychopathology series. In Handbook of Autism and Pervasive Developmental Disorder: Assessment, Diagnosis, and Treatment; Springer International Publishing: Cham, Switzerland, 2022; pp. 135–184. [Google Scholar]
- Katusic, M.Z.; Myers, S.M.; Weaver, A.L.; Voigt, R.G. IQ in Autism Spectrum Disorder: A Population-Based Birth Cohort Study. Pediatrics 2021, 148, e2020049899. [Google Scholar] [CrossRef] [PubMed]
- Hassiotis, A.; Emerson, E.; Wieland, J.; Bertelli, M.O. Borderline Intellectual Functioning. In Textbook of Psychiatry for Intellectual Disability and Autism Spectrum Disorder; Springer International Publishing: Cham, Switzerland, 2022; pp. 95–106. [Google Scholar]
- Loucas, T.; Charman, T.; Pickles, A.; Simonoff, E.; Chandler, S.; Meldrum, D.; Baird, G. Autistic symptomatology and language ability in autism spectrum disorder and specific language impairment. J. Child Psychol. Psychiatry 2008, 49, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, J.; El-Raziq, M.A.; Castroviejo, E.; Durrleman, S.; Ferré, S.; Grama, I.; Hendriks, P.; Kissine, M.; Manenti, M.; Marinis, T.; et al. Language in autism: Domains, profiles and co-occurring conditions. J. Neural Transm. 2023, 130, 433–457. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.M.; Shaw, K.A.; DiRienzo, M.; Durkin, M.S.; Esler, A.; Hall-Lande, J.; Wiggins, L.; Zahorodny, W.; Singer, A.; Maenner, M.J. The Prevalence and Characteristics of Children with Profound Autism, 15 Sites, United States, 2000–2016. Public Heath Rep. 2023, 138, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Posar, A.; Visconti, P. Update about “minimally verbal” children with autism spectrum disorder. Rev. Paul. Pediatr. 2022, 40, e2020158. [Google Scholar] [CrossRef] [PubMed]
- Bullen, J.C.; Zajic, M.C.; McIntyre, N.; Solari, E.; Mundy, P. Patterns of math and reading achievement in children and adolescents with autism spectrum disorder. Res. Autism Spectr. Disord. 2022, 92, 101933. [Google Scholar] [CrossRef]
- Chen, L.; Abrams, D.A.; Rosenberg-Lee, M.; Iuculano, T.; Wakeman, H.N.; Prathap, S.; Chen, T.; Menon, V. Quantitative Analysis of Heterogeneity in Academic Achievement of Children with Autism. Clin. Psychol. Sci. 2018, 7, 362–380. [Google Scholar] [CrossRef] [PubMed]
- Oswald, T.M.; Beck, J.S.; Iosif, A.; McCauley, J.B.; Gilhooly, L.J.; Matter, J.C.; Solomon, M. Clinical and Cognitive Characteristics Associated with Mathematics Problem Solving in Adolescents with Autism Spectrum Disorder. Autism Res. 2015, 9, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Atun-Einy, O.; Lotan, M.; Harel, Y.; Shavit, E.; Burstein, S.; Kempner, G. Physical Therapy for Young Children Diagnosed with Autism Spectrum Disorders–Clinical Frameworks Model in an Israeli Setting. Front. Pediatr. 2013, 1, 19. [Google Scholar] [CrossRef] [PubMed]
- Casartelli, L.; Molteni, M.; Ronconi, L. So close yet so far: Motor anomalies impacting on social functioning in autism spectrum disorder. Neurosci. Biobehav. Rev. 2016, 63, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Cook, J. From movement kinematics to social cognition: The case of autism. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150372. [Google Scholar] [CrossRef]
- Ohara, R.; Kanejima, Y.; Kitamura, M.; Izawa, P.K. Association between Social Skills and Motor Skills in Individuals with Autism Spectrum Disorder: A Systematic Review. Eur. J. Investig. Health Psychol. Educ. 2019, 10, 276–296. [Google Scholar] [CrossRef]
- Ming, X.; Brimacombe, M.; Wagner, G.C. Prevalence of motor impairment in autism spectrum disorders. Brain Dev. 2007, 29, 565–570. [Google Scholar] [CrossRef]
- Casartelli, L.; Federici, A.; Biffi, E.; Molteni, M.; Ronconi, L. Are We “Motorically” Wired to Others? High-Level Motor Computations and Their Role in Autism. Neuroscientist 2018, 24, 568–581. [Google Scholar] [CrossRef]
- Casartelli, L.; Chiamulera, C. The motor way: Clinical implications of understanding and shaping actions with the motor system in autism and drug addiction. Cogn. Affect. Behav. Neurosci. 2016, 16, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, M.W.; Sweeney, J.A. Sensorimotor dysfunctions as primary features of autism spectrum disorders. Sci. China Life Sci. 2015, 58, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Pramparo, T.; Gazestani, V.H.; Lombardo, M.V.; Pierce, K.; Lewis, N.E. The ASD Living Biology: From cell proliferation to clinical phenotype. Mol. Psychiatry 2019, 24, 88–107. [Google Scholar] [CrossRef] [PubMed]
- Hazlett, H.C.; Gu, H.; Munsell, B.C.; Kim, S.H.; Styner, M.; Wolff, J.J.; Elison, J.T.; Swanson, M.R.; Zhu, H.; Botteron, K.N.; et al. Early brain development in infants at high risk for autism spectrum disorder. Nature 2017, 542, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.D.; Piven, J. Brain and behavior development in autism from birth through infancy. Dialog. Clin. Neurosci. 2017, 19, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Vivanti, G.; Nuske, H.J. Autism, attachment, and social learning: Three challenges and a way forward. Behav. Brain Res. 2017, 325, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Grzadzinski, R.; Lord, C.; Sanders, S.J.; Werling, D.; Bal, V.H. Children with autism spectrum disorder who improve with fever: Insights from the Simons Simplex Collection. Autism Res. 2018, 11, 175–184. [Google Scholar] [CrossRef]
- Vivanti, G.; Rogers, S.J.; Dwyer, P.; Rivera, S. Early Learning in Autism; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–12. [Google Scholar]
- Anderson, D.K.; Liang, J.W.; Lord, C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. J. Child Psychol. Psychiatry 2014, 55, 485–494. [Google Scholar] [CrossRef]
- Jones, R.M.; Pickles, A.; Lord, C. Evaluating the quality of peer interactions in children and adolescents with autism with the Penn Interactive Peer Play Scale (PIPPS). Mol. Autism 2017, 8, 28. [Google Scholar] [CrossRef]
- Lord, C.; Risi, S.; DiLavore, P.S.; Shulman, C.; Thurm, A.; Pickles, A. Autism from 2 to 9 years of age. Arch. Gen. Psychiatry 2006, 63, 694–701. [Google Scholar] [CrossRef]
- Kanne, S.M.; Gerber, A.J.; Quirmbach, L.M.; Sparrow, S.S.; Cicchetti, D.V.; Saulnier, C.A. The Role of Adaptive Behavior in Autism Spectrum Disorders: Implications for Functional Outcome. J. Autism Dev. Disord. 2011, 41, 1007–1018. [Google Scholar] [CrossRef]
- Klin, A.; Saulnier, C.A.; Sparrow, S.S.; Cicchetti, D.V.; Volkmar, F.R.; Lord, C. Social and Communication Abilities and Disabilities in Higher Functioning Individuals with Autism Spectrum Disorders: The Vineland and the ADOS. J. Autism Dev. Disord. 2006, 37, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Flanagan, H.E.; Geier, J.D.; Freeman, N.L. Brief Report: The Vineland Adaptive Behavior Scales in Young Children with Autism Spectrum Disorders at Different Cognitive Levels. J. Autism Dev. Disord. 2009, 39, 1066–1078. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, J.L.; Gates, J.A.; Lerner, M.D. Friendship in school-age boys with autism spectrum disorders: A meta-analytic summary and developmental, process-based model. Psychol. Bull. 2016, 142, 601–622. [Google Scholar] [CrossRef] [PubMed]
- Roux, A.M.; Shattuck, P.T.; Rast, J.E.; Rava, J.A.; Anderson, K. National Autism Indicators Report: Transition into Young Adulthood; Life Course Outcomes Research Program; A.J. Drexel Autism Institute, Drexel University: Philadelphia, PA, USA, 2015. [Google Scholar]
- Petrina, N.; Carter, M.; Stephenson, J. The nature of friendship in children with autism spectrum disorders: A systematic review. Res. Autism Spectr. Disord. 2014, 8, 111–126. [Google Scholar] [CrossRef]
- Płatos, M.; Pisula, E. Friendship understanding in males and females on the autism spectrum and their typically developing peers. Res. Autism Spectr. Disord. 2021, 81, 101716. [Google Scholar] [CrossRef]
- Pecora, L.A.; Hancock, G.I.; Hooley, M.; Demmer, D.H.; Attwood, T.; Mesibov, G.B.; Stokes, M.A. Gender identity, sexual orientation and adverse sexual experiences in autistic females. Mol. Autism 2020, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Roux, A.M.; Rast, J.E.; Anderson, K.A.; Shattuck, P.T. National Autism Indicators Report: Developmental Disability Services and Outcomes in Adulthood; Life Course Outcomes Program; A.J. Drexel Autism Institute, Drexel University: Philadelphia, PA, USA, 2017. [Google Scholar]
- Rast, J.E.; Roux, A.M.; Anderson, K.A.; Croen, L.A.; Kuo, A.A.; Shea, L.L.; Shattuck, P.T. National Autism Indicators Report: Health and Health Care; Life Course Outcomes Program; A.J. Drexel Autism Institute, Drexel University: Philadelphia, PA, USA, 2020. [Google Scholar]
- Bitsika, V.; Sharpley, C.F. Stress, Anxiety and Depression Among Parents of Children with Autism Spectrum Disorder. Aust. J. Guid. Couns. 2004, 14, 151–161. [Google Scholar] [CrossRef]
- Bouma, R.; Schweitzer, R. The impact of chronic childhood illness on family stress: A comparison between autism and cystic fibrosis. J. Clin. Psychol. 1990, 46, 722–730. [Google Scholar] [CrossRef]
- Donenberg, G.; Baker, B.L. The impact of young children with externalizing behaviors on their families. J. Abnorm. Child Psychol. 1993, 21, 179–198. [Google Scholar] [CrossRef]
- Gray, D.E.; Holden, W.J. Psycho-social well-being among the parents of children with autism. Aust. N. Z. J. Dev. Disabil. 1992, 18, 83–93. [Google Scholar] [CrossRef]
- Gurney, J.G.; McPheeters, M.L.; Davis, M.M. Parental Report of Health Conditions and Health Care Use Among Children with and without Autism. Arch. Pediatr. Adolesc. Med. 2006, 160, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Kogan, M.D.; Strickland, B.B.; Blumberg, S.J.; Singh, G.K.; Perrin, J.M.; van Dyck, P.C. A National Profile of the Health Care Experiences and Family Impact of Autism Spectrum Disorder Among Children in the United States, 2005–2006. Pediatrics 2008, 122, e1149–e1158. [Google Scholar] [CrossRef]
- Liptak, G.S.; Stuart, T.; Auinger, P. Health Care Utilization and Expenditures for Children with Autism: Data from U.S. National Samples. J. Autism Dev. Disord. 2006, 36, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Schieve, L.A.; Boulet, S.L.; Kogan, M.D.; Yeargin-Allsopp, M.; Boyle, C.A.; Visser, S.N.; Blumberg, S.J.; Rice, C. Parenting aggravation and autism spectrum disorders: 2007 National Survey of Children’s Health. Disabil. Health J. 2011, 4, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Valicenti-McDermott, M.; Lawson, K.; Hottinger, K.; Seijo, R.; Schechtman, M.; Shulman, L.; Shinnar, S. Parental Stress in Families of Children with Autism and Other Developmental Disabilities. J. Child Neurol. 2015, 30, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Hartley, S.L.; Barker, E.T.; Seltzer, M.M.; Floyd, F.; Greenberg, J.; Orsmond, G.; Bolt, D. The relative risk and timing of divorce in families of children with an autism spectrum disorder. J. Fam. Psychol. 2010, 24, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Musetti, A.; Manari, T.; Dioni, B.; Raffin, C.; Bravo, G.; Mariani, R.; Esposito, G.; Dimitriou, D.; Plazzi, G.; Franceschini, C.; et al. Parental Quality of Life and Involvement in Intervention for Children or Adolescents with Autism Spectrum Disorders: A Systematic Review. J. Pers. Med. 2021, 11, 894. [Google Scholar] [CrossRef] [PubMed]
- Vasilopoulou, E.; Nisbet, J. The quality of life of parents of children with autism spectrum disorder: A systematic review. Res. Autism Spectr. Disord. 2016, 23, 36–49. [Google Scholar] [CrossRef]
- Vohra, R.; Madhavan, S.; Sambamoorthi, U.; Peter, C.S. Access to services, quality of care, and family impact for children with autism, other developmental disabilities, and other mental health conditions. Autism 2014, 18, 815–826. [Google Scholar] [CrossRef]
- Enea, V.; Rusu, D.M. Raising a Child with Autism Spectrum Disorder: A Systematic Review of the Literature Investigating Parenting Stress. J. Ment. Health Res. Intellect. Disabil. 2020, 13, 283–321. [Google Scholar] [CrossRef]
- Gomes, P.T.; Lima, L.H.; Bueno, M.K.; Araújo, L.A.; Souza, N.M. Autism in Brazil: A systematic review of family challenges and coping strategies. J. Pediatr. 2015, 91, 111–121. [Google Scholar] [CrossRef]
- Schnabel, A.; Youssef, G.J.; Hallford, D.J.; Hartley, E.J.; McGillivray, J.A.; Stewart, M.; Forbes, D.; Austin, D.W. Psychopathology in parents of children with autism spectrum disorder: A systematic review and meta-analysis of prevalence. Autism 2020, 24, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Glidden, D.; Bouman, W.P.; Jones, B.A.; Arcelus, J. Gender Dysphoria and Autism Spectrum Disorder: A Systematic Review of the Literature. Sex. Med. Rev. 2016, 4, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Kallitsounaki, A.; Williams, D.M. Autism Spectrum Disorder and Gender Dysphoria/Incongruence. A systematic Literature Review and Meta-Analysis. J. Autism Dev. Disord. 2023, 53, 3103–3117. [Google Scholar] [CrossRef] [PubMed]
- Koller, J.; David, T.; Bar, N.; Lebowitz, E.R. The Role of Family Accommodation of RRBs in Disruptive Behavior Among Children with Autism. J. Autism Dev. Disord. 2022, 52, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Buescher, A.V.S.; Cidav, Z.; Knapp, M.; Mandell, D.S. Costs of Autism Spectrum Disorders in the United Kingdom and the United States. JAMA Pediatr. 2014, 168, 721–728. [Google Scholar] [CrossRef]
- Croteau, C.; Mottron, L.; Dorais, M.; Tarride, J.-E.; Perreault, S. Use, costs, and predictors of psychiatric healthcare services following an autism spectrum diagnosis: Population-based cohort study. Autism 2019, 23, 2020–2030. [Google Scholar] [CrossRef]
- Bowman, K.S.; Suarez, V.D.; Weiss, M.J. Standards for Interprofessional Collaboration in the Treatment of Individuals with Autism. Behav. Anal. Pract. 2021, 14, 1191–1208. [Google Scholar] [CrossRef]
- Lustrea, A. Case management in autism spectrum disorders. In Perspective on Autistic Specturm Disorders; Al Ghazi, L., Zappaterra, T.T., Eds.; Edizioni ETS: Pisa, Italy, 2019; pp. 43–57. [Google Scholar]
- Zuckerman, K.E.; Lindly, O.J.; Bethell, C.D.; Kuhlthau, K. Family Impacts Among Children with Autism Spectrum Disorder: The Role of Health Care Quality. Acad. Pediatr. 2014, 14, 398–407. [Google Scholar] [CrossRef]
- Lawrence, C. Autism and Flexischooling A Shared Classroom and Homeschooling Approach; Jessica Kingsley: London, UK, 2012. [Google Scholar]
- Vivanti, G.; Bent, C.; Capes, K.; Upson, S.; Hudry, K.; Dissanayake, C. The Victorian ASELCC Team Characteristics of children on the autism spectrum who benefit the most from receiving intervention in inclusive versus specialised early childhood education settings. Autism Res. 2022, 15, 2200–2209. [Google Scholar] [CrossRef]
- Tobin, M.C.; Drager, K.D.; Richardson, L.F. A systematic review of social participation for adults with autism spectrum disorders: Support, social functioning, and quality of life. Res. Autism Spectr. Disord. 2014, 8, 214–229. [Google Scholar] [CrossRef]
- Sáez-Suanes, G.P.; Álvarez-Couto, M. Factors Associated with Quality of Life in Adults with Autism Spectrum Disorder: A Systematic Review. Rev. J. Autism Dev. Disord. 2021, 9, 1–13. [Google Scholar] [CrossRef]
- Cybulski, L.; Ashcroft, D.M.; Carr, M.J.; Garg, S.; Chew-Graham, C.A.; Kapur, N.; Webb, R.T. Risk factors for nonfatal self-harm and suicide among adolescents: Two nested case–control studies conducted in the UK Clinical Practice Research Datalink. J. Child Psychol. Psychiatry 2022, 63, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Hedley, D.; Uljarević, M. Systematic Review of Suicide in Autism Spectrum Disorder: Current Trends and Implications. Curr. Dev. Disord. Rep. 2018, 5, 65–76. [Google Scholar] [CrossRef]
- da Silva, J.H.; Hayashi, M.C.P.I. Estudo bibliométrico da produção científica sobre a associação de pais e amigos dos excepcionais. Rev. Educ. Espec. 2018, 31, 65. [Google Scholar] [CrossRef]
- Mottron, L.; Bzdok, D. Autism spectrum heterogeneity: Fact or artifact? Mol. Psychiatry 2020, 25, 3178–3185. [Google Scholar] [CrossRef] [PubMed]
- Charman, T.; Pickles, A.; Simonoff, E.; Chandler, S.; Loucas, T.; Baird, G. IQ in children with autism spectrum disorders: Data from the Special Needs and Autism Project (SNAP). Psychol. Med. 2011, 41, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.; Mandy, W.; Elliott, D.; White, R.; Pittwood, T.; Ford, T. Selection bias on intellectual ability in autism research: A cross-sectional review and meta-analysis. Mol. Autism 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Lovaas, O.I. The development of a treatment-research project for developmentally disabled and autistic children. J. Appl. Behav. Anal. 1993, 26, 617–630. [Google Scholar] [CrossRef]
- Dawson, G.; Rogers, S.; Munson, J.; Smith, M.; Winter, J.; Greenson, J.; Donaldson, A.; Varley, J. Randomized, Controlled Trial of an Intervention for Toddlers with Autism: The Early Start Denver Model. Pediatrics 2010, 125, e17–e23. [Google Scholar] [CrossRef]
- Waddington, H.; Reynolds, J.E.; Macaskill, E.; Curtis, S.; Taylor, L.J.; Whitehouse, A.J. The effects of JASPER intervention for children with autism spectrum disorder: A systematic review. Autism 2021, 25, 2370–2385. [Google Scholar] [CrossRef]
- Pierce, K.; Schreibman, L. Increasing complex social behaviors in children with autism: Effects of peer-implemented pivotal response training. J. Appl. Behav. Anal. 1995, 28, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Odom, S.L.; Boyd, B.A.; Hall, L.J.; Hume, K. Evaluation of Comprehensive Treatment Models for Individuals with Autism Spectrum Disorders. J. Autism Dev. Disord. 2010, 40, 425–436, Erratum in J. Autism Dev. Disord. 2010, 40, 437. [Google Scholar] [CrossRef] [PubMed]
- Ingersoll, B.; Wainer, A. Initial Efficacy of Project ImPACT: A Parent-Mediated Social Communication Intervention for Young Children with ASD. J. Autism Dev. Disord. 2013, 43, 2943–2952. [Google Scholar] [CrossRef] [PubMed]
- Estes, A.; Munson, J.; Rogers, S.J.; Greenson, J.; Winter, J.; Dawson, G. Long-Term Outcomes of Early Intervention in 6-Year-Old Children with Autism Spectrum Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 580–587. [Google Scholar] [CrossRef]
- Acar, S.; Chen, C.-I.; Xie, H. Parental involvement in developmental disabilities across three cultures: A systematic review. Res. Dev. Disabil. 2021, 110, 103861. [Google Scholar] [CrossRef] [PubMed]
- Movahedazarhouligh, S. Parent-implemented interventions and family-centered service delivery approaches in early intervention and early childhood special education. Early Child Dev. Care 2019, 191, 1–12. [Google Scholar] [CrossRef]
- Almasri, N.A.; An, M.; Palisano, R.J. Parents’ Perception of Receiving Family-Centered Care for Their Children with Physical Disabilities: A Meta-Analysis. Phys. Occup. Ther. Pediatr. 2018, 38, 427–443. [Google Scholar] [CrossRef] [PubMed]
- An, M.; Palisano, R.J.; Yi, C.-H.; Chiarello, L.A.; Dunst, C.J.; Gracely, E.J. Effects of a Collaborative Intervention Process on Parent Empowerment and Child Performance: A Randomized Controlled Trial. Phys. Occup. Ther. Pediatr. 2019, 39, 1–15. [Google Scholar] [CrossRef]
- An, M.; Palisano, R.J.; Yi, C.-H.; Chiarello, L.A.; Dunst, C.J.; Gracely, E.J. Effects of a Collaborative Intervention Process on Parent–Therapist Interaction: A Randomized Controlled Trial. Phys. Occup. Ther. Pediatr. 2019, 39, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Borji, R.; Laatar, R.; Zarrouk, N.; Sahli, S.; Rebai, H. Cognitive-motor interference during standing stance across different postural and cognitive tasks in individuals with Down syndrome. Res. Dev. Disabil. 2023, 139, 104562. [Google Scholar] [CrossRef] [PubMed]
- Jabouille, F.; Billot, M.; Hermand, E.; Lemonnier, E.; Perrochon, A. Balance rehabilitation for postural control in children with Autism Spectrum Disorder: A two-case report study. Physiother. Theory Pract. 2023, 39, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Leone, C.; Feys, P.; Moumdjian, L.; D’amico, E.; Zappia, M.; Patti, F. Cognitive-motor dual-task interference: A systematic review of neural correlates. Neurosci. Biobehav. Rev. 2017, 75, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Reilly, D.S.; Woollacott, M.H.; van Donkelaar, P.; Saavedra, S. The Interaction Between Executive Attention and Postural Control in Dual-Task Conditions: Children with Cerebral Palsy. Arch. Phys. Med. Rehabil. 2008, 89, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T.L.; Snider, J.; Amit, M.; Ng, T.N.; Townsend, J.; Chukoskie, L. An Objective System for Quantifying the Effect of Cognitive Load on Movement in Individuals with Autism Spectrum Disorder. In Proceedings of the 2019 9th International IEEE/EMBS Conference on Neural Engineering (NER), San Francisco, CA, USA, 20–23 March 2019; pp. 1042–1045. [Google Scholar]
- Torres, E.B.; Denisova, K. Motor noise is rich signal in autism research and pharmacological treatments. Sci. Rep. 2016, 6, 37422. [Google Scholar] [CrossRef] [PubMed]
- Van Biesen, D.; Jacobs, L.; McCulloch, K.; Janssens, L.; Vanlandewijck, Y.C. Cognitive-motor dual-task ability of athletes with and without intellectual impairment. J. Sports Sci. 2018, 36, 513–521. [Google Scholar] [CrossRef]
- Wolpert, D.M.; Doya, K.; Kawato, M. A unifying computational framework for motor control and social interaction. Philos. Trans. R. Soc. B Biol. Sci. 2003, 358, 593–602. [Google Scholar] [CrossRef]
- Ahar, S.; Ghadiri, F. The Effect of Motor Imagery Training on Motor Proficiency of Children with Autism Spectrum Disorder: A Clinical Trial Study. J. Rafsanjan Univ. Med. Sci. 2021, 20, 801–816. [Google Scholar] [CrossRef]
- Kalyuga, S. Schema acquisition and sources of cognitive load. In Cognitive Load Theory; Cambridge University Press: Cambridge, UK, 2010; pp. 48–64. [Google Scholar]
- Sweller, J.; Ayres, P.; Kalyuga, S. Cognitive Load Theory; Springer: New York, NY, USA, 2011. [Google Scholar]
- Bleyenheuft, Y.; Ebner-Karestinos, D.; Surana, B.; Paradis, J.; Sidiropoulos, A.; Renders, A.; Friel, K.M.; Brandao, M.; Rameckers, E.; Gordon, A.M. Intensive upper- and lower-extremity training for children with bilateral cerebral palsy: A quasi-randomized trial. Dev. Med. Child Neurol. 2017, 59, 625–633. [Google Scholar] [CrossRef]
- Figueiredo, P.R.P.; Mancini, M.C.; Feitosa, A.M.; Teixeira, C.M.M.F.; Guerzoni, V.P.D.; Elvrum, A.G.; Ferre, C.L.; Gordon, A.M.; Brandão, M.B. Hand–arm bimanual intensive therapy and daily functioning of children with bilateral cerebral palsy: A randomized controlled trial. Dev. Med. Child Neurol. 2020, 62, 1274–1282. [Google Scholar] [CrossRef]
- Hodgson, R.; Biswas, M.; Palmer, S.; Marshall, D.; Rodgers, M.; Stewart, L.; Simmonds, M.; Rai, D.; Le Couteur, A. Intensive behavioural interventions based on applied behaviour analysis (ABA) for young children with autism: A cost-effectiveness analysis. PLoS ONE 2022, 17, e0270833. [Google Scholar] [CrossRef] [PubMed]
- Jackman, M.; Sakzewski, L.; Morgan, C.; Boyd, R.N.; Brennan, S.E.; Langdon, K.; Toovey, R.A.M.; Greaves, S.; Thorley, M.; Novak, I. Interventions to improve physical function for children and young people with cerebral palsy: International clinical practice guideline. Dev. Med. Child Neurol. 2021, 64, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, F.; Feldman, I.; Lavelle, T.A.; Skokauskas, N. The cost-effectiveness of treatments for attention deficit-hyperactivity disorder and autism spectrum disorder in children and adolescents: A systematic review. Eur. Child Adolesc. Psychiatry 2022, 31, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Ingersoll, B.; Dvortcsak, A. Teaching Social Communication to Children with Autism and Other Developmental Delays (2-Book Set): The Project ImPACT Guide to Coaching Parents and The Project ImPACT Manual for Parents, 2nd ed.; Guilford Press: New York, NY, USA, 2019. [Google Scholar]
- Fenning, R.M.; Butter, E.M.; Macklin, E.A.; Norris, M.; Hammersmith, K.J.; McKinnon-Bermingham, K.; Chan, J.; Stephenson, K.G.; Albright, C.; Scherr, J.; et al. Parent Training for Dental Care in Underserved Children with Autism: A Randomized Controlled Trial. Pediatrics 2022, 149, e2021050691. [Google Scholar] [CrossRef]
- Kazdin, A.E. Behavior Modification in Applied Settings, 7th ed.; Waveland Press: Long Grove, IL, USA, 2012; Available online: https://books.google.com.br/books?hl=pt-BR&lr=&id=6dEYAAAAQBAJ&oi=fnd&pg=PR1&dq=Behavior+modification+in+applied+setting+&ots=5J8UT4s2u7&sig=jLJjKcduyEaHBnm8D8v_da-hMJg#v=onepage&q=Behavior%20modification%20in%20applied%20setting&f=false (accessed on 24 January 2024).
- Loffi, R.G. Exoesqueleto Flexível Baseado nas Linhas Miofasciais e Padrões Geométricos Gerais. Depositante: TREINI Biotecnologia LTDA. Patente brasileira nº BR102018009935-3 A2, 2019. [Google Scholar]
- Stecco, A.; Giordani, F.; Fede, C.; Pirri, C.; De Caro, R.; Stecco, C. From Muscle to the Myofascial Unit: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 4527. [Google Scholar] [CrossRef] [PubMed]
- Hadad, B.-S.; Schwartz, S. Perception in autism does not adhere to Weber’s law. eLife 2019, 8, e42223. [Google Scholar] [CrossRef] [PubMed]
- Williams, Z.J.; He, J.L.; Cascio, C.J.; Woynaroski, T.G. A review of decreased sound tolerance in autism: Definitions, phenomenology, and potential mechanisms. Neurosci. Biobehav. Rev. 2021, 121, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Damiano, D.L.; Forssberg, H. International initiatives to improve the lives of children with developmental disabilities. Dev. Med. Child Neurol. 2019, 61, 1121. [Google Scholar] [CrossRef]
- Fivush, R. The Development of Autobiographical Memory. Annu. Rev. Psychol. 2011, 62, 559–582. [Google Scholar] [CrossRef]
- Nelson, K.; Fivush, R. The Emergence of Autobiographical Memory: A Social Cultural Developmental Theory. Psychol. Rev. 2004, 111, 486–511. [Google Scholar] [CrossRef]
- Kendall, P.C.; Braswell, L. Cognitive-Behavioral Therapy for Impulsive Children; Guilford Press: New York, NY, USA, 1993; Available online: https://books.google.com.br/books?hl=pt-BR&lr=&id=McRPHK-c7CoC&oi=fnd&pg=PA11&dq=+Cognitive-behavioral+therapy+for+impulsive+children.+Guilford+Press.&ots=2_0Ln5awcg&sig=gsRsCSrw-lCQEeYzGtb7_eIkE5Q#v=onepage&q=Cognitive-behavioral%20therapy%20for%20impulsive%20children.%20Guilford%20Press.&f=false (accessed on 24 January 2024).
- Nezu, A.M.; Nezu, C. Entrenamiento en solución de problemas. In Manual de Técnicas de Terapia y Modificación de Conducta; Caballo, V.E., Ed.; Siglo Veintinuno: Madrid, Spain, 2020; pp. 527–554. [Google Scholar]
- Santacreu, J. El entrenamiento en autoinstrucciones. In Manual de Técnicas de Terapia y Modificación de Conducta; Caballo, V.E., Ed.; Siglo Veintinuno: Madrid, Spain, 2020; pp. 607–626. [Google Scholar]
- Crick, N.R.; Dodge, K.A. A review and reformulation of social information-processing mechanisms in children’s social adjustment. Psychol. Bull. 1994, 151, 74. [Google Scholar] [CrossRef]
- Dodge, K.A. Social-Cognitive Mechanisms in the Development of Conduct Disorder and Depression. Annu. Rev. Psychol. 1993, 44, 559–584. [Google Scholar] [CrossRef] [PubMed]
- Fritz, A.; Ehlert, A.; Balzer, L. Development of mathematical concepts as basis for an elaborated mathematical understanding. S. Afr. J. Child. Educ. 2013, 3, 38–67. [Google Scholar]
- Freitas, F.R.; Herzog, M.; Haase, V.G.; Fritz, A. Compreensão conceitual do número no diagnóstico e intervenção para as dificuldades de aprendizagem na aritmética. In Diferenças Individuais, Família, Currículo e Intervenções; Chapter Pedagogia do Sucesso; Ampla: Belo Horizonte, Brazil, 2022. [Google Scholar]
- Ziv, Y.; Hadad, B.S.; Khateeb, Y. Social Information Processing in Preschool Children Diagnosed with Autism Spectrum Disorder. J. Autism Dev. Disord. 2013, 44, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.W.; Franklin, S.; Crerar, A. Neuropsychology and the remediation of disorders of spoken language. Hove (UK): Erl-baumn. In Cognitive Neuropsychology and Cognitive Rehabilitation; Lawrence Erlbaum Associates, Inc.: Mahwah, NJ, USA, 1994; pp. 287–315. [Google Scholar]
- Friedmann, N.; Coltheart, M. Types of Development Dyslexia; Bar-On, A., Ravid, D., Dattner, E., Eds.; Macquarie University: Berlin, Germany; De Gruyter: Boston, MA, USA, 2018; pp. 721–752. Available online: https://researchers.mq.edu.au/en/publications/types-of-development-dyslexia (accessed on 24 January 2024).
- Moura, R.; Haase, V.G.; Lopes-Silva, J.B.; Batista, L.T.; de Freitas, F.R.; Bahnmuller, J.; Moeller, K. Reading and Writing Words and Numbers: Similarities, Differences, and Implications. Loughborough University. 2021. Available online: https://www.degruyter.com/document/doi/10.1515/9783110661941-015/html (accessed on 24 January 2024).
- Temple, C. Developmental Cognitive Neuropsychology; Psychology Press: Hove, UK, 1997. [Google Scholar]
- Dawes, E.; Leitão, S.; Claessen, M.; Kane, R. A randomized controlled trial of an oral inferential comprehension intervention for young children with developmental language disorder. Child Lang. Teach. Ther. 2018, 35, 39–54. [Google Scholar] [CrossRef]
- Hessling, A.; Schuele, C.M. Individualized Narrative Intervention for School-Age Children with Specific Language Impairment. Lang. Speech, Hearth Serv. Sch. 2020, 51, 687–705. [Google Scholar] [CrossRef] [PubMed]
- Saywitz, K.J.; Snyder, L. Narrative elaboration: Test of a new procedure for interviewing children. J. Consult. Clin. Psychol. 1996, 64, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Spencer, T.D.; Kajian, M.; Petersen, D.B.; Bilyk, N. Effects of an Individualized Narrative Intervention on Children’s Storytelling and Comprehension Skills. J. Early Interv. 2013, 35, 243–269. [Google Scholar] [CrossRef]
- Trojano, L.; Grossi, D. ‘Pure’ Constructional Apraxia—A Cognitive Analysis of a Single Case. Behav. Neurol. 1998, 11, 43–49. [Google Scholar] [CrossRef]
- Roncato, S.; Sartori, G.; Masterson, J.; Rumiati, R. Constructional apraxia: An information processing analysis. Cogn. Neuropsychol. 1987, 4, 113–129. [Google Scholar] [CrossRef]
- Van Sommers, P. Drawing and Cognition: Descriptive and Experimental Studies of Graphic Production Processes; Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Van Sommers, P.V. A system for drawing and drawing-related neuropsychology. Cogn. Neuropsychol. 1989, 6, 117–164. [Google Scholar] [CrossRef]
- Vincent, L.B.; Openden, D.; Gentry, J.A.; Long, L.A.; Matthews, N.L. Promoting Social Learning at Recess for Children with ASD and Related Social Challenges. Behav. Anal. Pract. 2017, 11, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Ketcheson, L.; Hauck, J.; Ulrich, D. The effects of an early motor skill intervention on motor skills, levels of physical activity, and socialization in young children with autism spectrum disorder: A pilot study. Autism 2017, 21, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Ke, F.; Moon, J. Virtual collaborative gaming as social skills training for high-functioning autistic children. Br. J. Educ. Technol. 2018, 49, 728–741. [Google Scholar] [CrossRef]
- Prince, J. Implementation of the Power Card Strategy to Increase Appropriate Social Commenting of Children with autism Spectrum Disorder during Game Play. Master Dissertation, Temple University, Philadelphia, PA, USA, 2018. [Google Scholar]
- Tsai, W.-T.; Lee, I.-J.; Chen, C.-H. Inclusion of third-person perspective in CAVE-like immersive 3D virtual reality role-playing games for social reciprocity training of children with an autism spectrum disorder. Univers. Access Inf. Soc. 2020, 20, 375–389. [Google Scholar] [CrossRef]
- Willingham, D. Why Don’t Students Like School? A Cognitive Scientist Answers Questions about How the Mind Works and What It Means for the Classroom; Wiley: New York, NY, USA, 2021. [Google Scholar]
- Barrouillet, P.; Camos, V.; Perruchet, P.; Seron, X. ADAPT: A Developmental, Asemantic, and Procedural Model for Transcoding from Verbal to Arabic Numerals. Psychol. Rev. 2004, 111, 368–394. [Google Scholar] [CrossRef]
- Rosenthal, M.; Wallace, G.L.; Lawson, R.; Wills, M.C.; Dixon, E.; Yerys, B.E.; Kenworthy, L. Impairments in real-world executive function increase from childhood to adolescence in autism spectrum disorders. Neuropsychology 2013, 27, 13–18. [Google Scholar] [CrossRef]
- Debodinance, E.; Maljaars, J.; Noens, I.; Noortgate, W.V.D. Interventions for toddlers with autism spectrum disorder: A meta-analysis of single-subject experimental studies. Res. Autism Spectr. Disord. 2017, 36, 79–92. [Google Scholar] [CrossRef]
- Franz, L.; Goodwin, C.D.; Rieder, A.; Matheis, M.; Damiano, D.L. Early intervention for very young children with or at high likelihood for autism spectrum disorder: An overview of reviews. Dev. Med. Child Neurol. 2022, 64, 1063–1076. [Google Scholar] [CrossRef]
- Morris, E.K. A case study in the misrepresentation of applied behavior analysis in autism: The gernsbacher lectures. Behav. Anal. 2009, 32, 205–240. [Google Scholar] [CrossRef]
- Paynter, J.; Sulek, R.; Westerveld, M. The Importance of Evidence Based Practices and Autism. In Handbook of Autism and Pervasive Developmental Disorder; Springer: Berlin/Heidelberg, Germany, 2022; pp. 579–598. [Google Scholar]
- Lovaas, O.I. Behavioral treatment and normal educational and intellectual functioning in young autistic children. J. Consult. Clin. Psychol. 1987, 55, 3–9. [Google Scholar] [CrossRef]
- Lovaas, O.I.; Koegel, R.; Simmons, J.Q.; Long, J.S. Some generalization and follow-up measures on autistic children in behavior therapy1. J. Appl. Behav. Anal. 1973, 6, 131–165. [Google Scholar] [CrossRef] [PubMed]
- Baum, W.M. Compreender o Behaviorismo—3.ed.: Comportamento, Cultura e Evolução; Artmed Editora: Belo Horizonte, Brazil, 2018; Available online: https://books.google.com.br/books?hl=pt-BR&lr=&id=dIxyDwAAQBAJ&oi=fnd&pg=PT6&dq=Compreender+o+Behaviorismo:+Com (accessed on 24 January 2024).
- Cannon, J.; O’Brien, A.M.; Bungert, L.; Sinha, P. Prediction in Autism Spectrum Disorder: A Systematic Review of Empirical Evidence. Autism Res. 2021, 14, 604–630. [Google Scholar] [CrossRef] [PubMed]
- Eigsti, I.-M. A Review of Embodiment in Autism Spectrum Disorders. Front. Psychol. 2013, 4, 224. [Google Scholar] [CrossRef] [PubMed]
- Hellendoorn, A.; Wijnroks, L.; Leseman, P.P.M. Unraveling the nature of autism: Finding order amid change. Front. Psychol. 2015, 6, 359. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.; Kiebel, S.; Friston, K.; Kiebel, S. Predictive coding under the free-energy principle. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.J.; Brumback, A.C. Rethinking Stereotypies in Autism. Semin. Pediatr. Neurol. 2021, 38, 100897. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, P.; Giallongo, L.; Milintenda, G.; Cannarozzo, M. Autism, autistic traits and creativity: A systematic review and meta-analysis. Cogn. Process. 2021, 22, 1–36, Erratum in Cogn. Process. 2021, 22, 733.. [Google Scholar] [CrossRef]
- Wu, X.; Deng, H.; Jian, S.; Chen, H.; Li, Q.; Gong, R.; Wu, J. Global trends and hotspots in the digital therapeutics of autism spectrum disorders: A bibliometric analysis from 2002 to 2022. Front. Psychiatry 2023, 14, 1126404. [Google Scholar] [CrossRef]
- Annelise, A.J.; Costa, A.J.; Silva, J.B.L.; Chagas, P.P.; Krinzinger, H.; Lonneman, J.; Willmes, K.; Wood, G.; Haase, V.G. A hand full of numbers: A role for offloading in arithmetics learning? Front. Psychol. 2011, 2, 368. [Google Scholar] [CrossRef] [PubMed]
- Batstra, L.; Neeleman, J.; Hadders-Algra, M. The neurology of learning and behavioural problems in pre-adolescent children. Acta Psychiatr. Scand. 2003, 108, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Mundy, P. A review of joint attention and social-cognitive brain systems in typical development and autism spectrum disorder. Eur. J. Neurosci. 2018, 47, 497–514. [Google Scholar] [CrossRef] [PubMed]
- Dawson, G.; Toth, K.; Abbott, R.; Osterling, J.; Munson, J.; Estes, A.; Liaw, J. Early Social Attention Impairments in Autism: Social Orienting, Joint Attention, and Attention to Distress. Dev. Psychol. 2004, 40, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Dawson, G.; Munson, J.; Estes, A.; Osterling, J.; McPartland, J.; Toth, K.; Carver, L.; Abbott, R. Neurocognitive Function and Joint Attention Ability in Young Children with Autism Spectrum Disorder Versus Developmental Delay. Child Dev. 2002, 73, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Özçalışkan, S.; Goldin-Meadow, S. Gesture is at the cutting edge of early language development. Cognition 2005, 96, B101–B113. [Google Scholar] [CrossRef] [PubMed]
- Uzgiris, I.C. Two Functions of Imitation During Infancy. Int. J. Behav. Dev. 1981, 4, 1–12. [Google Scholar] [CrossRef]
- Bailes, A.F.; Greve, K.; Schmitt, L.C. Changes in Two Children with Cerebral Palsy After Intensive Suit Therapy: A Case Report. Pediatr. Phys. Ther. 2010, 22, 76–85. [Google Scholar] [CrossRef]
- Purnamasari, N.; Bachtiar, F.; Puspitha, R.A. The effectiveness of motor-cognitive dual-task training in reducing risk falls on elderly. Enfermería Clínica 2020, 30, 317–321. [Google Scholar] [CrossRef]
- Ko, M.-S.; Lee, J.-A.; Kang, S.-Y.; Jeon, H.-S. Effect of Adeli suit treatment on gait in a child with cerebral palsy: A single-subject report. Physiother. Theory Pract. 2014, 31, 275–282. [Google Scholar] [CrossRef]
- Matthews, M.J.; Watson, M.; Richardson, B. Effects of Dynamic Elastomeric Fabric Orthoses on Children with Cerebral Palsy. Prosthetics Orthot. Int. 2009, 33, 339–347. [Google Scholar] [CrossRef]
- Bar-Haim, S.; Harries, N.; Belokopytov, M.; Frank, A.; Copeliovitch, L.; Kaplanski, J.; Lahat, E. Comparison of efficacy of Adeli suit and neurodevelopmental treatments in children with cerebral palsy. Dev. Med. Child Neurol. 2006, 48, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Almeida, K.M.; Fonseca, S.T.; Figueiredo, P.R.; Aquino, A.A.; Mancini, M.C. Effects of interventions with therapeutic suits (clothing) on impairments and functional limitations of children with cerebral palsy: A systematic review. Braz. J. Phys. Ther. 2017, 21, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Levin, S.M. The tensegrity-truss as a model for spine mechanics: Biotensegrity. J. Mech. Med. Biol. 2002, 02, 375–388. [Google Scholar] [CrossRef]
- Ingber, D.E. Tensegrity and mechanotransduction. J. Bodyw. Mov. Ther. 2008, 12, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Turvey, M.T.; Fonseca, S.T. The Medium of Haptic Perception: A Tensegrity Hypothesis. J. Mot. Behav. 2014, 46, 143–187. [Google Scholar] [CrossRef] [PubMed]
- Busquet-Vanderheyden, M. As Cadeias Fisiológicas—A Cadeia Visceral: Tórax, Garganta e Boca: Descrição e Tratamento; Método Busquet: Curitiba, Brazil, 2020; Available online: https://books.google.com.br/books?hl=pt-BR&lr=&id=nsjgDwAAQBAJ&oi=fnd&pg=PT4&dq=as+cadeias+fisiol (accessed on 24 January 2024).
- Myers, T.W. Anatomy Trains; Churchill Livingstone: Oxford, UK, 2001; 332p. [Google Scholar]
- Stecco, C.; Porzionato, A.; Macchi, V.; Tiengo, C.; Parenti, A.; Aldegheri, R.; Delmas, V.; De Caro, R. Histological characteristics of the deep fascia of the upper limb. Italy J. Anat. Embryol. 2006, 111, 105–110. [Google Scholar]
- Stecco, A.; Macchi, V.; Stecco, C.; Porzionato, A.; Day, J.A.; Delmas, V.; De Caro, R. Anatomical study of myofascial continuity in the anterior region of the upper limb. J. Bodyw. Mov. Ther. 2009, 13, 53–62. [Google Scholar] [CrossRef]
- Schleip, R.; Müller, D.G. Training principles for fascial connective tissues: Scientific foundation and suggested practical applications. J. Bodyw. Mov. Ther. 2013, 17, 103–115. [Google Scholar] [CrossRef]
- Schleip, R.; Bayer, J. Fascial Fitness: How to Be Vital, Elastic and Dynamic in Everyday Life and Sport; Lotus Pub: Lotus, CA, USA, 2017; 224p. [Google Scholar]
- Fede, C.; Petrelli, L.; Guidolin, D.; Porzionato, A.; Pirri, C.; Fan, C.; De Caro, R.; Stecco, C. Evidence of a new hidden neural network into deep fasciae. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Stecco, C. Functional Atlas of the Human Fascial System; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014; Available online: https://books.google.com.br/books?hl=pt-BR&lr=&id=8eDTBQAAQBAJ&oi=fnd&pg=PP1&dq=+Functional+atlas+of+the+human+fascial+system.&ots=HEfpTwL99-&sig=6-uLwccpXaN-sFouHVsFqigku_U#v=onepage&q=Functional%20atlas%20of%20the%20human%20fasc (accessed on 24 January 2024).
- Fede, C.; Petrelli, L.; Pirri, C.; Neuhuber, W.; Tiengo, C.; Biz, C.; De Caro, R.; Schleip, R.; Stecco, C. Innervation of human superficial fascia. Front. Neuroanat. 2022, 16, 981426. [Google Scholar] [CrossRef]
- Stecco, C.; Sfriso, M.M.; Porzionato, A.; Rambaldo, A.; Albertin, G.; Macchi, V.; De Caro, R. Microscopic anatomy of the visceral fasciae. J. Anat. 2017, 231, 121–128. [Google Scholar] [CrossRef]
- Mosconi, M.W.; Mohanty, S.; Greene, R.K.; Cook, E.H.; Vaillancourt, D.E.; Sweeney, J.A. Feedforward and Feedback Motor Control Abnormalities Implicate Cerebellar Dysfunctions in Autism Spectrum Disorder. J. Neurosci. 2015, 35, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Serdarevic, F.; Ghassabian, A.; van Batenburg-Eddes, T.; White, T.; Blanken, L.M.E.; Jaddoe, V.W.V.; Verhulst, F.C.; Tiemeier, H. Infant muscle tone and childhood autistic traits: A longitudinal study in the general population. Autism Res. 2017, 10, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, T.A.H.; Józsa, L.; Kannus, P.; Järvinen, T.L.N.; Järvinen, M. Organization and distribution of intramuscular connective tissue in normal and immobilized skeletal muscles. An immunohistochemical, polarization and scanning electron microscopic study. J. Muscle Res. Cell Motil. 2002, 23, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Reeves, N.D.; Narici, M.V.; Maganaris, C.N. Myotendinous plasticity to ageing and resistance exercise in humans. Exp. Physiol. 2006, 91, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Proske, U.; Gandevia, S.C. The kinaesthetic senses. J. Physiol. 2009, 587, 4139–4146. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, A.; Slack, H.G.B. The metabolism of collagen from liver, bone, skin and tendon in the normal rat. Biochem. J. 1953, 53, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Lei nº9.656, de 3 de Junho de 1998. Dispõe Sobre osPlanos e Seguros Privados de Assistência à Saúde. Diário Oficial da União. 4 June 1998. Available online: https://www.planalto.gov.br/ccivil_03/leis/l9656.htm (accessed on 29 January 2024).
- Law, M.; Baptiste, S.; Carswell, A.; McColl, M.A.; Polatajko, H.; Pollock, N. Canadian Occupational Performance Measure, 4th ed.; CAOT Publications ACE: Ottowa, ON, Canada, 2005. [Google Scholar]
- Constantino, J.N.; Gruber, C.P. Social Responsiveness Scale: SRS-2; Pearson: Sydney, Australia, 2012. [Google Scholar]
- Borges, L.; Hauck-Filho, N. Escala de Responsividade Social (SRS-2); Pearson: Sydney, Australia, 2020. [Google Scholar]
- Di Rezze, B.; Rosenbaum, P.; Zwaigenbaum, L.; Hidecker, M.J.C.; Stratford, P.; Cousins, M.; Camden, C.; Law, M. Developing a classification system of social communication functioning of preschool children with autism spectrum disorder. Dev. Med. Child Neurol. 2016, 58, 942–948. [Google Scholar] [CrossRef]
- Angelini, A.L.; Alves, I.C.B.; Custdio, E.M.; Duarte, W.F.; Duarte, J.L.M. Manual: Matrizes Progressivas Coloridas de Raven; Centro Editor de Testes e Pesquisas em Psicologia: São Paulo, Brazil, 1999. [Google Scholar]
- Raven, J.; Raven, J.C.; Court, J.H. Matrizes Progressivas Coloridas de Raven: CPM; Pearson: São Paulo, Brazil, 2018; 142p. [Google Scholar]
- Goodman, R. The Strengths and Difficulties Questionnaire: A Research Note. J. Child Psychol. Psychiatry 1997, 38, 581–586. [Google Scholar] [CrossRef]
- Fleitlich, B.; Cortázar, P.G.; Goodman, R. Questionário de Capacidades e Dificuldades (SDQ). Infanto Rev. Neuropsiquiatr. Infanc. Adolesc 2000, 44–50. [Google Scholar]
- Haley, S.M.; Coster, W.J.; Dumas, H.M.; Fragala-Pinkham, M.A.; Moed, R. PEDI-CAT: Development, Standardization and Administration Manual; Boston University: Boston, MA, USA, 2012. [Google Scholar]
- Franjoine, M.R.; Gunther, J.S.; Taylor, M.J. Pediatric balance scale: A modified version of the berg balance scale for the school-age child with mild to moderate motor impairment. Pediatr. Phys. Ther. Off. Publ. Sect. Pediatr. Am. Phys. Ther. Assoc. 2003, 15, 114–128. [Google Scholar] [CrossRef]
- Paillard, T.; Noé, F. Techniques and Methods for Testing the Postural Function in Healthy and Pathological Subjects. BioMed Res. Int. 2015, 2015, 891390. [Google Scholar] [CrossRef]
- Martín-Díaz, P.; Carratalá-Tejada, M.; Molina-Rueda, F.; Cuesta-Gómez, A. Reliability and agreement of the timed up and go test in children and teenagers with autism spectrum disorder. Eur. J. Pediatr. 2023, 182, 3577–3585. [Google Scholar] [CrossRef]
- Ulrich, D.A.; Soppelsa, R.; Albaret, J.M. Test of Gross Motor Development Examiner’s Manual; Pro-Ed, Inc.: Austin, TX, USA, 2000. [Google Scholar]
- Poole, J.L.; Burtner, P.A.; Torres, T.A.; McMullen, C.K.; Markham, A.; Marcum, M.L.; Anderson, J.B.; Qualls, C. Measuring Dexterity in Children Using the Nine-hole Peg Test. J. Hand Ther. 2005, 18, 348–351. [Google Scholar] [CrossRef] [PubMed]



| Component | Description | References |
|---|---|---|
| Evidence-based interdisciplinary practice | MIG is implemented in close collaboration among professionals from multiple specialties, making joint decisions with the family, and formulating a common intervention plan with measurable objectives and periodic monitoring of the achievement and reformulation of goals. | Atun-Einy et al., 2013 [57]; Bowman et al., 2021 [105]; Hume et al., 2021 [2]; Lustrea, 2019 [106]; Steinbrenner et al., 2020 [2]. |
| Biopsychosocial approach | MIG uses interventions at the WHO’s five levels of biopsychosocial impacts. | Bolte et al., 2019 [43]; Schiariti et al., 2018 [44]; Silva et al., 2013 [45]; WHO, 2001 [46]. |
| Family-centered practice and social validity | MIG implements a decision-making process in close collaboration with the family. | Almasri, An and Palisano, 2018 [127]; An et al., 2017 [128]; An et al., 2019. [129]. |
| Bottom-up therapeutic approaches | MIG uses a flexible therapeutic suit based on myofascial trails with the aim of stabilizing postural tone, thus freeing up processing resources for socio-cognitive learning. | Borji et al., 2023 [130]; Jabouille et al., 2023 [131]; Leone et al., 2017 [132]; Reilly et al., 2008 [133]; Simmons et al., 2019 [134]; Torres and Denisova, 2016 [135]; Van Biesen et al., 2018 [136]; Wolpert et al., 2003 [137]. |
| Top-down therapeutic approaches | MIG uses explicit instruction and development of cognitive schemas, imagery and sociomotor synchronization to promote social, emotional and cognitive skills, providing structure for action and decision making. | Ahar and Ghadiri, 2021 [138]; Kalyuga, 2010 [139]; Sweller et al.; 2011 [140]. |
| Intensive and prolonged training | MIG assumes that significant results at the body structure and function level, involving tissue remodeling and activity-dependent neuroplasticity, require intense and prolonged intervention. | Bleyenheuft, et al., 2015 [141]; Figueiredo et al., 2020 [142]; Hodgson et al., 2022 [143]; Jackman et al., 2021 [144]; Sampaio et al., 2021 [145]. |
| Naturalistic learning environment | MIG assumes that interventions in the child’s living environments or similar environments promote social learning and generalization. | Bruinsma et al., 2019 [8]. |
| Concrete and playful materials and activities | MIG assumes that concrete and playful materials and activities promote motivation and engagement in interventions. | Bruinsma et al., 2019 [8]. |
| Parent-mediated interventions | MIG assumes that parent-mediated interventions facilitate generalization in the child’s living environment and reduce costs. | Ingersoll and Dvortcsak, 2019 [146]. |
| Manualization | MIG operationalizes its procedures through teaching materials provided to professionals. | Odom et al., 2010 [122] |
| Supervision and continued education | MIG offers online supervision to professionals and continues training through regularly held courses and conferences. | Odom et al., 2010 [122] |
| Care management through app | MIG uses an mobile app (MIG+) especially developed to manage interdisciplinary healthcare efficiently. MIG is based on the assumption that the agility and integration of information transmitted by the family and the technical team positively impact healthcare. | Fenning et al., 2022 [147]. |
| Purpose | Unit | Description | Theoretical–Methodological Foundation | References |
|---|---|---|---|---|
| Logistics | Therapeutic suit (MIG Flex) mounting space | Spaces to store and assemble the flexible therapeutic suit (MIG Flex). | Promoting postural stability, adequate regulation of muscle tone and quality of movements performed. | Loffi, 2019 [149]; Stecco et al., 2023 [150]. |
| Environmental reactivity workup | Stimulus–control, controlled instability, and fixation rooms | Spaces to work on reducing distractors and focus and attention activities to achieve partial independence. | Graded exposure and desensitization for hypersensitivity and attention training | Hadad and Schwartz, 2019 [151]; Williams et al., 2021 [152]. |
| Infant intervention unit | Baby room | Early intervention | Early intervention | Damiano and Forssberg, 2021 [153]; Grzadzinski et al., 2018 [69]. |
| Naturalistic learning units | Home | Daily living activities and autobiography | The child works on daily life skills (such as eating, dressing, personal hygiene, using the bathroom, brushing teeth and taking a shower, for example) and, at the same time, prepares their autobiography. Daily living skills training is based on the cognitive–behavioral model of self-instruction in problem solving. Autobiographical elaboration allows the child to incorporate their disabilities as a part of their self and, at the same time, develop a positive self-concept. | Fivush, 2011 [154]; Nelson and Fivush, 2004 [155]; Kendal and Braswell, 1993 [156]; Nezu and Nezu, 2020 [157]; Santacreu, 2020 [158]. |
| Supermarket | Shopping at the supermarket | The cognitive supermarket shopping script allows the child develops linguistic and conceptual skills of categorization and vocabulary, numerical–arithmetic, social and planning skills. | Crick and Dodge, 1994 [159]; Dodge, 1993 [160]; Fritz et al., 2013 [161]; Freitas et al., 2022 [162]; Kendal and Braswell, 1993 [156]; Nezu and Nezu, 2020 [157]; Santacreu, 2020 [158]; Ziv et al., 2014 [163]. | |
| School, Castle of Letters and Numbers | Oral lexical comprehension and expression Reading and writing words and numbers | Oral and written activities related to words and numbers are based on cognitive–neuropsychological models of lexical processing. | Ellis et al., 1994 [164]; Friedmann and Coltheart, 2018 [165]; Moura et al., 2021 [166]; Temple, 1997 [167]. | |
| Castle of Tales | Oral narratives | The child learns to analyze and elaborate upon oral narratives based on the categories of story grammar. Oral narratives constitute an important precursor to reading comprehension. | Dawes et al., 2019 [168]; Hessling and Schuele, 2020 [169]; Saywitz and Snyder, 1996 [170]; Spencer et al., 2013 [171]. | |
| Desenhix (“Drawix”) | Visuospatial, visuoconstructive and graphomotor skills underlying drawing. Figurative representation of the human body. Graphic narratives. | The child learns to use graphic strategies to represent the human body, emotions and social situations created in the form of comics. | Fivush, 2011 [154]; Grossi and Trojano, 1999 [172]; Roncato et al., 1987 [173]; Nelson & Fivush, 2004 [155]; Saywitz and Snyder, 1996 [170]; Van Sommers, 1984 [174]; Van Sommers, 1989, [175]. | |
| Fitness space (sports court) and Social Rules Gym | Basic sports skills and participation in team sports and rule-based social/cooperative games. | Operant conditioning and modeling strategies are used to develop basic sports skills and participation in group sports. | Wang et al., 2022 [4]; Vincent et al., 2018 [176]; Ketcheson et al., 2017 [177]. | |
| Galaxy of the Future | Role-playing game. | Participation skills, group strategies and development of gesture imitation skills are developed through role-playing games. | Ke and Moon, 2018 [178]; Prince, 2018 [179]; Tsai et al., 2021 [180]. |
| Domain | Instrument | Description | Reference |
|---|---|---|---|
| Autism symptoms | Social Responsiveness Scale (SRS-2) | The SRS-2 aims to assess symptoms related to autism spectrum disorder (ASD) as well as to classify them into mild, moderate and severe levels. Its assessment is made globally and specifically through six subcategories of symptoms. These subcategories are social perception, social cognition, social communication, social motivation, restrictive and repetitive patterns and social communication and interaction. | Constantino and Gruber (2012) [232]; Borges and Hauck-Filho (2020) [233] |
| Support level | Autism Classification System of Functioning: Social Communication (ACSF:SC) | ACSF is a five-level descriptive system based on the International Classification of Functioning, Disability and Health (ICF) that provides a standardized way to report a child’s/youth’s social communication abilities in two situations: when they are performing at their best (capacity) and how they usually perform (typical performance). | Di Rezze et al., 2016 [234] |
| General cognitive ability | Raven’s CPM | CPM is a 36-item test used to estimate the nonverbal reasoning of children aged from 6 years to 11 years and 11 months. | Angelini et al., 1999 [235]; Raven, Raven and Court, 2018 [236] |
| Behavioral disorders | Strengths and Difficulties Questionnaire (SDQ) | The SDQ is a brief behavioral screening questionnaire for 2–17-year-olds, and is divided into five subscales: prosocial behavior problems, hyperactivity, emotional, behavioral and relationship problems. | Goodman, 1997 [237]; Fleitlich and cols., 2000 [238] |
| Family needs and priorities | Canadian Occupational Performance Measure (COPM) | The COPM is a client-centered outcome measure to identify and prioritize everyday issues that restrict the participation of individuals. This measure focuses on self-care, leisure and productivity. | Law et al., 2005 [231] |
| Activity and participation | Pediatric Evaluation of Disability Inventory Computer Adaptive Test (PEDI-CAT) | The PEDI-CAT is a computer adaptive caregiver report which measures daily activities, mobility, social/cognitive and responsibility with the aim of identifying functional delay and examining the improvement of an individual child after intervention. | Haley et al., 2012 [239] |
| Motor abilities | Pediatric balance scale (PBS) | PBS is a 14-item, criterion-referenced measure based on the Berg Balance Scale that examines functional balance in the context of everyday tasks in the pediatric population. | Franjoine et al., 2003 [240] |
| Inertial sensor of standing balance (linear and angular oscillations, BaiobitTM) | This is a tool used for measuring postural oscillations of the center of mass (CM) during bipedal balance or single-pedal position, eyes open or closed, to evaluate the patient’s postural control. The main variables measured using this unit are: (a) the area/surface of the ellipse, (b) path length, (c) displacement amplitude and (d) speed. | Paillard and Noé, 2015 [241] | |
| Timed Up and Go (TUG) | A tool used in clinical practice with children and teenagers to assess gait and dynamic balance. | Martín-Díaz et al., 2023 [242] | |
| Test of gross motor development (TGMD-2) | The TGMD-2 is a discriminative, norm-referenced test used to assess the level of competence in the motor skills involving large muscle groups that produce the force needed to move the trunk and upper and lower limbs. | Ulrich, Soppelsa and Albaret, 2000 [243] | |
| Nine-hole peg test (9HPT) | 9HPT is a standardized, quantitative assessment used to measure finger dexterity. | Poole et al., 2005 [244] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loffi, R.G.; Cruz, T.K.F.; Paiva, G.M.; Souto, D.O.; Barreto, S.R.; Santana, P.A.N.; Nascimento, A.A.A.C.; Costa, F.R.M.; Cota, E.B.; Haase, V.G. Theoretical–Methodological Foundations for the Global Integration Method (Método de Integração Global—MIG) in the Treatment of Autism Spectrum Disorder. Children 2024, 11, 191. https://doi.org/10.3390/children11020191
Loffi RG, Cruz TKF, Paiva GM, Souto DO, Barreto SR, Santana PAN, Nascimento AAAC, Costa FRM, Cota EB, Haase VG. Theoretical–Methodological Foundations for the Global Integration Method (Método de Integração Global—MIG) in the Treatment of Autism Spectrum Disorder. Children. 2024; 11(2):191. https://doi.org/10.3390/children11020191
Chicago/Turabian StyleLoffi, Renato Guimarães, Thalita Karla Flores Cruz, Giulia Moreira Paiva, Deisiane Oliveira Souto, Simone Rosa Barreto, Patrícia Aparecida Neves Santana, Amanda Aparecida Alves Cunha Nascimento, Fabiana Rachel Martins Costa, Elisa Braz Cota, and Vitor Geraldi Haase. 2024. "Theoretical–Methodological Foundations for the Global Integration Method (Método de Integração Global—MIG) in the Treatment of Autism Spectrum Disorder" Children 11, no. 2: 191. https://doi.org/10.3390/children11020191