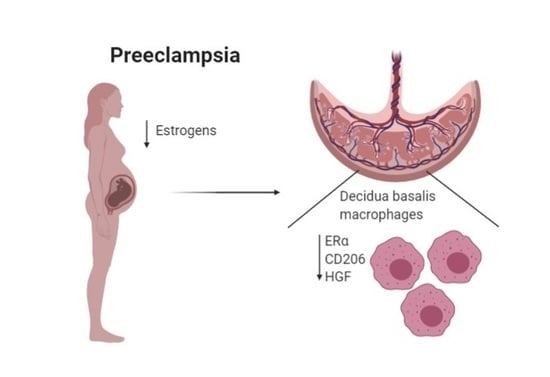
Expression of Estrogen Receptor α by Decidual Macrophages in Preeclampsia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation of Decidual Macrophages
2.3. Flow Cytometry Analysis
2.4. RNA Extraction, Reverse Transcription Reaction, RT-PCR and the Network Analysis
2.5. Western Blot Analysis
2.6. Immunohistochemistry
2.7. Statistical Analysis
3. Results
3.1. Decidual Macrophages in Primary Culture
3.2. Downregulation of ERα and HGF Levels in Decidual Macrophages
3.3. ERα and CD206 Immunostaining in Placental Tissue Sections
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “great Obstetrical Syndromes” are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, S.; Lemoine, E.; Granger, J.; Karumanchi, S.A. Preeclampsia: Pathophysiology, challenges, and perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.J.; Graham, C.H. The role of macrophages in utero-placental interactions during normal and pathological pregnancy. Immunol. Investig. 2008, 37, 535–564. [Google Scholar] [CrossRef]
- Yang, F.; Zheng, Q.; Jin, L. Dynamic function and composition changes of immune cells during normal and pathological pregnancy at the maternal-fetal interface. Front. Immunol. 2019, 10, 2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F.; Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative stress in preeclampsia and placental diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef] [Green Version]
- Amaral, L.M.; Wallace, K.; Owens, M.; LaMarca, B. Pathophysiology and current clinical management of preeclampsia. Curr. Hypertens. Rep. 2017, 19, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibai, B.; Dekker, G.; Kupferminc, M. Pre-Eclampsia. Lancet 2005, 365, 785–799. [Google Scholar] [CrossRef]
- Toniolo, A.; Fadini, G.P.; Tedesco, S.; Cappellari, R.; Vegeto, E.; Maggi, A.; Avogaro, A.; Bolego, C.; Cignarella, A. Alternative activation of human macrophages is rescued by estrogen treatment in vitro and impaired by menopausal status. J. Clin. Endocrinol. Metab. 2015, 100, E50–E58. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.; Hu, Z.; Zeng, K.; Yin, Y.; Zhao, M.; Chen, M.; Chen, Q. The reduction in circulating levels of estrogen and progesterone in women with preeclampsia. Pregnancy Hypertens. 2018, 11, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Bussen, S.; Bussen, D. Influence of the vascular endothelial growth factor on the development of severe pre-eclampsia or HELLP syndrome. Arch. Gynecol. Obstet. 2011, 284, 551–557. [Google Scholar] [CrossRef]
- Gruber, C.J.; Tschugguel, W.; Schneeberger, C.; Huber, J.C. Mechanisms of disease: Production and actions of estrogens. N. Engl. J. Med. 2002, 346, 340–352. [Google Scholar] [CrossRef]
- Fahlén, M.; Zhang, H.; Löfgren, L.; Masironi, B.; Von Schoultz, E.; Von Schoultz, B.; Sahlin, L. Expression of estrogen receptors in relation to hormone levels and the nottingham prognostic index. Anticancer Res. 2016, 36, 2839–2847. [Google Scholar] [PubMed]
- Nilsson, S.; Mäkelä, S.; Treuter, E.; Tujague, M.; Thomsen, J.; Andersson, G.; Enmark, E.; Pettersson, K.; Warner, M.; Gustafsson, J.Å. Mechanisms of estrogen action. Physiol. Rev. 2001, 81, 1535–1565. [Google Scholar] [CrossRef] [PubMed]
- Jobe, S.O.; Tyler, C.T.; Magness, R.R. Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertension 2013, 61, 480–487. [Google Scholar] [CrossRef]
- Hertig, A.; Liere, P.; Chabbert-Buffet, N.; Fort, J.; Pianos, A.; Eychenne, B.; Cambourg, A.; Schumacher, M.; Berkane, N.; Lefevre, G.; et al. Steroid profiling in preeclamptic women: Evidence for aromatase deficiency. Am. J. Obstet. Gynecol. 2010, 203, 477.e1–477.e9. [Google Scholar] [CrossRef]
- Dou, C.; Ding, N.; Zhao, C.; Hou, T.; Kang, F.; Cao, Z.; Liu, C.; Bai, Y.; Dai, Q.; Ma, Q.; et al. Estrogen deficiency-mediated M2 macrophage osteoclastogenesis contributes to M1/M2 ratio alteration in ovariectomized osteoporotic mice. J. Bone Miner. Res. 2018, 33, 899–908. [Google Scholar] [CrossRef] [Green Version]
- Sul, O.J.; Hyun, H.J.; Rajasekaran, M.; Suh, J.H.; Choi, H.S. Estrogen enhances browning in adipose tissue by M2 macrophage polarization via heme oxygenase-1. J. Cell. Physiol. 2020, 236. [Google Scholar] [CrossRef]
- Campbell, L.; Emmerson, E.; Williams, H.; Saville, C.R.; Krust, A.; Chambon, P.; Mace, K.A.; Hardman, M.J. Estrogen receptor-alpha promotes alternative macrophage activation during cutaneous repair. J. Investig. Dermatol. 2014, 134, 2447–2457. [Google Scholar] [CrossRef] [Green Version]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, J.; Qi, Q.R.; Li, Y.; Day, R.; Makhoul, J.; Magness, R.R.; Chen, D.B. Estrogen receptors and estrogen-induced uterine vasodilation in pregnancy. Int. J. Mol. Sci. 2020, 21, 4349. [Google Scholar] [CrossRef]
- Heikkinen, J.; Möttönen, M.; Komi, J.; Alanen, A.; Lassila, O. Phenotypic characterization of human decidual macrophages. Clin. Exp. Immunol. 2003, 131, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Simon, P. Q-Gene: Processing quantitative real-time RT-PCR data. Bioinformatics 2003, 19, 1439–1440. [Google Scholar] [CrossRef] [Green Version]
- Amaral, L.M.; Cunningham, M.W.; Cornelius, D.C.; LaMarca, B. Preeclampsia: Long-Term consequences for vascular health. Vasc. Health Risk Manag. 2015, 11, 403–415. [Google Scholar] [PubMed] [Green Version]
- Nobakht, M.; Gh, B.F. Application of metabolomics to preeclampsia diagnosis. Syst. Biol. Reprod. Med. 2018, 64, 324–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillay, P.; Moodley, K.; Moodley, J.; Mackraj, I. Placenta-Derived exosomes: Potential biomarkers of preeclampsia. Int. J. Nanomed. 2017, 12, 8009–8023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houser, B.L. Decidual macrophages and their roles at the maternal-fetal interface. Yale J. Biol. Med. 2012, 85, 105–118. [Google Scholar]
- Milosevic-Stevanovic, J.; Krstic, M.; Radovic-Janosevic, D.; Popovic, J.; Tasic, M.; Stojnev, S. Number of decidual natural killer cells & macrophages in pre-eclampsia. Indian J. Med. Res. 2016, 144, 823–830. [Google Scholar]
- Al-Khafaji, L.A.; Al-Yawer, M.A. Localization and counting of CD68-labelled macrophages in placentas of normal and preeclamptic women. In Proceedings of the AIP Conference, Baghdad, Iraq, 21 September 2017; Volume 1888, p. 20011. [Google Scholar]
- Cupurdija, K.; Azzola, D.; Hainz, U.; Gratchev, A.; Heitger, A.; Takikawa, O.; Goerdt, S.; Wintersteiger, R.; Dohr, G.; Sedlmayr, P. Macrophages of human first trimester decidua express markers associated to alternative activation. Am. J. Reprod. Immunol. 2004, 51, 117–122. [Google Scholar] [CrossRef]
- Gustafsson, C.; Mjösberg, J.; Matussek, A.; Geffers, R.; Matthiesen, L.; Berg, G.; Sharma, S.; Buer, J.; Ernerudh, J. Gene expression profiling of human decidual macrophages: Evidence for immunosuppressive phenotype. PLoS ONE 2008, 3, e2078. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.; Jenmalm, M.C.; Matussek, A.; Geffers, R.; Berg, G.; Ernerudh, J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J. Immunol. 2011, 187, 3671–3682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Ye, Y.; Zhang, J.; Ruan, C.-C.; Gao, P.-J. Immune imbalance is associated with the development of preeclampsia. Medicine (Baltim.) 2019, 98, e15080. [Google Scholar] [CrossRef]
- Vishnyakova, P.; Elchaninov, A.; Fatkhudinov, T.; Sukhikh, G. Role of the monocyte-macrophage system in normal pregnancy and preeclampsia. Int. J. Mol. Sci. 2019, 20, 3695. [Google Scholar] [CrossRef] [Green Version]
- Schonkeren, D.; Van Der Hoorn, M.L.; Khedoe, P.; Swings, G.; Van Beelen, E.; Claas, F.; Van Kooten, C.; De Heer, E.; Scherjon, S. Differential distribution and phenotype of decidual macrophages in preeclamptic versus control pregnancies. Am. J. Pathol. 2011, 178, 709–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheeler, K.C.; Jena, M.K.; Pradhan, B.S.; Nayak, N.; Das, S.; Hsu, C.D.; Wheeler, D.S.; Chen, K.; Nayak, N.R. VEGF may contribute to macrophage recruitment and M2 polarization in the decidua. PLoS ONE 2018, 13, e0191040. [Google Scholar] [CrossRef] [PubMed]
- Imamura, R.; Matsumoto, K. Hepatocyte growth factor in physiology and infectious diseases. Cytokine 2017, 98, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staud, F.; Karahoda, R. Trophoblast: The central unit of fetal growth, protection and programming. Int. J. Biochem. Cell Biol. 2018, 105, 35–40. [Google Scholar] [CrossRef]
- Furugori, K.; Kurauchi, O.; Itakura, A.; Kanou, Y.; Murata, Y.; Mizutani, S.; Seo, H.; Tomoda, Y.; Nakamura, T. Levels of hepatocyte growth factor and its messenger ribonucleic acid in uncomplicated pregnancies and those complicated by preeclampsia. J. Clin. Endocrinol. Metab. 1997, 82, 2726–2730. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Shono, J.I.; Suzuki, T.; Sawano, S.; Anderson, J.E.; Do, M.K.Q.; Ohtsubo, H.; Mizunoya, W.; Sato, Y.; Nakamura, M.; et al. Implication of anti-inflammatory macrophages in regenerative moto-neuritogenesis: Promotion of myoblast migration and neural chemorepellent semaphorin 3A expression in injured muscle. Int. J. Biochem. Cell Biol. 2014, 54, 272–285. [Google Scholar] [CrossRef]
- Zhang, Y.H.; He, M.; Wang, Y.; Liao, A.H. Modulators of the balance between M1 and M2 macrophages during pregnancy. Front. Immunol. 2017, 8, 120. [Google Scholar] [CrossRef] [Green Version]
- Berkane, N.; Liere, P.; Oudinet, J.-P.; Hertig, A.; Lefèvre, G.; Pluchino, N.; Schumacher, M.; Chabbert-Buffet, N. From pregnancy to preeclampsia: A key role for estrogens. Endocr. Rev. 2017, 38, 123–144. [Google Scholar] [CrossRef]
- Reshef, T.; Taylor, H.; Burney, R.; Mooney, S.; Giudice, L. Endocrinology of Pregnancy—Endotext—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK278962/ (accessed on 3 September 2020).
- Gratchev, A. TGF-β signalling in tumour associated macrophages. Immunobiology 2017, 222, 75–81. [Google Scholar] [CrossRef]
- Riccardi, C.; Levi-Schaffer, F.; Tiligada, E. (Eds.) Immunopharmacology and Inflammation; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-77657-6. [Google Scholar]
- Zhang, F.; Wang, H.; Wang, X.; Jiang, G.; Liu, H.; Zhang, G.; Wang, H.; Fang, R.; Bu, X.; Cai, S.; et al. TGF-β induces M2-like macrophage polarization via SNAILmediated suppression of a pro-inflammatory phenotype. Oncotarget 2016, 7, 52294–52306. [Google Scholar] [CrossRef] [Green Version]
- Mandalà, M. Influence of estrogens on uterine vascular adaptation in normal and preeclamptic pregnancies. Int. J. Mol. Sci. 2020, 21, 2592. [Google Scholar] [CrossRef] [Green Version]
- Ihionkhan, C.E.; Chambliss, K.L.; Gibson, L.L.; Hahner, L.D.; Mendelsohn, M.E.; Shaul, P.W. Estrogen causes dynamic alterations in endothelial estrogen receptor expression. Circ. Res. 2002, 91, 814–820. [Google Scholar] [CrossRef] [Green Version]
- Murphy, A.J.; Guyre, P.M.; Wira, C.R.; Pioli, P.A. Estradiol regulates expression of estrogen receptor ERα46 in human macrophages. PLoS ONE 2009, 4, e5539. [Google Scholar] [CrossRef] [Green Version]
- Robertson, J.A.; Farnell, Y.; Lindahl, L.S.; Ing, N.H. Estradiol up-regulates estrogen receptor messenger ribonucleic acid in endometrial carcinoma (Ishikawa) cells by stabilizing the message. J. Mol. Endocrinol. 2002, 29, 125–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, G.; Zhu, X.; Guo, C.; Yang, Y.; Han, T.; Chen, L.; Yin, W.; Gao, P.; Zhang, H.; Geng, J.; et al. Differential expression of estradiol and estrogen receptor α in severe preeclamptic pregnancies compared with normal pregnancies. Mol. Med. Rep. 2013, 7, 981–985. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Zhou, L.; Mao, X.; Tong, C.; Chen, X.; Zhao, D.; Baker, P.N.; Xia, Y.; Zhang, H. Association of a reduction of G-Protein coupled receptor 30 expression and the pathogenesis of preeclampsia. Mol. Med. Rep. 2017, 16, 5997–6003. [Google Scholar] [CrossRef] [Green Version]
- Molvarec, A.; Vér, Á.; Fekete, A.; Rosta, K.; Derzbach, L.; Derzsy, Z.; Karádi, I.; Rigó, J. Association between estrogen receptor α (ESR1) gene polymorphisms and severe preeclampsia. Hypertens. Res. 2007, 30, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Phiel, K.L.; Henderson, R.A.; Adelman, S.J.; Elloso, M.M. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol. Lett. 2005, 97, 107–113. [Google Scholar] [CrossRef]
- Laffont, S.; Seillet, C.; Guéry, J.C. Estrogen receptor-dependent regulation of dendritic cell development and function. Front. Immunol. 2017, 8, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mascanfroni, I.D.; Yeste, A.; Vieira, S.M.; Burns, E.J.; Patel, B.; Sloma, I.; Wu, Y.; Mayo, L.; Ben-Hamo, R.; Efroni, S.; et al. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat. Immunol. 2013, 14, 1054–1063. [Google Scholar] [CrossRef]
- Liang, Y.; Xie, H.; Wu, J.; Liu, D.; Yao, S. Villainous role of estrogen in macrophage-nerve interaction in endometriosis. Reprod. Biol. Endocrinol. 2018, 16, 122. [Google Scholar] [CrossRef] [Green Version]
- Khan, K.N.; Kitajima, M.; Hiraki, K.; Fujishita, A.; Sekine, I.; Ishimaru, T.; Masuzaki, H. Review article: Immunopathogenesis of pelvic endometriosis: Role of hepatocyte growth factor, macrophages and ovarian steroids. Am. J. Reprod. Immunol. 2008, 60, 383–404. [Google Scholar] [CrossRef] [Green Version]
- Khan, K.N.; Masuzaki, H.; Fujishita, A.; Kitajima, M.; Sekine, I.; Matsuyama, T.; Ishimaru, T. Estrogen and progesterone receptor expression im macrophages and regulation of hepatocyte growth factor by ovarian steroids in women with endometriosis. Hum. Reprod. 2005, 20, 2004–2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinn, J.A.; Munoz, F.M.; Gonik, B.; Frau, L.; Cutland, C.; Mallett-Moore, T.; Kissou, A.; Wittke, F.; Das, M.; Nunes, T.; et al. Preterm birth: Case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine 2016, 34, 6047–6056. [Google Scholar]



| Characteristics | Control | lPE | ePE |
|---|---|---|---|
| Number | 10 | 10 | 10 |
| Maternal age, years | 33.9 ± 3.5 | 34.6 ± 5.2 | 33.7 ± 6.3 |
| Gestational age at the moment of c/s, weeks | 38.6 ± 0.7 | 36.8 ± 1.4 * | 30.2 ± 3.4 * |
| Systolic blood pressure, mm Hg | 115.1 ± 5.2 | 149.5 ± 26.1 * | 158.1 ± 16.4 * |
| Diastolic blood pressure, mm Hg | 73.6 ± 3.0 | 94.8 ± 13.3 * | 99.6 ± 11.5 * |
| Proteinuria, g/L | not detected | 1.567 ± 2.2 | 2.516 ± 2.2 |
| Newborn mass, g | 3551.5 ± 469.02 | 2261.4 ± 463.7 * | 1325.1 ± 56.2 * |
| Newborn gender (Male/Female) | 4/6 | 3/7 | 4/6 |
| Intrauterine growth restriction, number of cases | 0 | 5 | 3 |
| Diabetes cases | 0 | 0 | 0 |
| Hypertension prior to the pregnancy, number of cases | 0 | 2 | 2 |
| Previous pregnancies, min–max and most frequent value in group | 0–41 and 2 | 0–30 | 0–20 |
| Miscarriage, number of cases | 0 | 2 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vishnyakova, P.; Poltavets, A.; Nikitina, M.; Midiber, K.; Mikhaleva, L.; Muminova, K.; Potapova, A.; Khodzhaeva, Z.; Pyregov, A.; Elchaninov, A.; et al. Expression of Estrogen Receptor α by Decidual Macrophages in Preeclampsia. Biomedicines 2021, 9, 191. https://doi.org/10.3390/biomedicines9020191
Vishnyakova P, Poltavets A, Nikitina M, Midiber K, Mikhaleva L, Muminova K, Potapova A, Khodzhaeva Z, Pyregov A, Elchaninov A, et al. Expression of Estrogen Receptor α by Decidual Macrophages in Preeclampsia. Biomedicines. 2021; 9(2):191. https://doi.org/10.3390/biomedicines9020191
Chicago/Turabian StyleVishnyakova, Polina, Anastasiya Poltavets, Maria Nikitina, Konstantin Midiber, Liudmila Mikhaleva, Kamilla Muminova, Alena Potapova, Zulfiya Khodzhaeva, Alexey Pyregov, Andrey Elchaninov, and et al. 2021. "Expression of Estrogen Receptor α by Decidual Macrophages in Preeclampsia" Biomedicines 9, no. 2: 191. https://doi.org/10.3390/biomedicines9020191