UV-B Filter Octylmethoxycinnamate Alters the Vascular Contractility Patterns in Pregnant Women with Hypothyroidism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Preparation of HUA Rings for Vascular Reactivity Studies
2.3. Arterial Contractility Experiments
2.4. Primary Cultures of HUA Smooth Muscle Cells (HUASMCs)
2.5. Preparation of HUASMC for Vascular Reactivity Studies
2.6. Cellular Contractility Experiments
2.7. Drugs, Chemicals, and Solutions
2.8. Statistical Analysis
2.9. Molecular Docking Studies
3. Results
3.1. Contractility Experiments in HUA
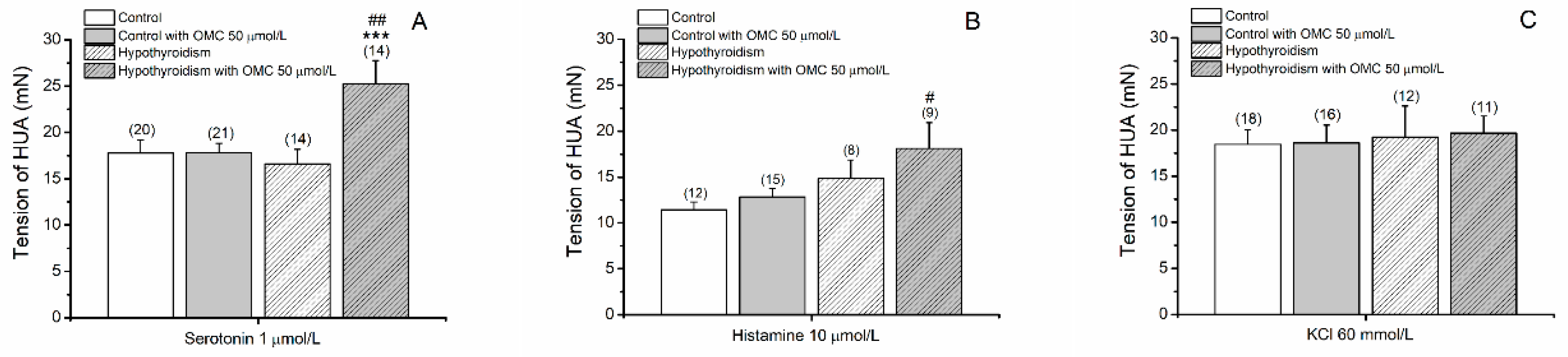
3.1.1. Tension Measurements of Arteries Contracted with 5-HT, His and KCl
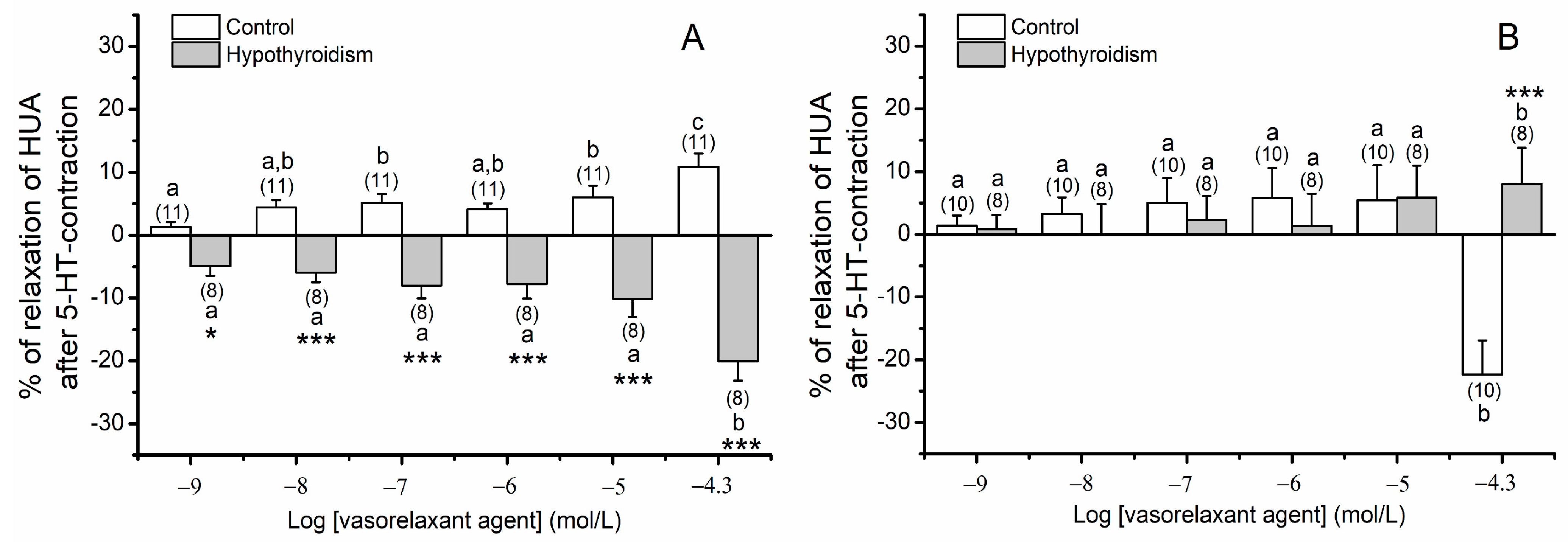
3.1.2. Effects of OMC on Arteries Contracted with 5-HT
3.1.3. Effects of OMC on Arteries Contracted with His
3.1.4. Effects of OMC on Arteries Contracted with KCl
3.2. Contractility Experiments in HUASMC
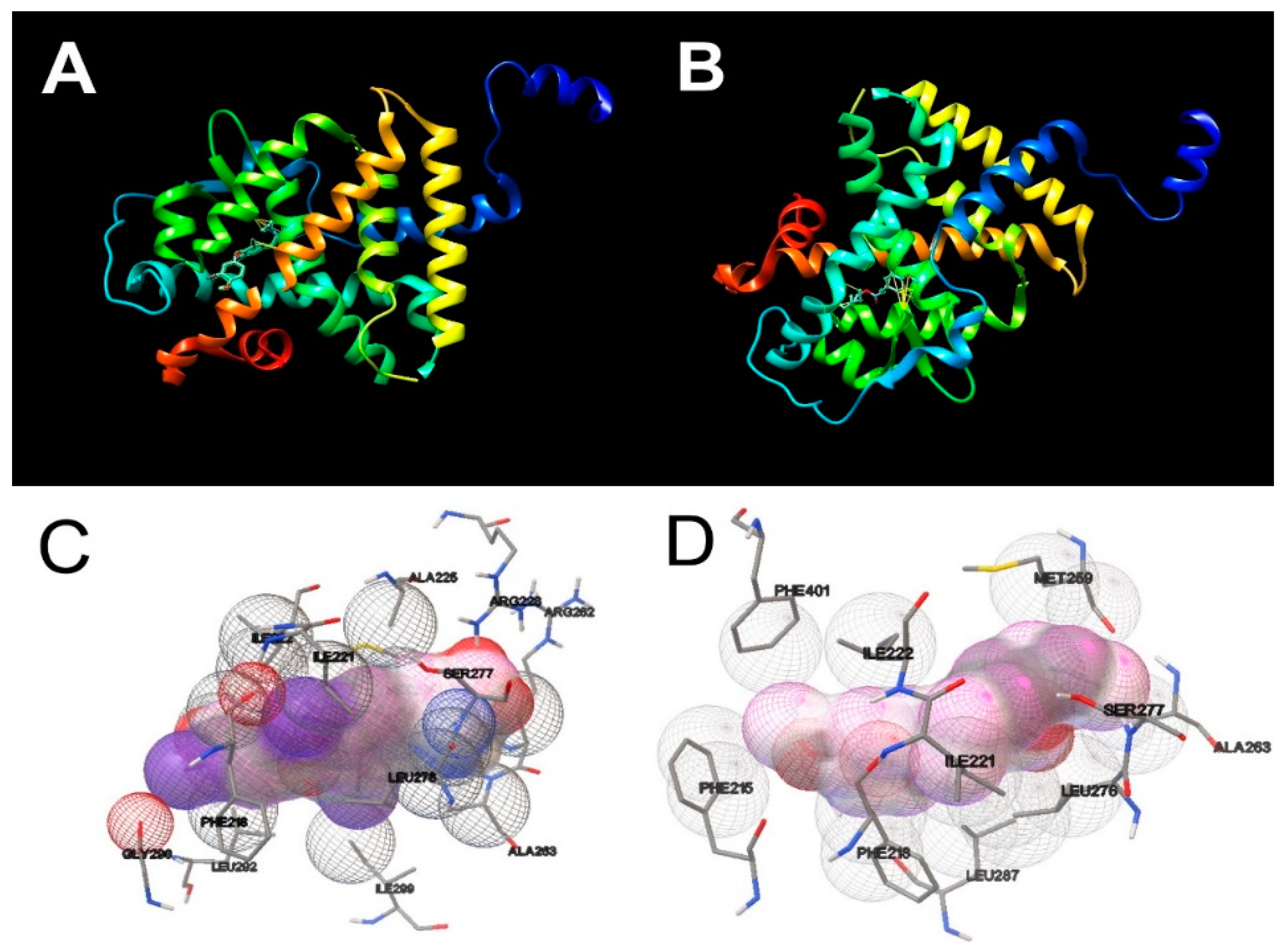
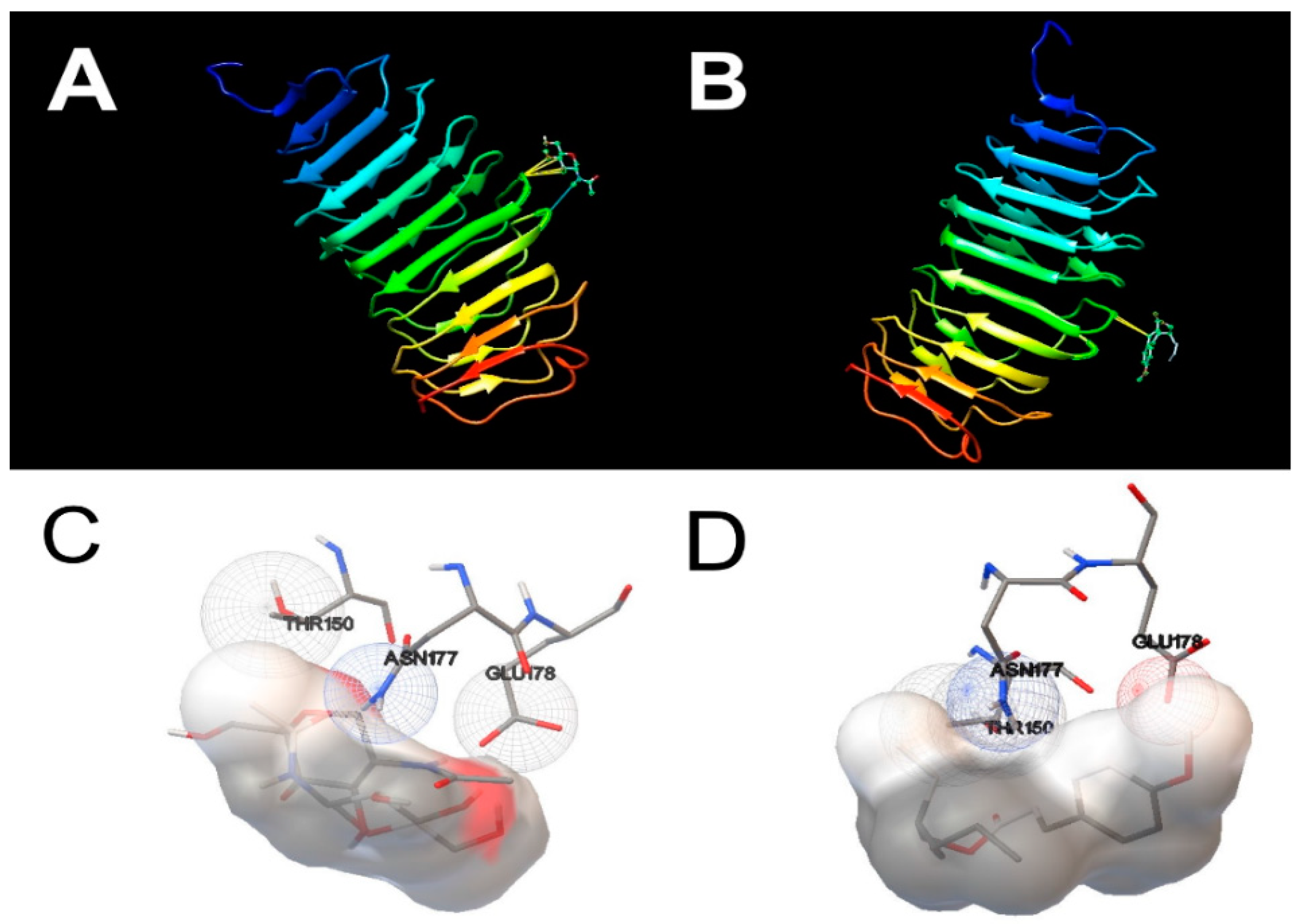
3.3. Molecular Docking Simulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelley, A.S.; Banker, M.; Goodrich, J.M.; Dolinoy, D.C.; Burant, C.; Domino, S.E.; Smith, Y.R.; Song, P.X.K.; Padmanabhan, V. Early pregnancy exposure to endocrine disrupting chemical mixtures are associated with inflammatory changes in maternal and neonatal circulation. Sci. Rep. 2019, 9, 5422. [Google Scholar] [CrossRef] [Green Version]
- Suh, S.; Pham, C.; Smith, J.; Mesinkovska, N.A. The banned sunscreen ingredients and their impact on human health: A systematic review. Int. J. Dermatol. 2020. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Jugan, M.-L.; Levi, Y.; Blondeau, J.-P. Endocrine disruptors and thyroid hormone physiology. Biochem. Pharmacol. 2010, 79, 939–947. [Google Scholar] [CrossRef] [Green Version]
- Padula, A.M.; Monk, C.; Brennan, P.A.; Borders, A.; Barrett, E.S.; McEvoy, C.T.; Foss, S.; Desai, P.; Alshawabkeh, A.; Wurth, R. A review of maternal prenatal exposures to environmental chemicals and psychosocial stressors—Implications for research on perinatal outcomes in the ECHO program. J. Perinatol. 2020, 40, 10–24. [Google Scholar] [CrossRef]
- Rager, J.E.; Bangma, J.; Carberry, C.; Chao, A.; Grossman, J.; Lu, K.; Manuck, T.A.; Sobus, J.R.; Szilagyi, J.; Fry, R.C. Review of the environmental prenatal exposome and its relationship to maternal and fetal health. Reprod. Toxicol. 2020, 98, 1–12. [Google Scholar] [CrossRef]
- Marie-Pierre, S.-R.; Cabut, S.; Vendittelli, F.; Sauvant-Rochat, M.-P. Changes in Cosmetics Use during Pregnancy and Risk Perception by Women. Int. J. Environ. Res. Public Health 2016, 13, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanner, E.M.; Hallerbäck, M.U.; Wikström, S.; Lindh, C.; Kiviranta, H.; Gennings, C.; Bornehag, C.-G. Early prenatal exposure to suspected endocrine disruptor mixtures is associated with lower IQ at age seven. Environ. Int. 2020, 134, 105185. [Google Scholar] [CrossRef]
- Ghassabian, A.; Trasande, L. Disruption in Thyroid Signaling Pathway: A Mechanism for the Effect of Endocrine-Disrupting Chemicals on Child Neurodevelopment. Front. Endocrinol. 2018, 9, 204. [Google Scholar] [CrossRef]
- Vancamp, P.; Houbrechts, A.M.; Darras, V.M. Insights from zebrafish deficiency models to understand the impact of local thyroid hormone regulator action on early development. Gen. Comp. Endocrinol. 2019, 279, 45–52. [Google Scholar] [CrossRef]
- Street, M.E.; Bernasconi, S. Endocrine-Disrupting Chemicals in Human Fetal Growth. Int. J. Mol. Sci. 2020, 21, 1430. [Google Scholar] [CrossRef] [Green Version]
- Jabbar, A.; Pingitore, A.; Pearce, S.H.S.; Zaman, A.J.A.; Iervasi, A.P.G.; Razvi, A.J.S.H.S.P.A.Z.S. Thyroid hormones and cardiovascular disease. Nat. Rev. Cardiol. 2017, 14, 39–55. [Google Scholar] [CrossRef]
- Razvi, S.; Jabbar, A.; Pingitore, A.; Danzi, S.; Biondi, B.; Klein, I.; Peeters, R.; Zaman, A.; Iervasi, G. Thyroid Hormones and Cardiovascular Function and Diseases. J. Am. Coll. Cardiol. 2018, 71, 1781–1796. [Google Scholar] [CrossRef]
- Lorigo, M.; Cairrao, E. Antioxidants as stabilizers of UV filters: An example for the UV-B filter octylmethoxycinnamate. Biomed. Dermatol. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Ferraris, F.K.; Garcia, E.B.; Chaves, A.D.S.; De Brito, T.M.; Doro, L.H.; Da Silva, N.M.F.; Alves, A.S.; Pádua, T.A.; Henriques, M.D.G.M.O.; Machado, T.S.C.; et al. Exposure to the UV Filter Octyl Methoxy Cinnamate in the Postnatal Period Induces Thyroid Dysregulation and Perturbs the Immune System of Mice. Front. Endocrinol. 2020, 10. [Google Scholar] [CrossRef]
- Benson, H.A.E.; Sarveiya, V.; Risk, S.; Roberts, M.S. Influence of anatomical site and topical formulation on skin penetration of sunscreens. Ther. Clin. Risk Manag. 2005, 1, 209–218. [Google Scholar]
- Krause, M.; Klit, A.; Jensen, M.B.; Søeborg, T.; Frederiksen, H.; Schlumpf, M.; Lichtensteiger, W.; Skakkebaek, N.E.; Drzewiecki, K.T. Sunscreens: Are they beneficial for health? An overview of endocrine disrupting properties of UV-filters. Int. J. Androl. 2012, 35, 424–436. [Google Scholar] [CrossRef]
- Kunz, P.Y.; Fent, K. Multiple hormonal activities of UV filters and comparison of in vivo and in vitro estrogenic activity of ethyl-4-aminobenzoate in fish. Aquat. Toxicol. 2006, 79, 305–324. [Google Scholar] [CrossRef]
- Schlumpf, M.; Kypke, K.; Wittassek, M.; Angerer, J.; Mascher, H.; Mascher, D.; Vökt, C.; Birchler, M.; Lichtensteiger, W. Exposure patterns of UV filters, fragrances, parabens, phthalates, organochlor pesticides, PBDEs, and PCBs in human milk: Correlation of UV filters with use of cosmetics. Chemosphere 2010, 81, 1171–1183. [Google Scholar] [CrossRef]
- Negreira, N.; Rodríguez, I.; Rubí, E.; Cela, R. Determination of selected UV filters in indoor dust by matrix solid-phase dispersion and gas chromatography–tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 5895–5902. [Google Scholar] [CrossRef]
- Zwiener, C.; Ichardson, S.D.R.; Arini, D.M.D.E.M.; Grummt, T.; Launer, T.G.; Rimmel, F.R.H.F. Drowning in Disinfection Byproducts? Assessing Swimming Pool Water. Environ. Sci. Technol. 2006, 41, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.S.D.; Gago-Ferrero, P.; Llorca, M.; Barceló, D. Analysis of UV filters in tap water and other clean waters in Spain. Anal. Bioanal. Chem. 2011, 402, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Loraine, G.; Pettigrove, M.E. Seasonal Variations in Concentrations of Pharmaceuticals and Personal Care Products in Drinking Water and Reclaimed Wastewater in Southern California. Environ. Sci. Technol. 2006, 40, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.L.; Lim, H.W. Review of environmental effects of oxybenzone and other sunscreen active ingredients. J. Am. Acad. Dermatol. 2019, 80, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Lorigo, M.; Mariana, M.; Cairrao, E. Photoprotection of ultraviolet-B filters: Updated review of endocrine disrupting properties. Steroids 2018, 131, 46–58. [Google Scholar] [CrossRef]
- Siller, A.; Blaszak, S.C.; Lazar, M.; Harken, E.O. Update About the Effects of the Sunscreen Ingredients Oxybenzone and Octinoxate on Humans and the Environment. Plast. Surg. Nurs. 2019, 39, 157–160. [Google Scholar] [CrossRef]
- Janjua, N.R.; Kongshoj, B.; Andersson, A.-M.; Wulf, H.C. Sunscreens in human plasma and urine after repeated whole-body topical application. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 456–461. [Google Scholar] [CrossRef]
- Schlumpf, M.; Kypke, K.; Vökt, C.C.; Birchler, M.; Durrer, S.; Faass, O.; Ehnes, C.; Fuetsch, M.; Gaille, C.; Henseler, M.; et al. Corrigendum. Chim. Int. J. Chem. 2008, 62, 688. [Google Scholar] [CrossRef]
- Vela-Soria, F.; Gallardo-Torres, M.; Ballesteros, O.; Díaz, C.; Pérez, J.; Navalón, A.; Vinggaard, A.M.; Olea, N. Assessment of parabens and ultraviolet filters in human placenta tissue by ultrasound-assisted extraction and ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2017, 1487, 153–161. [Google Scholar] [CrossRef]
- Schmutzler, C.; Gotthardt, I.; Hofmann, P.J.; Radovic, B.; Kovacs, G.; Stemmler, L.; Nobis, I.; Bacinski, A.; Mentrup, B.; Ambrugger, P.; et al. Endocrine Disruptors and the Thyroid Gland—A Combined in Vitro and in Vivo Analysis of Potential New Biomarkers. Environ. Health Perspect. 2007, 115, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Song, M.-K.; Choi, H.-S.; Ryu, J.-C. Monitoring of deiodinase deficiency based on transcriptomic responses in SH-SY5Y cells. Arch. Toxicol. 2013, 87, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Janjua, N.R.; Kongshoj, B.; Petersen, J.H.; Wulf, H. Sunscreens and thyroid function in humans after short-term whole-body topical application: A single-blinded study. Br. J. Dermatol. 2007, 156, 1080–1082. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Carlé, A. Levothyroxine Formulations: Pharmacological and Clinical Implications of Generic Substitution. Adv. Ther. 2019, 36, 59–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cairrao, E.; Álvarez, E.; Santos-Silva, A.J.; Verde, I. Potassium channels are involved in testosterone-induced vasorelaxation of human umbilical artery. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2007, 376, 375–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorigo, M.; Quintaneiro, C.; Lemos, M.C.; Martinez-De-Oliveira, J.; Breitenfeld, L.; Cairrao, E. UV-B Filter Octylmethoxycinnamate Induces Vasorelaxation by Ca2+ Channel Inhibition and Guanylyl Cyclase Activation in Human Umbilical Arteries. Int. J. Mol. Sci. 2019, 20, 1376. [Google Scholar] [CrossRef] [Green Version]
- Schlumpf, M.; Cotton, B.; Conscience, M.; Haller, V.; Steinmann, B.; Lichtensteiger, W. In vitro and in vivo estrogenicity of UV screens. Environ. Health Perspect. 2001, 109, 239–244. [Google Scholar] [CrossRef]
- Sharma, A.; Bányiová, K.; Babica, P.; El Yamani, N.; Collins, A.R.; Čupr, P. Different DNA damage response of cis and trans isomers of commonly used UV filter after the exposure on adult human liver stem cells and human lymphoblastoid cells. Sci. Total Environ. 2017, 593, 18–26. [Google Scholar] [CrossRef]
- Nečasová, A.; Bányiová, K.; Literák, J.; Čupr, P. New probabilistic risk assessment of ethylhexyl methoxycinnamate: Comparing the genotoxic effects oftrans- andcis-EHMC. Environ. Toxicol. 2016, 32, 569–580. [Google Scholar] [CrossRef]
- Cairrão, E.; Santos-Silva, A.J.; Alvarez, E.; Correia, I.; Verde, I. Isolation and culture of human umbilical artery smooth muscle cells expressing functional calcium channels. Vitr. Cell. Dev. Biol. Anim. 2009, 45, 175–184. [Google Scholar] [CrossRef]
- Lorigo, M.; Mariana, M.; Feiteiro, J.; Cairrao, E. How is the human umbilical artery regulated? J. Obstet. Gynaecol. Res. 2018, 44, 1193–1201. [Google Scholar] [CrossRef] [Green Version]
- Mariana, M.; Feiteiro, J.; Cairrao, E.; Verde, I. Mifepristone is a Vasodilator Due to the Inhibition of Smooth Muscle Cells L-Type Ca2+ Channels. Reprod. Sci. 2015, 23, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benvenga, S.; Di Bari, F.; Vita, R. Undertreated hypothyroidism due to calcium or iron supplementation corrected by oral liquid levothyroxine. Endocrine 2017, 56, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, P.H.; Volpe, S.L. Effect of iron supplementation on thyroid hormone levels and resting metabolic rate in two college female athletes: A case study. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Shakir, K.M.M.; Chute, J.P.; Aprill, B.S.; Lazarus, A.A. Ferrous sulfate-induced increase in requirement for thyroxine in a patient with primary hypothyroidism. South. Med. J. 1997, 90, 637–639. [Google Scholar] [CrossRef]
- Ng, J.M.; Wakil, A.; Dawson, A.; Masson, E.A.; Allan, B.J.; Lindow, S.W.; Krishnan, R.; Wardell, S.; Igzeer, Y. Levothyroxine and iron in pregnancy: Right dose, wrong time? Endocr. Abstr. 2009, 19, P356. [Google Scholar]
- Quan, A.; Leung, S.W.; Lao, T.T.; Man, R.Y. 5-hydroxytryptamine and thromboxane A2 as physiologic mediators of human umbilical artery closure. J. Soc. Gynecol. Investig. 2003, 10, 490–495. [Google Scholar] [CrossRef]
- Lovren, F.; Li, X.-F.; Lytton, J.; Triggle, C.R. Functional characterization and m-RNA expression of 5-HT receptors mediating contraction in human umbilical artery. Br. J. Pharmacol. 1999, 127, 1247–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Silva, A.J.; Cairrao, E.; Marques, B.; Verde, I. Regulation of human umbilical artery contractility by different serotonin and histamine receptors. Reprod. Sci. 2009, 16, 1175–1185. [Google Scholar] [CrossRef] [Green Version]
- Santos-Silva, A.J.; Cairrao, E.; Verde, I. Study of the mechanisms regulating human umbilical artery contractility. Health 2010, 2, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Hawley, J.; Rubin, P.; Hill, S.J. Distribution of receptors mediating phosphoinositide hydrolysis in cultured human umbilical artery smooth muscle and endothelial cells. Biochem. Pharmacol. 1995, 49, 1005–1011. [Google Scholar] [CrossRef]
- Santos-Silva, A.J.; Cairrao, E.; Morgado, M.; Álvarez, E.; Verde, I. PDE4 and PDE5 regulate cyclic nucleotides relaxing effects in human umbilical arteries. Eur. J. Pharmacol. 2008, 582, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, E.; Cairrao, E.; Morgado, M.; Morais, C.; Verde, I. Testosterone and Cholesterol Vasodilation of Rat Aorta Involves L-Type Calcium Channel Inhibition. Adv. Pharmacol. Sci. 2010, 2010, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kuo, I.Y.; Wölfle, S.E.; Hill, C.E. T-type calcium channels and vascular function: The new kid on the block? J. Physiol. 2011, 589, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Sikanderkhel, S.; Gui, J.; Adeniyi, A.-R.; O’Dell, K.; Erickson, M.; Malpartida, J.; Mufti, Z.; Khan, T.; Mufti, H.; et al. Thyroid and Cardiovascular Disease: A Focused Review on the Impact of Hyperthyroidism in Heart Failure. Cardiol. Res. 2020, 11, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Osuna, P.M.; Udovcic, M.; Sharma, M.D. Hyperthyroidism and the Heart. Methodist DeBakey Cardiovasc. J. 2017, 13, 60–63. [Google Scholar] [CrossRef] [Green Version]
- Udovcic, M.; Pena, R.H.; Patham, B.; Tabatabai, L.; Kansara, A. Hypothyroidism and the Heart. Methodist DeBakey Cardiovasc. J. 2017, 13, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Klein, I.; Danzi, S. Thyroid Disease and the Heart. Curr. Probl. Cardiol. 2016, 41, 65–92. [Google Scholar] [CrossRef] [Green Version]
- Barreto-Chaves, M.L.M.; Monteiro, P.D.S.; Furstenau, C.R. Acute actions of thyroid hormone on blood vessel biochemistry and physiology. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 300–303. [Google Scholar] [CrossRef]
- Tian, L.; Ni, J.; Guo, T.; Liu, J.; Dang, Y.; Guo, Q.; Zhang, L. TSH stimulates the proliferation of vascular smooth muscle cells. Endocrine 2014, 46, 651–658. [Google Scholar] [CrossRef]
- Makino, A.; Wang, H.; Scott, B.T.; Yuan, J.X.-J.; Dillmann, W.H. Thyroid hormone receptor-α and vascular function. Am. J. Physiol. Physiol. 2012, 302, C1346–C1352. [Google Scholar] [CrossRef] [PubMed]
- Grais, I.M.; Sowers, J.R. Thyroid and the Heart. Am. J. Med. 2014, 127, 691–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, I.; Ojamaa, K. Thyroid Hormone and the Cardiovascular System. N. Engl. J. Med. 2001, 344, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, T.; Tsunekawa, K.; Seki, K.; Mori, M.; Murakami, M. Regulation of iodothyronine deiodinase and roles of thyroid hormones in human coronary artery smooth muscle cells. Atherosclerosis 2006, 186, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Ojamaa, K.; Klemperer, J.D.; Klein, I. Acute Effects of Thyroid Hormone on Vascular Smooth Muscle. Thyroid 1996, 6, 505–512. [Google Scholar] [CrossRef]
- Fukuyama, K.; Ichiki, T.; Takeda, K.; Tokunou, T.; Iino, N.; Masuda, S.; Ishibashi, M.; Egashira, K.; Shimokawa, H.; Hirano, K.; et al. Downregulation of Vascular Angiotensin II Type 1 Receptor by Thyroid Hormone. Hypertension 2003, 41, 598–603. [Google Scholar] [CrossRef] [Green Version]
- Carrillo-Sepúlveda, M.A.; Ceravolo, G.S.; Fortes, Z.B.; Carvalho, M.H.; Tostes, R.; Laurindo, F.R.; Webb, R.C.; Barreto-Chaves, M.L.M. Thyroid hormone stimulates NO production via activation of the PI3K/Akt pathway in vascular myocytes. Cardiovasc. Res. 2009, 85, 560–570. [Google Scholar] [CrossRef] [Green Version]
- Demirel, M.; Gürsoy, G.; Yıldız, M. Does Treatment of Either Hypothyroidy or Hyperthyroidy Affect Diurnal Blood Pressure. Arch. Iran. Med. 2017, 20, 572–580. [Google Scholar]
- Hiroi, Y.; Kim, H.-H.; Ying, H.; Furuya, F.; Huang, Z.; Simoncini, T.; Noma, K.; Ueki, K.; Nguyen, N.-H.; Scanlan, T.S.; et al. Rapid nongenomic actions of thyroid hormone. Proc. Nat. Acad. Sci. USA 2006, 103, 14104–14109. [Google Scholar] [CrossRef] [Green Version]
- Kuzman, J.; Gerdes, A.; Kobayashi, S.; Liang, Q. Thyroid hormone activates Akt and prevents serum starvation-induced cell death in neonatal rat cardiomyocytes. J. Mol. Cell. Cardiol. 2005, 39, 841–844. [Google Scholar] [CrossRef]
- Napoli, R.; Apuzzi, V.; Bosso, G.; D’Anna, C.; De Sena, A.; Pirozzi, C.; Marano, A.; Lupoli, G.A.; Cudemo, G.; Oliviero, U.; et al. Recombinant Human Thyrotropin Enhances Endothelial-Mediated Vasodilation of Conduit Arteries. J. Clin. Endocrinol. Metab. 2009, 94, 1012–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwaveling, J.; Pfaffendorf, M.; Zwieten, P.A. The direct effects of thyroid hormones on rat mesenteric resistance arteries. Fundam. Clin. Pharmacol. 1997, 11, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, T. Thyroid Hormone and Vascular Remodeling. J. Atheroscler. Thromb. 2016, 23, 266–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, S.; Murthy, M.; Ramteke, K.; Raparti, G. Thyroid: Disorders, disruptors and drugs. Int. J. Nutr. Pharmacol. Neurol. Dis. 2013, 3, 87. [Google Scholar] [CrossRef]
- Ao, J.; Yuan, T.; Gao, L.; Yu, X.; Zhao, X.; Tian, Y.; Ding, W.; Ma, Y.; Shen, Z. Organic UV filters exposure induces the production of inflammatory cytokines in human macrophages. Sci. Total. Environ. 2018, 635, 926–935. [Google Scholar] [CrossRef]
- Sheikh, I.A. Molecular interactions of thyroxine binding globulin and thyroid hormone receptor with estrogenic compounds 4-nonylphenol, 4-tert-octylphenol and bisphenol A metabolite (MBP). Life Sci. 2020, 253, 117738. [Google Scholar] [CrossRef]
- Ali, M.R.; Latif, R.; Davies, T.F.; Mezei, M. Monte Carlo loop refinement and virtual screening of the thyroid-stimulating hormone receptor transmembrane domain. J. Biomol. Struct. Dyn. 2014, 33, 1140–1152. [Google Scholar] [CrossRef] [Green Version]
- Satpathy, R. Application of Molecular Docking Methods on Endocrine Disrupting Chemicals: A Review. J. Appl. Biotechnol. Rep. 2020, 7, 74–80. [Google Scholar] [CrossRef]
- Gao, L.; Yuan, T.; Cheng, P.; Zhou, C.; Ao, J.; Wang, W.; Zhang, H. Organic UV filters inhibit multixenobiotic resistance (MXR) activity in Tetrahymena thermophila: Investigations by the Rhodamine 123 accumulation assay and molecular docking. Ecotoxicology 2016, 25, 1318–1326. [Google Scholar] [CrossRef]
- Couderq, S.; Leemans, M.; Fini, J.-B. Testing for thyroid hormone disruptors, a review of non-mammalian in vivo models. Mol. Cell. Endocrinol. 2020, 508, 110779. [Google Scholar] [CrossRef]
- Rolfo, A.; Nuzzo, A.M.; De Amicis, R.; Moretti, L.; Bertoli, S.; Leone, A. Fetal–Maternal Exposure to Endocrine Disruptors: Correlation with Diet Intake and Pregnancy Outcomes. Nutrients 2020, 12, 1744. [Google Scholar] [CrossRef] [PubMed]
- Campesi, I.; Franconi, F.; Montella, A.; Dessole, S.; Capobianco, G. Human Umbilical Cord: Information Mine in Sex-Specific Medicine. Life 2021, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-X.; Chen, L.; Meng, X.-Z.; Chen, B.-H.; Chen, S.-Q.; Zhao, Y.; Zhao, L.-F.; Liang, Y.; Zhang, Y. Exposure Levels of Environmental Endocrine Disruptors in Mother-Newborn Pairs in China and Their Placental Transfer Characteristics. PLoS ONE 2013, 8, e62526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.-L.; Chang, C.-C.; Shen, Y.-J.; Hung, J.-H.; Guo, B.-R.; Chuang, H.-Y.; Mao, I.-F. Quantification of prenatal exposure and maternal-fetal transfer of nonylphenol. Chemosphere 2008, 73, S239–S245. [Google Scholar] [CrossRef]
- Tan, B. Analysis of selected pesticides and alkylphenols in human cord blood by gas chromatograph-mass spectrometer. Talanta 2003, 61, 385–391. [Google Scholar] [CrossRef]
- De Llano, J.J.M.; Fuertes, G.; Torró, I.; Garcia-Vicent, C.; Fayos, J.L.; Lurbe, E. Birth weight and characteristics of endothelial and smooth muscle cell cultures from human umbilical cord vessels. J. Transl. Med. 2009, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Steinert, J.R.; Wyatt, A.W.; Poston, L.; Jacob, R.; Mann, G.E. Preeclampsia is associated with altered Ca 2+ regulation and nitric oxide production in human fetal venous endothelial cells. FASEB J. 2002, 16, 721–723. [Google Scholar] [CrossRef]
- Gokina, N.I.; Bevan, J.A. Histamine-induced depolarization: Ionic mechanisms and role in sustained contraction of rabbit cerebral arteries. Am. J. Physiol. Circ. Physiol. 2000, 278, H2094–H2104. [Google Scholar] [CrossRef]
- Mallozzi, M.; Bordi, G.; Garo, C.; Caserta, D. The effect of maternal exposure to endocrine disrupting chemicals on fetal and neonatal development: A review on the major concerns. Birth Defects Res. Part. C Embryo Today Rev. 2016, 108, 224–242. [Google Scholar] [CrossRef]
- Glória, S.; Marques, J.; Feiteiro, J.; Marcelino, H.; Verde, I.; Cairrao, E. Tributyltin role on the serotonin and histamine receptors in human umbilical artery. Toxicol. Vitr. 2018, 50, 210–216. [Google Scholar] [CrossRef]
- Tang, Z.-R.; Xu, X.-L.; Deng, S.-L.; Lian, Z.; Yu, K. Oestrogenic Endocrine Disruptors in the Placenta and the Fetus. Int. J. Mol. Sci. 2020, 21, 1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| S. No | Name | Abbreviation | PubChem ID | CASRN |
|---|---|---|---|---|
| 1 | 3,5,3′Triiodothyronine | T3 | 5920 | 6893-02-3 |
| 2 | 2-acetamido-2-deoxy-beta-d-glucopyranose | NAG | 24139 | 7512-17-6 |
| 3 | octylmethoxycinnamate | OMC | 5355130 | 5466-77-3 |
| Compound | TRHα | TSH |
|---|---|---|
| T3 | −10.80 | - |
| NAG | - | −1.61 |
| OMC_1 | −7.69 | 0.68 |
| OMC_2 | −7.67 | 0.69 |
| OMC_3 | −7.65 | 0.73 |
| OMC_4 | −7.63 | 0.96 |
| OMC_5 | −7.60 | 1.08 |
| OMC_6 | −7.56 | 1.16 |
| OMC_7 | −6.92 | 1.01 |
| OMC_8 | −7.35 | 1.11 |
| OMC_9 | −7.16 | 1.30 |
| OMC_10 | −6.71 | 1.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorigo, M.; Quintaneiro, C.; Breitenfeld, L.; Cairrao, E. UV-B Filter Octylmethoxycinnamate Alters the Vascular Contractility Patterns in Pregnant Women with Hypothyroidism. Biomedicines 2021, 9, 115. https://doi.org/10.3390/biomedicines9020115
Lorigo M, Quintaneiro C, Breitenfeld L, Cairrao E. UV-B Filter Octylmethoxycinnamate Alters the Vascular Contractility Patterns in Pregnant Women with Hypothyroidism. Biomedicines. 2021; 9(2):115. https://doi.org/10.3390/biomedicines9020115
Chicago/Turabian StyleLorigo, Margarida, Carla Quintaneiro, Luiza Breitenfeld, and Elisa Cairrao. 2021. "UV-B Filter Octylmethoxycinnamate Alters the Vascular Contractility Patterns in Pregnant Women with Hypothyroidism" Biomedicines 9, no. 2: 115. https://doi.org/10.3390/biomedicines9020115








