Role of Oxidative Stress in Heart Failure: Insights from Gene Transfer Studies
Abstract
:1. Introduction
1.1. General Aim of the Review
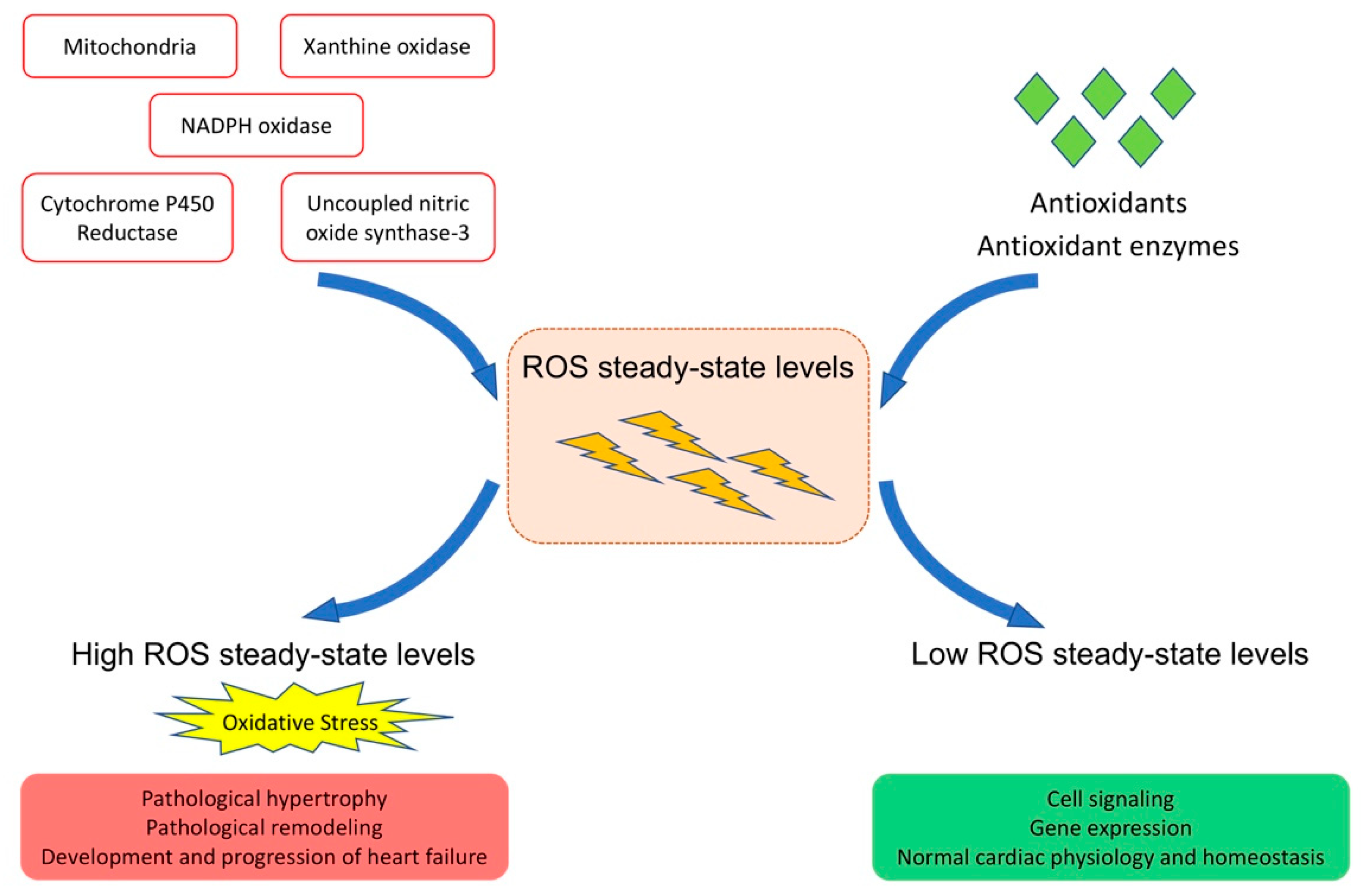
1.2. Reactive Oxygen Species
1.3. Role of ROS in Cardiac Physiology and Homeostasis
1.4. Oxidative Stress
1.5. Oxidative Stress and Heart Failure
2. Antioxidant Enzyme Gene Transfer
2.1. Gene Transfer of the Primary Antioxidant Enzymes
2.2. Thioredoxin Gene Transfer
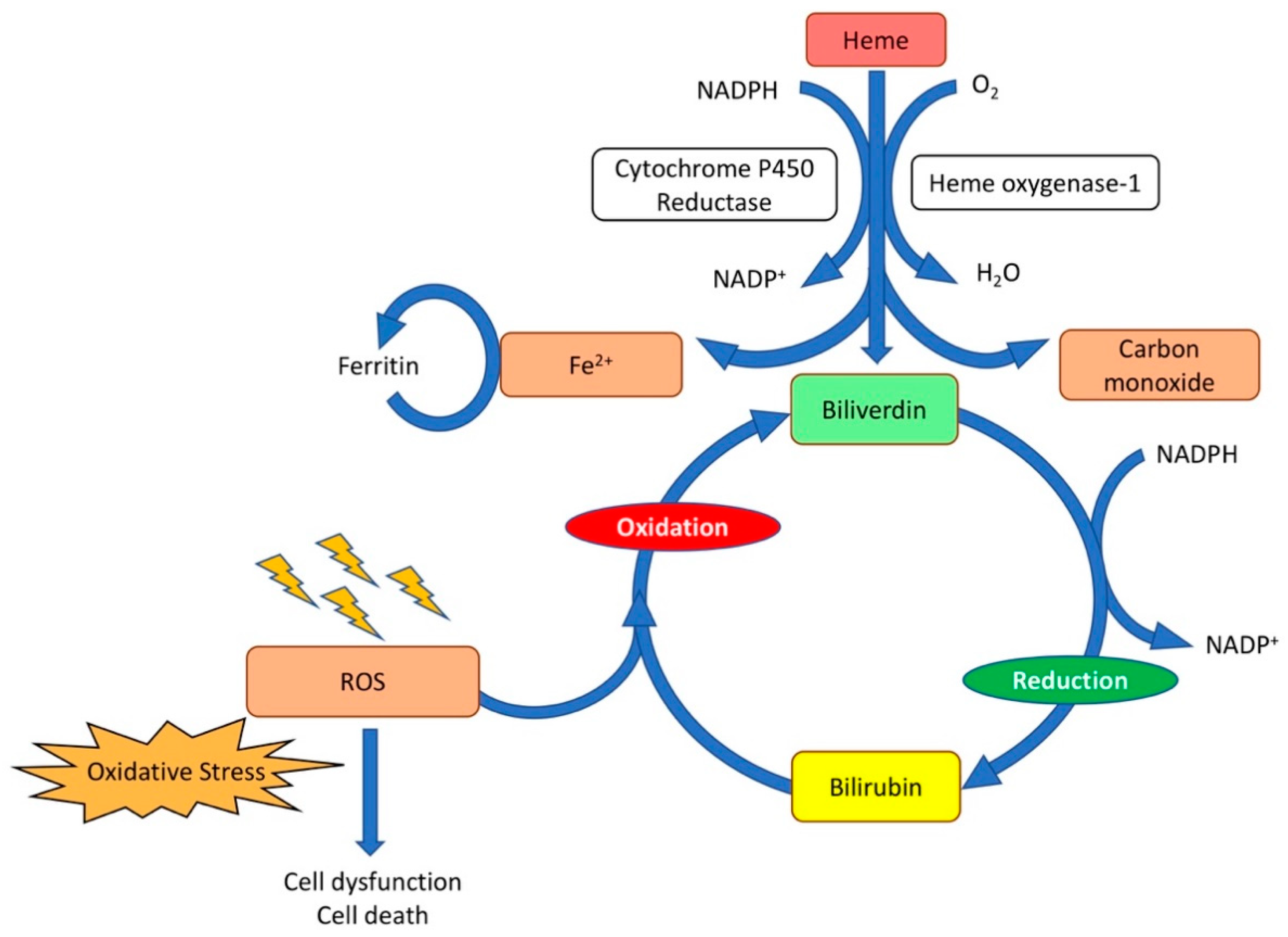
2.3. Heme Oxygenase-1 Gene Transfer
3. Metabolic Risk Factors, Oxidative Stress, and Heart Failure: Impact of Gene Therapy
4. Gene Transfer of MicroRNAs and Modulation of MicroRNA Activity to Reduce Oxidative Stress and to Prevent Heart Failure
4.1. MicroRNAs and Oxidative Stress
4.2. Impact of Oxidative Changes of MicroRNAs on Cardiac Hypertrophy and Heart Failure: Direct Evidence for a Causal Role of Oxidative Stress
4.3. MiR-152 Gene Transfer to Reduce Oxidative Stress and to Improve Cardiac Function in a Model of Doxorubicin-Induced Cardiomyopathy
4.4. MicroRNA-132 Inhibition Using Antagomirs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Radi, R. Peroxynitrite, a Stealthy Biological Oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, M.-H.; Bachschmid, M.M. Hypoxia–Reoxygenation Triggers Coronary Vasospasm in Isolated Bovine Coronary Arteries via Tyrosine Nitration of Prostacyclin Synthase. J. Exp. Med. 1999, 190, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Lauer, N.; Mülsch, A.; Kojda, G. The effect of peroxynitrite on the catalytic activity of soluble guanylyl cyclase. Free Radic. Biol. Med. 2001, 31, 1360–1367. [Google Scholar] [CrossRef]
- MacMillan-Crow, L.A.; Crow, J.P.; Thompson, J.A. Peroxynitrite-mediated inactivation of manganese superoxide dis-mutase involves nitration and oxidation of critical tyrosine residues. Biochemistry 1998, 37, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Gori, T.; Keaney, J.F., Jr.; Maack, C.; Daiber, A. Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implica-tions. Eur. Heart J. 2015, 36, 2555–2564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, C.X.; Anilkumar, N.; Zhang, M.; Brewer, A.C.; Shah, A.M. Redox signaling in cardiac myocytes. Free Radic. Biol. Med. 2011, 50, 777–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, S.G. Redox signaling: Hydrogen peroxide as intracellular messenger. Exp. Mol. Med. 1999, 31, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Klomsiri, C.; Karplus, P.; Poole, L.B. Cysteine-Based Redox Switches in Enzymes. Antioxid. Redox Signal. 2011, 14, 1065–1077. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Sun, W.; Zhang, Z.; Zheng, Y. The Role of Nrf2-Mediated Pathway in Cardiac Remodeling and Heart Failure. Oxidative Med. Cell. Longev. 2014, 2014, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Fourquet, S.; Guerois, R.; Biard, D.; Toledano, M.B. Activation of NRF2 by Nitrosative Agents and H2O2 Involves KEAP1 Disulfide Formation. J. Biol. Chem. 2010, 285, 8463–8471. [Google Scholar] [CrossRef] [Green Version]
- Seok, H.; Lee, H.; Lee, S.; Ahn, S.H.; Lee, H.-S.; Kim, G.-W.D.; Peak, J.; Park, J.; Cho, Y.K.; Jeong, Y.; et al. Position-specific oxidation of miR-1 encodes cardiac hypertrophy. Nat. Cell Biol. 2020, 584, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stresses and their classifications. Ukr. Biochem. J. 2015, 87, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiomi, T.; Tsutsui, H.; Matsusaka, H.; Murakami, K.; Hayashidani, S.; Ikeuchi, M.; Wen, J.; Kubota, T.; Utsumi, H.; Takeshita, A. Overexpression of Glutathione Peroxidase Prevents Left Ventricular Remodeling and Failure After Myocardial Infarction in Mice. Circulation 2004, 109, 544–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, I.; Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2016, 97, 245–262. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, T.; Chen, Y.; Ahokas, R.A.; Sun, Y. Oxidative stress mediates cardiac fibrosis by enhancing transforming growth factor-beta1 in hypertensive rats. Mol. Cell. Biochem. 2008, 317, 43–50. [Google Scholar] [CrossRef]
- Philip, J.L.; Razzaque, M.A.; Han, M.; Li, J.; Theccanat, T.; Xu, X.; Akhter, S.A. Regulation of mitochondrial oxidative stress by beta-arrestins in cultured human cardiac fibroblasts. Dis. Model Mech. 2015, 8, 1579–1589. [Google Scholar]
- Takimoto, E.; Kass, D.A. Role of Oxidative Stress in Cardiac Hypertrophy and Remodeling. Hypertens 2007, 49, 241–248. [Google Scholar] [CrossRef]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Ide, T.; Tsutsui, H.; Kinugawa, S.; Suematsu, N.; Hayashidani, S.; Ichikawa, K.; Utsumi, H.; Machida, Y.; Egashira, K.; Takeshita, A. Direct Evidence for Increased Hydroxyl Radicals Originating from Superoxide in the Failing Myocardium. Circ. Res. 2000, 86, 152–157. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Sato, Y.; Fujiwara, H.; Takatsu, Y. Cardiac troponin and heart failure in the era of high-sensitivity assays. J. Cardiol. 2012, 60, 160–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kociol, R.; Pang, P.; Gheorghiade, M.; Fonarow, G.; O’Connor, C.M.; Felker, G. Troponin Elevation in Heart Failure: Prevalence, Mechanisms, and Clinical Implications. J. Am. Coll. Cardiol. 2010, 56, 1071–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mair, J.; Lindahl, B.; Hammarsten, O.; Müller, C.; Giannitsis, E.; Huber, K.; Möckel, M.; Plebani, M.; Thygesen, K.; Jaffe, A.S. How is cardiac troponin released from injured myocardium? Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Barth, E. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J. Mol. Cell. Cardiol. 1992, 24, 669–681. [Google Scholar] [CrossRef]
- Schaper, J.; Meiser, E.; Stämmler, G. Ultrastructural morphometric analysis of myocardium from dogs, rats, hamsters, mice, and from human hearts. Circ. Res. 1985, 56, 377–391. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.; Ma, Y.; Zhang, W.; Bao, D.; Dong, W.; Lian, H.; Huang, L.; Zhang, L. Knockdown of cytochrome P450 2E1 inhibits oxidative stress and apoptosis in the cTnT(R141W) dilated cardi-omyopathy transgenic mice. Hypertension 2012, 60, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.-J.; Zhao, C.-L.; Ouyang, S.; Deng, K.-Q.; Zhu, L.; Montezano, A.C.; Zhang, C.; Hu, F.; Zhu, X.-Y.; Tian, S.; et al. Ca2+ -Dependent NOX5 (NADPH Oxidase 5) Exaggerates Cardiac Hypertrophy Through Reactive Oxygen Species Production. Hypertension 2020, 76, 827–838. [Google Scholar] [CrossRef]
- Forstermann, U.; Munzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [Green Version]
- Takimoto, E.; Champion, H.C.; Li, M.; Ren, S.; Rodriguez, E.R.; Tavazzi, B.; Lazzarino, G.; Paolocci, N.; Gabrielson, K.L.; Wang, Y.; et al. Oxidant stress from nitric oxide synthase–3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J. Clin. Investig. 2005, 115, 1221–1231. [Google Scholar] [CrossRef] [Green Version]
- Mondola, P.; Damiano, S.; Sasso, A.; Santillo, M. The Cu, Zn Superoxide Dismutase: Not Only a Dismutase Enzyme. Front. Physiol. 2016, 7, 594. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, S.; Crehuet, R.; Anglada, J.M.; Francisco, J.S.; Xia, Y. Two-step reaction mechanism reveals new antioxidant capability of cysteine disulfides against hydroxyl radical attack. Proc. Natl. Acad. Sci. USA 2020, 117, 18216–18223. [Google Scholar] [CrossRef] [PubMed]
- Konkalmatt, P.R.; Beyers, R.J.; O’Connor, D.M.; Xu, Y.; Seaman, M.E.; French, B.A. Cardiac-Selective Expression of Extracellular Superoxide Dismutase After Systemic Injection of Adeno-Associated Virus 9 Protects the Heart Against Post–Myocardial Infarction Left Ventricular Remodeling. Circ. Cardiovasc. Imaging 2013, 6, 478–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, K.M.R.; Smith, R.S.; Xu, Y.; French, B.A. A single direct injection into the left ventricular wall of an adeno-associated virus 9 (AAV9) vector ex-pressing extracellular superoxide dismutase from the cardiac troponin-T promoter protects mice against myocardial infarction. J. Gene Med. 2011, 13, 333–341. [Google Scholar] [CrossRef]
- Iida, S.; Chu, Y.; Francis, J.; Weiss, R.M.; Gunnett, C.A.; Faraci, F.; Heistad, D.D. Gene transfer of extracellular superoxide dismutase improves endothelial function in rats with heart failure. Am. J. Physiol. Circ. Physiol. 2005, 289, H525–H532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okutsu, M.; Call, J.A.; Lira, V.A.; Zhang, M.; Donet, J.A.; French, B.A.; Martin, K.S.; Peirce, S.; Rembold, C.M.; Annex, B.H.; et al. Extracellular Superoxide Dismutase Ameliorates Skeletal Muscle Abnormalities, Cachexia, and Exercise Intolerance in Mice with Congestive Heart Failure. Circ. Heart Fail. 2014, 7, 519–530. [Google Scholar] [CrossRef] [Green Version]
- Woo, Y.J.; Zhang, J.C.; Vijayasarathy, C.; Zwacka, R.; Englehardt, J.F.; Gardner, T.J.; Sweeney, H.L. Recombinant adenovirus-mediated cardiac gene transfer of superoxide dismutase and catalase attenuates postischemic contractile dysfunction. Circulation 1998, 98, 255–260. [Google Scholar]
- Matsushima, S.; Kinugawa, S.; Ide, T.; Matsusaka, H.; Inoue, N.; Ohta, Y.; Yokota, T.; Sunagawa, K.; Tsutsui, H. Overexpression of glutathione peroxidase attenuates myocardial remodeling and preserves diastolic function in diabetic heart. Am. J. Physiol. Circ. Physiol. 2006, 291, H2237–H2245. [Google Scholar] [CrossRef] [Green Version]
- Ardanaz, N.; Yang, X.-P.; Cifuentes, M.E.; Haurani, M.J.; Jackson, K.W.; Liao, T.-D.; Carretero, O.A.; Pagano, P.J. Lack of Glutathione Peroxidase 1 Accelerates Cardiac-Specific Hypertrophy and Dysfunction in Angiotensin II Hypertension. Hypertension 2010, 55, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, Y.; Shioji, K.; Nakamura, H.; Masutani, H.; Yodoi, J. From Oxygen Sensing to Heart Failure: Role of Thioredoxin. Antioxid. Redox Signal. 2007, 9, 689–699. [Google Scholar] [CrossRef]
- Watson, W.H.; Yang, X.; Choi, Y.E.; Jones, D.P.; Kehrer, J.P. Thioredoxin and Its Role in Toxicology. Toxicol. Sci. 2004, 78, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Ago, T.; Sadoshima, J. Thioredoxin and ventricular remodeling. J. Mol. Cell. Cardiol. 2006, 41, 762–773. [Google Scholar] [CrossRef] [Green Version]
- Das, K.C.; Das, C.K. Thioredoxin, a singlet oxygen quencher and hydroxyl radical scavenger: Redox independent func-tions. Biochem. Biophys. Res. Commun. 2000, 277, 443–447. [Google Scholar] [CrossRef]
- Shioji, K.; Kishimoto, C.; Nakamura, H.; Masutani, H.; Yuan, Z.; Oka, S.-I.; Yodoi, J. Overexpression of Thioredoxin-1 in Transgenic Mice Attenuates Adriamycin-Induced Cardiotoxicity. Circulation 2002, 106, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.M.; Thirunavukkarasu, M.; Penumathsa, S.V.; Koneru, S.; Zhan, L.; Maulik, G.; Sudhakaran, P.R.; Maulik, N. Thioredoxin-1 gene therapy enhances angiogenic signaling and reduces ventricular remodeling in in-farcted myocardium of diabetic rats. Circulation 2010, 121, 1244–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balla, G.; Jacob, H.S.; Balla, J.; Rosenberg, M.; Nath, K.; Apple, F.; Eaton, J.W.; Vercellotti, G.M. Ferritin: A cytoprotective antioxidant strategem of endothelium. J. Biol. Chem. 1992, 267, 18148–18153. [Google Scholar] [CrossRef]
- Poss, K.D.; Tonegawa, S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc. Natl. Acad. Sci. USA 1997, 94, 10925–10930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocker, R.; Yamamoto, Y.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Bilirubin Is an Antioxidant of Possible Physiological Importance. Science 1987, 235, 1043–1046. [Google Scholar] [CrossRef]
- Wagener, F.A.D.T.G.; Volk, H.-D.; Willis, D.; Abraham, N.G.; Soares, M.; Adema, G.J.; Figdor, C. Different Faces of the Heme-Heme Oxygenase System in Inflammation. Pharmacol. Rev. 2003, 55, 551–571. [Google Scholar] [CrossRef] [Green Version]
- Katori, M.; Buelow, R.; Ke, B.; Ma, J.; Coito, A.J.; Iyer, S.; Southard, D.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Heme Oxygenase-1 Overexpression Protects Rat Hearts from Cold Ischemia/Reperfusion Injury Via an Antiapoptotic Pathway. Transplantation 2002, 73, 287–292. [Google Scholar] [CrossRef]
- Sedlak, T.W.; Snyder, S.H. Messenger molecules and cell death: Therapeutic implications. JAMA 2006, 295, 81–89. [Google Scholar] [CrossRef]
- Balla, J.; Jacob, H.S.; Balla, G.; Nath, K.; Eaton, J.W.; Vercellotti, G.M. Endothelial-cell heme uptake from heme proteins: Induction of sensitization and desensitization to oxidant damage. Proc. Natl. Acad. Sci. USA 1993, 90, 9285–9289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otterbein, L.E.; Foresti, R.; Motterlini, R. Heme Oxygenase-1 and Carbon Monoxide in the Heart: The Balancing Act Be-tween Danger Signaling and Pro-Survival. Circ. Res. 2016, 118, 1940–1959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, A.S.; Figueiredo-Pereira, C.; Vieira, H.L. Carbon monoxide and mitochondria-modulation of cell metabolism, redox response and cell death. Front. Physiol. 2015, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, L.G.; Agrawal, R.; Zhang, L.; Rezvani, M.; Mangi, A.A.; Ehsan, A.; Griese, D.P.; Dell’Acqua, G.; Mann, M.J.; Oyama, J.; et al. Gene therapy strategy for long-term myocardial protection using adeno-associated virus-mediated delivery of heme oxygenase gene. Circulation 2002, 105, 602–607. [Google Scholar] [CrossRef] [Green Version]
- Hinkel, R.; Lange, P.; Petersen, B.; Gottlieb, E.; Ng, J.K.M.; Finger, S.; Horstkotte, J.; Lee, S.; Thormann, M.; Knorr, M.; et al. Heme Oxygenase-1 Gene Therapy Provides Cardioprotection Via Control of Post-Ischemic Inflammation: An Experimental Study in a Pre-Clinical Pig Model. J. Am. Coll. Cardiol. 2015, 66, 154–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Simpson, J.A.; Brunt, K.R.; Ward, C.A.; Hall, S.R.R.; Kinobe, R.T.; Barrette, V.; Tse, M.Y.; Pang, S.C.; Pachori, A.S.; et al. Preemptive heme oxygenase-1 gene delivery reveals reduced mortality and preservation of left ventricular function 1 yr after acute myocardial infarction. Am. J. Physiol. Circ. Physiol. 2007, 293, H48–H59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Hamid, T.; Keith, R.J.; Zhou, G.; Partridge, C.R.; Xiang, X.; Kingery, J.R.; Lewis, R.K.; Li, Q.; Rokosh, G.; et al. Cardioprotective and Antiapoptotic Effects of Heme Oxygenase-1 in the Failing Heart. Circulation 2010, 121, 1912–1925. [Google Scholar] [CrossRef] [Green Version]
- Collino, M.; Pini, A.; Mugelli, N.; Mastroianni, R.; Bani, D.; Fantozzi, R.; Papucci, L.; Fazi, M.; Masini, E. Beneficial effect of prolonged heme oxygenase 1 activation in a rat model of chronic heart failure. Dis. Model. Mech. 2013, 6, 1012–1020. [Google Scholar] [CrossRef] [Green Version]
- Csonka, C.; Sárközy, M.; Pipicz, M.; Dux, L.; Csont, T. Modulation of Hypercholesterolemia-Induced Oxidative/Nitrative Stress in the Heart. Oxidative Med. Cell. Longev. 2015, 2016, 1–23. [Google Scholar] [CrossRef]
- Papatheodorou, L.; Weiss, N. Vascular Oxidant Stress and Inflammation in Hyperhomocysteinemia. Antioxid. Redox Signal. 2007, 9, 1941–1958. [Google Scholar] [CrossRef]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef]
- Roberts, C.K.; Sindhu, K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef]
- Mishra, M.; de Geest, B. High-Density Lipoprotein-Targeted Therapies for Heart Failure. Biomedicines 2020, 8, 620. [Google Scholar] [CrossRef]
- De Geest, B.; Mishra, M. Role of high-density lipoproteins in cardioprotection and in reverse remodeling: Therapeutic implications. Biochim. et Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2021, 1866, 159022. [Google Scholar] [CrossRef]
- Sengupta, S.; Wehbe, C.; Majors, A.K.; Ketterer, M.E.; DiBello, P.M.; Jacobsen, D.W. Relative Roles of Albumin and Ceruloplasmin in the Formation of Homocystine, Homocysteine-Cysteine-mixed Disulfide, and Cystine in Circulation. J. Biol. Chem. 2001, 276, 46896–46904. [Google Scholar] [CrossRef] [Green Version]
- Heinecke, J.W.; Rosen, H.; Suzuki, L.A.; Chait, A. The role of sulfur-containing amino acids in superoxide production and modification of low density lipoprotein by arterial smooth muscle cells. J. Biol. Chem. 1987, 262, 10098–10103. [Google Scholar] [CrossRef]
- Muthuramu, I.; Singh, N.; Amin, R.; Nefyodova, E.; Debasse, M.; Van Horenbeeck, I.; Jacobs, F.; De Geest, B. Selective homocysteine-lowering gene transfer attenuates pressure overload-induced cardiomyopathy via reduced oxidative stress. J. Mol. Med. 2015, 93, 609–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthuramu, I.; Jacobs, F.; Singh, N.; Gordts, S.C.; De Geest, B. Selective Homocysteine Lowering Gene Transfer Improves Infarct Healing, Attenuates Remodelling, and Enhances Diastolic Function after Myocardial Infarction in Mice. PLoS ONE 2013, 8, e63710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, M.M.; Moresco, R.N.; Duarte, T.; Santi, A.; Bagatini, M.D.; Da Cruz, I.B.; Schetinger, M.R.; Loro, V.L. Oxidative stress in hypercholesterolemia and its association with Ala16Val superoxide dismutase gene polymorphism. Clin. Biochem. 2010, 43, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Farnaghi, S.; Prasadam, I.; Cai, G.; Friis, T.; Du, Z.; Crawford, R.; Mao, X.; Xiao, Y. Protective effects of mitochondria-targeted antioxidants and statins on cholesterol-induced osteoarthritis. FASEB J. 2017, 31, 356–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, C.H.; Cosso, G.R.; Alberici, C.L.; Maciel, N.E.; Salerno, G.A.; Dorighello, G.G.; Velho, A.J.; De Faria, E.C.; Vercesi, A.E. Oxidative stress in atherosclerosis-prone mouse is due to low antioxidant capacity of mitochondria. FASEB J. 2005, 19, 278–280. [Google Scholar] [CrossRef]
- Muthuramu, I.; Mishra, M.; Aboumsallem, J.P.; Postnov, A.; Gheysens, O.; De Geest, B. Cholesterol lowering attenuates pressure overload-induced heart failure in mice with mild hypercholesterolemia. Aging 2019, 11, 6872–6891. [Google Scholar] [CrossRef]
- Muthuramu, I.; Amin, R.; Postnov, A.; Mishra, M.; Aboumsallem, J.P.; Dresselaers, T.; Himmelreich, U.; Van Veldhoven, P.P.; Gheysens, O.; Jacobs, F.; et al. Cholesterol-Lowering Gene Therapy Counteracts the Development of Non-ischemic Cardiomyopathy in Mice. Mol. Ther. 2017, 25, 2513–2525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Craeyveld, E.; Jacobs, F.; Gordts, S.C.; De Geest, B. Low-density lipoprotein receptor gene transfer in hypercholesterolemic mice improves cardiac function after myocardial infarction. Gene Ther. 2011, 19, 860–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.G.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative Stress in Obesity: A Critical Component in Human Diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aboumsallem, J.P.; Muthuramu, I.; Mishra, M.; De Geest, B. Cholesterol-Lowering Gene Therapy Prevents Heart Failure with Preserved Ejection Fraction in Obese Type 2 Diabetic Mice. Int. J. Mol. Sci. 2019, 20, 2222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soran, H.; Schofield, J.D.; Durrington, P.N. Antioxidant properties of HDL. Front. Pharmacol. 2015, 6, 222. [Google Scholar] [CrossRef] [Green Version]
- Mackness, M.; Arrol, S.; Abbott, C.; Durrington, P. Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase. Atheroscler. 1993, 104, 129–135. [Google Scholar] [CrossRef]
- De Geest, B.; Stengel, D.; Landeloos, M.; Lox, M.; Le Gat, L.; Collen, D.; Holvoet, P.; Ninio, E. Effect of overexpression of human apo A-I in C57BL/6 and C57BL/6 apo E-deficient mice on 2 lipoprotein-associated enzymes, platelet-activating factor acetylhydrolase and paraoxonase. Comparison of adenovirus-mediated human apo A-I gene transfer and human apo A-I transgenesis. Arter. Thromb Vasc. Biol. 2000, 20, 68–75. [Google Scholar]
- Tabet, F.; Rye, K.-A. High-density lipoproteins, inflammation and oxidative stress. Clin. Sci. 2009, 116, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Van Linthout, S.; Spillmann, F.; Riad, A.; Trimpert, C.; Lievens, J.; Meloni, M.; Escher, F.; Filenberg, E.; Demir, O.; Li, J.; et al. Human Apolipoprotein A-I Gene Transfer Reduces the Development of Experimental Diabetic Cardiomyopathy. Circulation 2008, 117, 1563–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Linthout, S.; Spillmann, F.; Lorenz, M.; Meloni, M.; Jacobs, F.; Egorova, M.; Stangl, V.; De Geest, B.; Schultheiss, H.-P.; Tschope, C. Vascular-Protective Effects of High-Density Lipoprotein Include the Downregulation of the Angiotensin II Type 1 Receptor. Hypertension 2009, 53, 682–687. [Google Scholar] [CrossRef]
- Wenzel, P.; Munzel, T. From menace to marvel: High-density lipoprotein prevents endothelial nitric oxide synthase uncoupling in diabetes mellitus by angiotensin II type 1 receptor downregulation. Hypertension 2009, 53, 587–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, R.; Muthuramu, I.; Aboumsallem, J.P.; Mishra, M.; Jacobs, F.; De Geest, B. Selective HDL-Raising Human Apo A-I Gene Therapy Counteracts Cardiac Hypertrophy, Reduces Myocardial Fibrosis, and Improves Cardiac Function in Mice with Chronic Pressure Overload. Int. J. Mol. Sci. 2017, 18, 2012. [Google Scholar] [CrossRef] [Green Version]
- Christison, J.; Karjalainen, A.; Brauman, J.; Bygrave, F.; Stocker, R. Rapid reduction and removal of HDL- but not LDL-associated cholesteryl ester hydroperoxides by rat liver perfused in situ. Biochem. J. 1996, 314, 739–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Eck, M.; Hoekstra, M.; Hildebrand, R.B.; Yaong, Y.; Stengel, D.; Kruijt, J.K.; Sattler, W.; Tietge, U.J.; Ninio, E.; Van Berkel, T.J.; et al. Increased Oxidative Stress in Scavenger Receptor BI Knockout Mice with Dysfunctional HDL. Arter. Thromb. Vasc. Biol. 2007, 27, 2413–2419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigotti, A.; Trigatti, B.L.; Penman, M.; Rayburn, H.; Herz, J.; Krieger, M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc. Natl. Acad. Sci. USA 1997, 94, 12610–12615. [Google Scholar] [CrossRef] [Green Version]
- Linton, M.F.; Tao, H.; Linton, E.F.; Yancey, P.G. SR-BI: A Multifunctional Receptor in Cholesterol Homeostasis and Atherosclerosis. Trends Endocrinol. Metab. 2017, 28, 461–472. [Google Scholar] [CrossRef]
- Muthuramu, I.; Amin, R.; Aboumsallem, J.P.; Mishra, M.; Robinson, E.L.; De Geest, B. Hepatocyte-Specific SR-BI Gene Transfer Corrects Cardiac Dysfunction in Scarb1 -Deficient Mice and Improves Pressure Overload-Induced Cardiomyopathy. Arter. Thromb. Vasc. Biol. 2018, 38, 2028–2040. [Google Scholar] [CrossRef] [Green Version]
- Mishra, M.; Muthuramu, I.; de Geest, B. HDL dysfunction, function, and heart failure. Aging 2019, 11, 293–294. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alles, J.; Fehlmann, T.; Fischer, U.; Backes, C.; Galata, V.; Minet, M.; Hart, M.; Abu-Halima, M.; Grässer, F.A.; Lenhof, H.-P.; et al. An estimate of the total number of true human miRNAs. Nucleic Acids Res. 2019, 47, 3353–3364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latronico, M.V.G.; Condorelli, G. MicroRNAs and cardiac pathology. Nat. Rev. Cardiol. 2009, 6, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Kura, B.; Bacova, B.S.; Kalocayova, B.; Sykora, M.; Slezak, J. Oxidative Stress-Responsive MicroRNAs in Heart Injury. Int. J. Mol. Sci. 2020, 21, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali Sheikh, M.S.; Salma, U.; Zhang, B.; Chen, J.; Zhuang, J.; Ping, Z. Diagnostic, Prognostic, and Therapeutic Value of Circulating miRNAs in Heart Failure Patients Associated with Oxidative Stress. Oxid Med. Cell. Longev. 2016, 2016, 5893064. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z. The Guideline of the Design and Validation of MiRNA Mimics. Methods Mol. Biol. 2011, 676, 211–223. [Google Scholar] [CrossRef]
- Ebert, M.S.; Sharp, P.A. MicroRNA sponges: Progress and possibilities. RNA 2010, 16, 2043–2050. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-W.; Zhang, C.; Lee, K.-C.; He, X.-J.; Lü, Z.-Q.; Huang, C.; Wu, Q.-C. Adenovirus-Mediated Gene Transfer of microRNA-21 Sponge Inhibits Neointimal Hyperplasia in Rat Vein Grafts. Int. J. Biol. Sci. 2017, 13, 1309–1319. [Google Scholar] [CrossRef] [Green Version]
- Gentner, B.; Schira, G.; Giustacchini, A.; Amendola, M.; Brown, B.D.; Ponzoni, M.; Naldini, L. Stable knockdown of microRNA in vivo by lentiviral vectors. Nat. Chem. Biol. 2008, 6, 63–66. [Google Scholar] [CrossRef]
- Simms, C.; Zaher, H.S. Quality control of chemically damaged RNA. Cell. Mol. Life Sci. 2016, 73, 3639–3653. [Google Scholar] [CrossRef] [Green Version]
- Robson, A. Oxidation of miRNAs by ROS leads to cardiac hypertrophy. Nat. Rev. Cardiol. 2020, 17, 678. [Google Scholar] [CrossRef] [PubMed]
- De Geest, B.; Mishra, M. Doxorubicin-induced cardiomyopathy: TERT gets to the heart of the matter. Mol. Ther. 2021, 29, 1363–1365. [Google Scholar] [CrossRef]
- Kang, Y.J.; Chen, Y.; Epstein, P. Suppression of Doxorubicin Cardiotoxicity by Overexpression of Catalase in the Heart of Transgenic Mice. J. Biol. Chem. 1996, 271, 12610–12616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.A.; Johnson, D.A.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Vargas, M.R.; Chen, P.C. The Nrf2-ARE pathway: An indicator and modulator of oxidative stress in neurodegeneration. Ann. N. Y. Acad. Sci. 2008, 1147, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-B.; Lai, X.; Guo, X.-F. Activation of Nrf2 by miR-152 Inhibits Doxorubicin-Induced Cardiotoxicity via Attenuation of Oxidative Stress, Inflammation, and Apoptosis. Oxidative Med. Cell. Longev. 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Itoh, K.; Tong, K.I.; Yamamoto, M. Molecular mechanism activating nrf2–keap1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 2004, 36, 1208–1213. [Google Scholar] [CrossRef]
- Masson, S.; Batkai, S.; Beermann, J.; Bär, C.; Pfanne, A.; Thum, S.; Magnoli, M.; Balconi, G.; Nicolosi, G.L.; Tavazzi, L.; et al. Circulating microRNA-132 levels improve risk prediction for heart failure hospitalization in patients with chronic heart failure. Eur. J. Heart Fail. 2018, 20, 78–85. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, D.; Wang, Q.; Shen, D.; Wang, Y.; Chen, B.; Zhang, J.; Gai, L. MiR-132 Inhibits Expression of SIRT1 and Induces Pro-inflammatory Processes of Vascular Endothelial Inflammation through Blockade of the SREBP-1c Metabolic Pathway. Cardiovasc. Drugs Ther. 2014, 28, 303–311. [Google Scholar] [CrossRef]
- Strum, J.C.; Johnson, J.H.; Ward, J.; Xie, H.; Feild, J.; Hester, A.; Alford, A.; Waters, K.M. MicroRNA 132 Regulates Nutritional Stress-Induced Chemokine Production through Repression of SirT. Mol. Endocrinol. 2009, 23, 1876–1884. [Google Scholar] [CrossRef] [Green Version]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Chen, B.; Zang, W.; Wang, J.; Huang, Y.; He, Y.; Yan, L.; Liu, J.; Zheng, W. The chemical biology of sirtuins. Chem. Soc. Rev. 2015, 44, 5246–5264. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Guarente, L. Sirtuins at a glance. J. Cell Sci. 2011, 124, 833–838. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Gao, X.; Wei, W. The crosstalk between Sirt1 and Keap1/Nrf2/ARE antioxidative pathway forms a positive feedback loop to inhibit FN and TGF-beta1 expressions in rat glomerular mesangial cells. Exp. Cell Res. 2017, 361, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Gurd, B.J. Deacetylation of PGC-1α by SIRT1: Importance for skeletal muscle function and exercise-induced mitochondrial biogenesis. Appl. Physiol. Nutr. Metab. 2011, 36, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S. PGC-1alpha, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid. Med. Cell. Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef] [Green Version]
- Ucar, A.; Gupta, S.K.; Fiedler, J.; Erikci, E.; Kardasinski, M.; Batkai, S.; Dangwal, S.; Kumarswamy, R.; Bang, C.; Holzmann, A. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat. Commun. 2012, 3, 1078. [Google Scholar] [CrossRef]
- Kops, G.; Dansen, T.B.; Polderman, P.E.; Saarloos, I.; Wirtz, K.W.A.; Coffer, P.J.; Huang, T.-T.; Bos, J.L.; Medema, R.; Burgering, B. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nat. Cell Biol. 2002, 419, 316–321. [Google Scholar] [CrossRef]
- Olmos, Y.; Valle, I.; Borniquel, S.; Tierrez, A.; Soria, E.; Lamas, S.; Monsalve, M. Mutual Dependence of Foxo3a and PGC-1α in the Induction of Oxidative Stress Genes. J. Biol. Chem. 2009, 284, 14476–14484. [Google Scholar] [CrossRef] [Green Version]
- Condorelli, G.; Ferrante, G. MicroRNA-132 Inhibition Prevents Myocardial Hypertrophy and Heart Failure in Pigs: Making Sense Out of Antisense. J. Am. Coll. Cardiol. 2021, 77, 2936–2938. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, K.; Liu, L.; Li, S. MicroRNA-132 promotes oxidative stress-induced pyroptosis by targeting sirtuin 1 in myocardial ischaemia-reperfusion injury. Int. J. Mol. Med. 2020, 45, 1942–1950. [Google Scholar] [CrossRef]
- Batkai, S.; Genschel, C.; Viereck, J.; Rump, S.; Bär, C.; Borchert, T.; Traxler, D.; Riesenhuber, M.; Spannbauer, A.; Lukovic, D.; et al. CDR132L improves systolic and diastolic function in a large animal model of chronic heart failure. Eur. Heart J. 2021, 42, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Hinkel, R.; Batkai, S.; Bähr, A.; Bozoglu, T.; Straub, S.; Borchert, T.; Viereck, J.; Howe, A.; Hornaschewitz, N.; Oberberger, L.; et al. AntimiR-132 Attenuates Myocardial Hypertrophy in an Animal Model of Percutaneous Aortic Constriction. J. Am. Coll. Cardiol. 2021, 77, 2923–2935. [Google Scholar] [CrossRef] [PubMed]
- Täubel, J.; Hauke, W.; Rump, S.; Viereck, J.; Batkai, S.; Poetzsch, J.; Rode, L.; Weigt, H.; Genschel, C.; Lorch, U.; et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: Results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur. Heart J. 2021, 42, 178–188. [Google Scholar] [CrossRef]
- Jones, N.; Hobbs, F.R.; Taylor, C.J. Prognosis following a diagnosis of heart failure and the role of primary care: A review of the literature. BJGP Open 2017, 1, 101013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Geest, B.; Mishra, M. Role of Oxidative Stress in Heart Failure: Insights from Gene Transfer Studies. Biomedicines 2021, 9, 1645. https://doi.org/10.3390/biomedicines9111645
De Geest B, Mishra M. Role of Oxidative Stress in Heart Failure: Insights from Gene Transfer Studies. Biomedicines. 2021; 9(11):1645. https://doi.org/10.3390/biomedicines9111645
Chicago/Turabian StyleDe Geest, Bart, and Mudit Mishra. 2021. "Role of Oxidative Stress in Heart Failure: Insights from Gene Transfer Studies" Biomedicines 9, no. 11: 1645. https://doi.org/10.3390/biomedicines9111645






