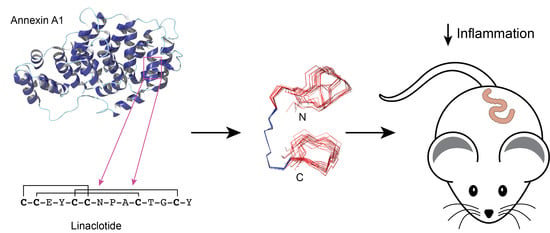
Engineering of an Anti-Inflammatory Peptide Based on the Disulfide-Rich Linaclotide Scaffold
Abstract
:1. Introduction
2. Experimental
2.1. Peptide Synthesis and Purification
2.2. NMR Spectroscopy and Structural Analysis
2.3. TNBS Colitis Assay
2.4. Tissue p-IκB-α (Ser32) and p-NF-κB p65 (Ser536) Measurements
3. Results
3.1. Peptide Design and Synthesis
3.2. Structural Analysis
3.3. TNBS Mouse Colitis Model
3.4. Tissue p-IκB-α (Ser32) and p-NF-κB p65 (Ser536) Measurements
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Yang, Y.L.; Hua, K.F.; Chuang, P.H.; Wu, S.H.; Wu, K.Y.; Chang, F.R.; Wu, Y.C. New cyclic peptides from the seeds of Annona squamosa L. and their anti-inflammatory activities. J. Agric. Food Chem. 2008, 56, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Svangård, E.; Göransson, U.; Hocaoglu, Z.; Gullbo, J.; Larsson, R.; Claeson, P.; Bohlin, L. Cytotoxic cyclotides from Viola tricolor. J. Nat. Prod. 2004, 67, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, K.R.; McKee, T.C.; Bokesch, H.R. Anti-HIV cyclotides. Curr. Protein Peptide Sci. 2004, 5, 331–340. [Google Scholar] [CrossRef]
- Tam, J.P.; Lu, Y.A.; Yang, J.L.; Chiu, K.W. An unusual structural motif of antimicrobial peptides containing end-to-end macrocycle and cystine-knot disulfides. Proc. Natl. Acad. Sci. USA 1999, 96, 8913–8918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, M.F.S.; Fensterseifer, I.C.M.; Migliolo, L.; Sousa, D.A.; de Capdville, G.; Arboleda-Valencia, J.W.; Colgrave, M.L.; Craik, D.J.; Magalhaes, B.S.; Dias, S.C.; et al. Identification and structural characterization of novel cyclotide with activity against an insect pest of sugar cane. J. Biol. Chem. 2012, 287, 134–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adessi, C.; Soto, C. Converting a peptide into a drug: Strategies to improve stability and bioavailability. Curr. Med. Chem. 2002, 9, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Ito, C.; Ribeiro, R.C.; Behm, F.G.; Raimondi, S.C.; Pui, C.H.; Campana, D. Cyclosporine A induces apoptosis in childhood acute lymphoblastic leukemia cells. Blood 1998, 91, 1001–1007. [Google Scholar] [PubMed]
- Miljanich, G.P. Ziconotide: Neuronal calcium channel blocker for treating severe chronic pain. Curr. Med. Chem. 2004, 11, 3029–3040. [Google Scholar] [CrossRef] [PubMed]
- Basus, V.J.; Nadasdi, L.; Ramachandran, J.; Miljanich, G.P. Solution structure of omega-conotoxin MVIIA using 2D NMR spectroscopy. FEBS Lett. 1995, 370, 163–169. [Google Scholar] [CrossRef]
- Rao, S.S.C.; Quigley, E.M.M.; Shiff, S.J.; Lavins, B.J.; Kurtz, C.B.; MacDougall, J.E.; Currie, M.G.; Johnston, J.M. Effect of linaclotide on severe abdominal symptoms in patients with irritable bowel syndrome with constipation. Clin. Gastroenterol. Hepatol. 2014, 12, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Lembo, A.J.; Lavins, B.J.; Shiff, S.J.; Kurtz, C.B.; Currie, M.G.; MacDougall, J.E.; Jia, X.D.; Shao, J.Z.; Fitch, D.A.; et al. Linaclotide for irritable bowel syndrome with constipation: A 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am. J. Gastroenterol. 2012, 107, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Cobos Caceres, C.; Bansal, P.S.; Navarro, S.; Wilson, D.; Don, L.; Giacomin, P.; Loukas, A.; Daly, N.L. An engineered cyclic peptide alleviates symptoms of inflammation in a murine model of inflammatory bowel disease. J. Biol. Chem. 2017, 292, 10288–10294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luckett, S.; Garcia, R.S.; Barker, J.J.; Konarev, A.V.; Shewry, P.R.; Clarke, A.R.; Brady, R.L. High-resolution structure of a potent, cyclic proteinase inhibitor from sunflower seeds. J. Mol. Biol. 1999, 290, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Daly, N.L.; Chen, Y.K.; Foley, F.M.; Bansal, P.S.; Bharathi, R.; Clark, R.J.; Sommerhoff, C.P.; Craik, D.J. The absolute structural requirement for a proline in the P3’-position of Bowman-Birk protease inhibitors is surmounted in the minimized SFTI-1 scaffold. J. Biol. Chem. 2006, 281, 23668–23675. [Google Scholar] [CrossRef] [PubMed]
- Lesner, A.; Legowska, A.; Wysocka, M.; Rolka, K. Sunflower trypsin inhibitor 1 as a molecular scaffold for drug discovery. Curr. Pharm. Des. 2011, 17, 4308–4317. [Google Scholar] [CrossRef] [PubMed]
- Ament, M.E. Inflammatory disease of the colon: Ulcerative colitis and Crohn’s colitis. J. Pediatr. 1975, 86, 322–334. [Google Scholar] [CrossRef]
- Navarro, S.; Ferreira, I.; Loukas, A. The hookworm pharmacopoeia for inflammatory diseases. Int. J. Parasitol. 2013, 43, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Molnar, T.; Farkas, K.; Szepes, Z.; Nagy, F.; Szucs, M.; Nyari, T.; Balint, A.; Wittmann, T. Long-term outcome of cyclosporin rescue therapy in acute, steroid-refractory severe ulcerative colitis. United Eur. Gastroenterol. J. 2014, 2, 108–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wangchuk, P.; Navarro, S.; Shepherd, C.; Keller, P.A.; Pyne, S.G.; Loukas, A. Diterpenoid alkaloids of Aconitum laciniatum and mitigation of inflammation by 14-O-acetylneoline in a murine model of ulcerative colitis. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Leoni, G.; Neumann, P.A.; Kamaly, N.; Quiros, M.; Nishio, H.; Jones, H.R.; Sumagin, R.; Hilgarth, R.S.; Alam, A.; Fredman, G.; et al. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J. Clin. Invest. 2015, 125, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Wüthrich, K. NMR studies of structure and function of biological macromolecules (Nobel lecture). J. Biomol. NMR 2003, 27, 13–39. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.L. Empirical analysis of backbone chemical shifts in proteins. Available online: http://www.bmrb.wisc.edu/published/Ikura_cs_study/part2_rc_aa_cs_stats.pdf (accessed on 6 November 2017).
- Güntert, P.; Buchner, L. Combined automated NOE assignment and structure calculation with CYANA. J. Biolmol. NMR 2015, 62, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Koradi, R.; Billeter, M.; Wüthrich, K. MOLMOL: A program for display and analysis of macromolecular structures. J. Mol. Graph. 1996, 14, 51–55. [Google Scholar] [CrossRef]
- Ferreira, I.; Smyth, D.; Gaze, S.; Aziz, A.; Giacomin, P.; Ruyssers, N.; Artis, D.; Laha, T.; Navarro, S.; Loukas, A.; et al. Hookworm excretory/secretory products induce interleukin-4 (il-4)(+) il-10(+) cd4(+) T cell responses and suppress pathology in a mouse model of colitis. Infect. Immun. 2013, 81, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Gariepy, J.; Lane, A.; Frayman, F.; Wilbur, D.; Robien, W.; Schoolnik, G.K.; Jardetzky, O. Structure of the toxic domain of Escherichia coli heat-stable enterotoxin ST I. Biochemistry 1986, 25, 7854–7866. [Google Scholar] [CrossRef] [PubMed]
- Güntert, P. Automated structure determination from NMR spectra. Eur. Biophys. J. 2009, 38, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Delaglio, F.; Cornilescu, G.; Bax, A. Talos+: A hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J. Biomol. NMR 2009, 44, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunasekera, S.; Foley, F.M.; Clark, R.J.; Sando, L.; Fabri, L.J.; Craik, D.J.; Daly, N.L. Engineering stabilized vascular endothelial growth factor-A antagonists: Synthesis, structural characterization, and bioactivity of grafted analogues of cyclotides. J. Med. Chem. 2008, 51, 7697–7704. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.; Gruber, C.W.; Cemazar, M.; Siatskas, C.; Tagore, P.; Payne, N.; Sun, G.Z.; Wang, S.H.; Bernard, C.C.; Craik, D.J. Molecular grafting onto a stable framework yields novel cyclic peptides for the treatment of multiple sclerosis. ACS Chem. Biol. 2014, 9, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, N.T.; Zhu, C.H.; Zhou, D.Y.; Nie, T.; Go, M.F.; Richards, R.J.; Rigas, B. MC-12, an annexin A1-based peptide, is effective in the treatment of experimental colitis. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Busby, R.W.; Bryant, A.P.; Bartolini, W.P.; Cordero, E.A.; Hannig, G.; Kessler, M.M.; Mahajan-Miklos, S.; Pierce, C.M.; Solinga, R.M.; Sun, L.J.; et al. Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. Eur. J. Pharmacol. 2010, 649, 328–335. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cobos, C.; Bansal, P.S.; Jones, L.; Wangchuk, P.; Wilson, D.; Loukas, A.; Daly, N.L. Engineering of an Anti-Inflammatory Peptide Based on the Disulfide-Rich Linaclotide Scaffold. Biomedicines 2018, 6, 97. https://doi.org/10.3390/biomedicines6040097
Cobos C, Bansal PS, Jones L, Wangchuk P, Wilson D, Loukas A, Daly NL. Engineering of an Anti-Inflammatory Peptide Based on the Disulfide-Rich Linaclotide Scaffold. Biomedicines. 2018; 6(4):97. https://doi.org/10.3390/biomedicines6040097
Chicago/Turabian StyleCobos, Claudia, Paramjit S. Bansal, Linda Jones, Phurpa Wangchuk, David Wilson, Alex Loukas, and Norelle L. Daly. 2018. "Engineering of an Anti-Inflammatory Peptide Based on the Disulfide-Rich Linaclotide Scaffold" Biomedicines 6, no. 4: 97. https://doi.org/10.3390/biomedicines6040097
APA StyleCobos, C., Bansal, P. S., Jones, L., Wangchuk, P., Wilson, D., Loukas, A., & Daly, N. L. (2018). Engineering of an Anti-Inflammatory Peptide Based on the Disulfide-Rich Linaclotide Scaffold. Biomedicines, 6(4), 97. https://doi.org/10.3390/biomedicines6040097