The Role of Gene Therapy in Premature Ovarian Insufficiency Management
Abstract
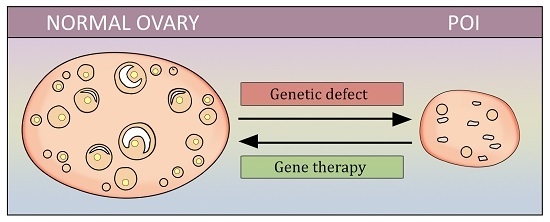
:1. Introduction
2. Gene Therapy for FSH-Receptor Defect Correction
3. Sal-Like 4 Genes as a Target of Gene Therapy in POI
4. Programmed Cell Death and Sphingomyelinase Gene
5. Neonatal Thymulin Gene Therapy
6. Basonuclin-1 Deficit as a Cause of POI
7. Future Directions
Author Contributions
Conflicts of Interest
References
- International Menopause Society. Available online: http://www.imsociety.org/menopause_terminology.php (accessed on 25 August 2018).
- Coulam, C.B.; Adamson, S.C.; Annegers, J.F. Incidence of premature ovarian failure. Obstet. Gynecol. 1986, 67, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Rees, M.; Purdie, D.W. Management of the Menopause, 5th ed.; Royal Society of Medicine: London, UK, 2009. [Google Scholar]
- Persani, L.; Rossetti, R.; Cacciatore, C. Genes involved in human premature ovarian failure. J. Mol. Endocrinol. 2010, 45, 257–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittenberger, M.D.; Hagerman, R.J.; Sherman, S.L.; McConkie-Rosell, A.; Welt, C.K.; Rebar, R.W.; Corrigan, E.C.; Simpson, J.L.; Nelson, L.M. The FMR1 premutation and reproduction. Fertil. Steril. 2007, 87, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, Z.J.; Qin, Y.; Shi, Y.; Wang, S.; Choi, Y.; Simpson, J.L.; Rajkovic, A. Transcription factor FIGLA is mutated in patients with premature ovarian failure. Am. J. Hum. Genet. 2008, 82, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, E.; Beck-Peccoz, P.; Persani, L. Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am. J. Hum. Genet. 2004, 75, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Touraine, P.; Beau, I.; Gougeon, A.; Meduri, G.; Desroches, A.; Pichard, C.; Detoeuf, M.; Paniel, B.; Prieur, M.; Zorn, J.R.; et al. New natural inactivating mutations of the follicle-stimulating hormone receptor: Correlations between receptor function and phenotype. Mol. Endocrinol. 1999, 13, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
- Nallathambi, J.; Moumné, L.; De Baere, E.; Beysen, D.; Usha, K.; Sundaresan, P.; Veitia, R.A. A novel polyalanine expansion in FOXL2: The first evidence for a recessive form of the blepharophimosis syndrome (BPES) associated with ovarian dysfunction. Hum. Genet. 2007, 121, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Shibanuma, K.; Tong, Z.B.; Vanderhoof, V.H.; Vanevski, K.; Nelson, L.M. Investigation of KIT gene mutations in women with 46,XX spontaneous premature ovarian failure. BMC Women’s Health 2002, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Chand, A.L.; Harrison, C.A.; Shelling, A.N. Inhibin and premature ovarian failure. Hum. Reprod. Update 2010, 16, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Caburet, S.; Arboleda, V.A.; Llano, E.; Overbeek, P.A.; Barbero, J.L.; Oka, K.; Harrison, W.; Vaiman, D.; Ben-Neriah, Z.; García-Tuñón, I.; et al. Mutant cohesin in premature ovarian failure. N. Engl. J. Med. 2014, 370, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Hehenkamp, W.J.; Volkers, N.A.; Broekmans, F.J.; de Jong, F.H.; Themmen, A.P.; Birnie, E.; Reekers, J.A.; Ankum, W.M. Loss of ovarian reserve after uterine artery embolization: A randomized comparison with hysterectomy. Hum. Reprod. 2007, 22, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Shuster, L.T.; Rhodes, D.J.; Gostout, B.S.; Grossardt, B.R.; Rocca, W.A. Premature menopause or early menopause: Long-term health consequences. Maturitas 2010, 65, 161–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera, C.M.; Grossardt, B.R.; Rhodes, D.J.; Brown, R.D., Jr.; Roger, V.L.; Melton, L.J., 3rd; Rocca, W.A. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause 2009, 16, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocca, W.A.; Grossardt, B.R.; de Andrade, M.; Malkasian, G.D.; Melton, L.J., 3rd. Survival patterns after oophorectomy in premenopausal women: A population-based cohort study. Lancet Oncol. 2006, 7, 821–828. [Google Scholar] [CrossRef]
- Nelson, L.M. Clinical practice. Primary ovarian insufficiency. N. Engl. J. Med. 2009, 360, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.M.; Anasti, J.N.; Kimzey, L.M.; Defensor, R.A.; Lipetz, K.J.; White, B.J.; Shawker, T.H.; Merino, M.J. Development of luteinized graafian follicles in patients with karyotypically normal spontaneous premature ovarian failure. J. Clin. Endocrinol. Metab. 1994, 79, 1470–1475. [Google Scholar] [PubMed]
- Popat, V.B.; Vanderhoof, V.H.; Calis, K.A.; Troendle, J.F.; Nelson, L.M. Normalization of serum luteinizing hormone levels in women with 46,XX spontaneous primary ovarian insufficiency. Fertil. Steril. 2008, 89, 429–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.J.; Schover, L.R.; Partridge, A.H.; Patrizio, P.; Wallace, W.H.; Hagerty, K.; Beck, L.N.; Brennan, L.V.; Oktay, K. American society of clinical oncology recommendations on fertility preservation in cancer patients. J. Clin. Oncol. 2006, 24, 2917–2931. [Google Scholar] [CrossRef] [PubMed]
- Anchan, R.M.; Ginsburg, E.S. Fertility concerns and preservation in younger women with breast cancer. Crit. Rev. Oncol. Hematol. 2010, 74, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Silber, S.; Kagawa, N.; Kuwayama, M.; Gosden, R. Duration of fertility after fresh and frozen ovary transplantation. Fertil. Steril. 2010, 94, 2191–2196. [Google Scholar] [CrossRef] [PubMed]
- Wuttke, W.; Seidlova-Wuttke, D. Herbal medicines for menopausal symptoms. Maturitas 2015, 81, 120. [Google Scholar] [CrossRef]
- Aittomäki, K.; Lucena, J.D.; Pakarinen, P.; Sistonen, P.; Tapanainen, J.; Gromoll, J.; Kaskikari, R.; Sankila, E.M.; Lehväslaiho, H.; Engel, A.R.; et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell 1995, 82, 959–968. [Google Scholar] [CrossRef] [Green Version]
- Tapanainen, J.S.; Aittomäki, K.; Min, J.; Vaskivuo, T.; Huhtaniemi, I.T. Men homozygous for an inactivating mutation of the follicle-stimulating hormone (FSH) receptor gene present variable suppression of spermatogenesis and fertility. Nat. Genet. 1997, 15, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Beau, I.; Touraine, P.; Meduri, G.; Gougeon, A.; Desroches, A.; Matuchansky, C.; Milgrom, E.; Kuttenn, F.; Misrahi, M. A novel phenotype related to partial loss of function mutations of the follicle stimulating hormone receptor. J. Clin. Investig. 1998, 102, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Doherty, E.; Pakarinen, P.; Tiitinen, A.; Kiilavuori, A.; Huhtaniemi, I.; Forrest, S.; Aittomäki, K. A Novel mutation in the FSH receptor inhibiting signal transduction and causing primary ovarian failure. J. Clin. Endocrinol. Metab. 2002, 87, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.A.; Achermann, J.C.; Pakarinen, P.; Kotlar, T.J.; Huhtaniemi, I.T.; Jameson, J.L.; Cheetham, T.D.; Ball, S.G. A novel loss of function mutation in exon 10 of the FSH receptor gene causing hypergonadotrophic hypogonadism: Clinical and molecular characteristics. Hum. Reprod. 2003, 18, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Talbert, L.M.; Raj, M.H.; Hammond, M.G.; Greer, T. Endocrine and immunologic studies in a patient with resistant ovary syndrome. Fertil. Steril. 1984, 42, 741–744. [Google Scholar] [CrossRef]
- Hallenbeck, P.L.; Stevenson, S.C. Targetable gene delivery vectors. Adv. Exp. Med. Biol. 2000, 465, 37–46. [Google Scholar] [PubMed]
- Al-Hendy, A.; Lee, E.J.; Wang, H.Q.; Copland, J.A. Gene therapy of uterine leiomyomas: Adenovirus-mediated expression of dominant negative estrogen receptor inhibits tumor growth in nude mice. Am. J. Obstet. Gynecol. 2004, 191, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.H.; Othman, E.E.; Hornung, D.; Al-Hendy, A. Gene therapy of benign gynecological diseases. Adv. Drug Deliv. Rev. 2009, 61, 822–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, M.; Zhang, D.; Salama, S.; Hamada, F.; Arafa, H.; Fouad, H.; Walker, C.; Al-Hendy, A. Towards fibroid gene therapy: Adenovirus-mediated delivery of herpes simplex virus 1 thymidine kinase gene/ganciclovir shrinks uterine leiomyoma in the Eker rat model. Gynecol. Obstet. Investig. 2009, 68, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Ghadami, M.; Salama, S.A.; Khatoon, N.; Chilvers, R.; Nagamani, M.; Chedrese, P.J.; Al-Hendy, A. Toward gene therapy of primary ovarian failure: Adenovirus expressing human FSH receptor corrects the Finnish C566T mutation. Mol. Hum. Reprod. 2008, 14, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ghadami, M.; El-Demerdash, E.; Salama, S.A.; Binhazim, A.A.; Archibong, A.E.; Chen, X.; Ballard, B.R.; Sairam, M.R.; Al-Hendy, A. Toward gene therapy of premature ovarian failure: Intraovarian injection of adenovirus expressing human FSH receptor restores folliculogenesis in FSHR(−/−) FORKO mice. Mol. Hum. Reprod. 2010, 16, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Dierich, A.; Sairam, M.R.; Monaco, L.; Fimia, G.M.; Gansmuller, A.; LeMeur, M.; Sassone-Corsi, P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: Targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc. Natl. Acad. Sci. USA 1998, 95, 13612–13617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danilovich, N.; Babu, P.S.; Xing, W.; Gerdes, M.; Krishnamurthy, H.; Sairam, M.R. Estrogen deficiency, obesity, and skeletal abnormalities in follicle-stimulating hormone receptor knockout (FORKO) female mice. Endocrinology 2000, 141, 4295–4308. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, D.; Münsterberg, A. The vertebrate spalt genes in development and disease. Dev. Biol. 2006, 293, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Deloukas, P.; Earthrowl, M.E.; Grafham, D.V.; Rubenfield, M.; French, L.; Steward, C.A.; Sims, S.K.; Jones, M.C.; Searle, S.; Scott, C.; et al. The DNA sequence and comparative analysis of human chromosome 10. Nature 2004, 429, 375–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohlhase, J.; Heinrich, M.; Liebers, M.; Archangelo, L.F.; Reardon, W.; Kispert, A. Cloning and expression analysis of SALL4, the murine homologue of the gene mutated in Okihiro syndrome. Cytogenet. Genome Res. 2002, 98, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, L.; Ni, F.; Song, J.; Wang, J.; Mu, Y.; Ma, X.; Cao, Y. Mutational analysis of SAL-Like 4 (SALL4) in Han Chinese women with premature ovarian failure. Mol. Hum. Reprod. 2009, 15, 557–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Li, L.; Xie, X.; Wang, J.; Yan, J.; Mu, Y.; Ma, X. Genetic variation of SAL-Like 4 (SALL4) in ventricular septal defect. Int. J. Cardiol. 2010, 145, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Aguila, J.R.; Liao, W.; Yang, J.; Avila, C.; Hagag, N.; Senzel, L.; Ma, Y. SALL4 is a robust stimulator for the expansion of hematopoietic stem cells. Blood 2011, 118, 576–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabban, J.T.; Zaloudek, C.J. A practical approach to immunohistochemical diagnosis of ovarian germ cell tumours and sex cord-stromal tumours. Histopathology 2013, 62, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Guo, S.; Allan, R.W.; Molberg, K.H.; Peng, Y. SALL4 is a novel sensitive and specific marker of ovarian primitive germ cell tumors and is particularly useful in distinguishing yolk sac tumor from clear cell carcinoma. Am. J. Surg. Pathol. 2009, 33, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Humphrey, P.A.; Allan, R.W. SALL4 is a novel sensitive and specific marker for metastatic germ cell tumors, with particular utility in detection of metastatic yolk sac tumors. Cancer 2009, 115, 2640–2651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, D.; Li, J.; Guo, C.C.; Allan, R.W.; Humphrey, P.A. SALL4 is a novel diagnostic marker for testicular germ cell tumors. Am. J. Surg. Pathol. 2009, 33, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Gnemmi, V.; Leteurtre, E.; Sudour-Bonnange, H.; Devisme, L.; Guettier, C.; Buob, D.; Leroy, X. SALL4 is a marker of the embryonal subtype of hepatoblastoma. Histopathology 2013, 63, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Roibon, N.; Katz, B.; Chaux, A.; Sharma, R.; Munari, E.; Faraj, S.F.; Illei, P.B.; Torbenson, M.; Netto, G.J. Immunohistochemical expression of SALL4 in hepatocellular carcinoma, a potential pitfall in the differential diagnosis of yolk sac tumors. Hum. Pathol. 2013, 44, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, T.; Kamiya, A.; Zeniya, M.; Chikada, H.; Hyuck, A.D.; Yamazaki, Y.; Wauthier, E.; Tajiri, H.; Miller, L.D.; Wang, X.W.; et al. Sal-like protein 4 (SALL4), a stem cell biomarker in liver cancers. Hepatology 2013, 57, 1469–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, S.S.; Yamashita, T.; Kondo, M.; Nio, K.; Hayashi, T.; Hara, Y.; Nomura, Y.; Yoshida, M.; Hayashi, T.; Oishi, N.; et al. The transcription factor SALL4 regulates stemness of EpCAM-positive hepatocellular carcinoma. J. Hepatol. 2014, 60, 127–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, K.J.; Gao, C.; Lim, J.S.; Yan, B.; Yang, H.; Dimitrov, T.; Kawasaki, A.; Ong, C.W.; Wong, K.F.; Lee, S.; et al. Oncofetal gene SALL4 in aggressive hepatocellular carcinoma. N. Engl. J. Med. 2013, 369, 1171–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, Y.; Tilly, J.L. Oocyte apoptosis: Like sand through an hourglass. Dev. Biol. 1999, 213, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Perez, G.I.; Robles, R.; Knudson, C.M.; Flaws, J.A.; Korsmeyer, S.J.; Tilly, J.L. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat. Genet. 1999, 21, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, T. Cell death genes may hold clues to preserving fertility after chemotherapy. J. Natl. Cancer Inst. 1999, 91, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Thompson, C.B. Apoptosis and disease: Regulation and clinical relevance of programmed cell death. Annu. Rev. Med. 1997, 48, 267–281. [Google Scholar] [PubMed]
- Thornberry, N.A.; Lazebnik, Y. Caspases: Enemies within. Science 1998, 281, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Cryns, V.; Yuan, J. Proteases to die for. Genes Dev. 1998, 12, 1551–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, J.; Chao, D.T.; Korsmeyer, S.J. BAX-induced cell death may not require interleukin-1β-converting enzyme-like proteases. Proc. Natl. Acad. Sci. USA 1996, 93, 14559–14563. [Google Scholar] [CrossRef] [PubMed]
- Kolesnick, R.N.; Krönke, M. Regulation of ceramide production and apoptosis. Annu. Rev. Physiol. 1998, 60, 643–665. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Cuvillier, O.; Edsall, L.C.; Kohama, T.; Menzeleev, R.; Olah, Z.; Olivera, A.; Pirianov, G.; Thomas, D.M.; Tu, Z.; et al. Sphingosine-1-phosphate in cell growth and cell death. Ann. N. Y. Acad. Sci. 1998, 845, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.; Kohama, T.; Edsall, L.; Nava, V.; Cuvillier, O.; Poulton, S.; Spiegel, S. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J. Cell Biol. 1999, 147, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Cuvillier, O.; Pirianov, G.; Kleuser, B.; Vanek, P.G.; Coso, O.A.; Gutkind, J.S.; Spiegel, S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 1996, 381, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Perez, G.I.; Paris, F.; Miranda, S.R.; Ehleiter, D.; Haimovitz-Friedman, A.; Fuks, Z.; Xie, Z.; Reed, J.C.; Schuchman, E.H.; et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat. Med. 2000, 6, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, K.; Erlich, S.; Perl, D.P.; Ferlinz, K.; Bisgaier, C.L.; Sandhoff, K.; Desnick, R.J.; Stewart, C.L.; Schuchman, E.H. Acid sphingomyelinase deficient mice: A model of types A and B Niemann-Pick disease. Nat. Genet. 1995, 10, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Norred, W.P.; Bacon, C.W.; Riley, R.T.; Merrill, A.H. Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J. Biol. Chem. 1991, 266, 14486–14490. [Google Scholar] [PubMed]
- Bose, R.; Verheij, M.; Haimovitz-Friedman, A.; Scotto, K.; Fuks, Z.; Kolesnick, R. Ceramide synthase mediates daunorubicin-induced apoptosis: An alternative mechanism for generating death signals. Cell 1995, 82, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Perez, G.I.; Tao, X.J.; Tilly, J.L. Fragmentation and death (a.k.a. apoptosis) of ovulated oocytes. Mol. Hum. Reprod. 1999, 5, 414–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Brocklyn, J.R.; Lee, M.J.; Menzeleev, R.; Olivera, A.; Edsall, L.; Cuvillier, O.; Thomas, D.M.; Coopman, P.J.; Thangada, S.; Liu, C.H.; et al. Dual actions of sphingosine-1-phosphate: Extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J. Cell Biol. 1998, 142, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Van Brocklyn, J.R.; Tu, Z.; Edsall, L.C.; Schmidt, R.R.; Spiegel, S. Sphingosine 1-phosphate-induced cell rounding and neurite retraction are mediated by the G protein-coupled receptor H218. J. Biol. Chem. 1999, 274, 4626–4632. [Google Scholar] [CrossRef] [PubMed]
- Edsall, L.C.; Pirianov, G.G.; Spiegel, S. Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation. J. Neurosci. 1997, 17, 6952–6960. [Google Scholar] [CrossRef] [PubMed]
- Rebar, R.W.; Morandini, I.C.; Erickson, G.F.; Petze, J.E. The hormonal basis of reproductive defects in athymic mice: Diminished gonadotropin concentrations in prepubertal females. Endocrinology 1981, 108, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, H.O.; Sorkin, E. Thymus involvement in female sexual maturation. Nature 1974, 249, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, S.P. ‘Nude’, a new hairless gene with pleiotropic effects in the mouse. Genet. Res. 1966, 8, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Lintern-Moore, S.; Pantelouris, E.M. Ovarian development in athymic nude mice. The size and composition of the follicle population. Mech. Ageing Dev. 1975, 4, 385–390. [Google Scholar] [CrossRef]
- Michael, S.D.; Taguchi, O.; Nishizuka, Y. Effect of neonatal thymectomy on ovarian development and plasma LH, FSH, GH and PRL in the mouse. Biol. Reprod. 1980, 22, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Nishizuka, Y.; Sakakura, T. Ovarian dysgenesis induced by neonatal thymectomy in the mouse. Endocrinology 1971, 89, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Brown, O.A.; Sosa, Y.E.; Dardenne, M.; Pleúau, J.M.; Goya, R.G. Studies on the gonadotropin-releasing activity of thymulin: Changes with age. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2000, 55, B170–B176. [Google Scholar] [CrossRef]
- Hinojosa, L.; García, L.; Domínguez, R.; Romano, M.C.; Damián-Matsumura, P.G.; Castillo, L.; Rosas, P. Effects of thymulin and GnRH on the release of gonadotropins by in vitro pituitary cells obtained from rats in each day of estrous cycle. Life Sci. 2004, 76, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.A.; Kendall, M.D.; Gillham, B.; Jones, M.T. The release of luteinizing hormone from pituitaries perifused with thymic extracts. Thymus 1988, 12, 253–264. [Google Scholar] [PubMed]
- Hinojosa, L.; Chavira, R.; Dominguez, R.; Rosas, P. Effects of thymulin on spontaneous puberty and gonadotrophin-induced ovulation in prepubertal normal and hypothymic mice. J. Endocrinol. 1999, 163, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Wise, T. In vitro and in vivo effects of thymulin on rat testicular steroid synthesis. J. Steroid Biochem. Mol. Biol. 1998, 66, 129–135. [Google Scholar] [CrossRef]
- Reggiani, P.C.; Barbeito, C.G.; Zuccolilli, G.O.; Cónsole, G.M.; Flamini, A.M.; Dardenne, M.; Goya, R.G. Neonatal thymulin gene therapy prevents ovarian dysgenesis and attenuates reproductive derangements in nude female mice. Endocrinology 2012, 153, 3922–3928. [Google Scholar] [CrossRef] [PubMed]
- Goya, R.G.; Reggiani, P.C.; Vesenbeckh, S.M.; Pléau, J.M.; Sosa, Y.E.; Cónsole, G.M.; Schade, R.; Henklein, P.; Dardenne, M. Thymulin gene therapy prevents the reduction in circulating gonadotropins induced by thymulin deficiency in mice. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E182–E187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Liu, Y.; Zhang, Z.; Lv, P.; Liu, Y.; Li, J.; Wu, Y.; Zhang, R.; Huang, Y.; Xu, G.; et al. Basonuclin 1 deficiency is a cause of primary ovarian insufficiency. Hum. Mol. Genet. 2018, 27, 3787–3800. [Google Scholar] [CrossRef] [PubMed]

| Idiopathic | |
|---|---|
| Primary | Chromosomal disease |
| Follicle-stimulating hormone-receptor (FSHR) gene polymorphism | |
| Mutation of inhibin B | |
| Autoimmune disorders | |
| Enzymatic defects | |
| Secondary | Cancer treatment (chemotherapy, radiotherapy) |
| Ovarian surgery | |
| Uterine artery embolization | |
| Infections (mumps etc.) |
| Physiologic | Pregnancy |
| Intrauterine adhesions | Asherman syndrome |
| Tuberculous endometritis | |
| Hypothalamic | Functional hypothalamic amenorrhea |
| Pituitary | Prolactinoma |
| Empty sella syndrome | |
| Sheehan syndrome | |
| Cushing syndrome | |
| Ovarian | Polycystic ovarian syndrome |
| Others | Hypothyroidism |
| Ovarian tumors | |
| Congenital adrenal hyperplasia | |
| Adrenal tumors |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atabiekov, I.; Hobeika, E.; Sheikh, U.; El Andaloussi, A.; Al-Hendy, A. The Role of Gene Therapy in Premature Ovarian Insufficiency Management. Biomedicines 2018, 6, 102. https://doi.org/10.3390/biomedicines6040102
Atabiekov I, Hobeika E, Sheikh U, El Andaloussi A, Al-Hendy A. The Role of Gene Therapy in Premature Ovarian Insufficiency Management. Biomedicines. 2018; 6(4):102. https://doi.org/10.3390/biomedicines6040102
Chicago/Turabian StyleAtabiekov, Ihor, Elie Hobeika, Ujalla Sheikh, Abdeljabar El Andaloussi, and Ayman Al-Hendy. 2018. "The Role of Gene Therapy in Premature Ovarian Insufficiency Management" Biomedicines 6, no. 4: 102. https://doi.org/10.3390/biomedicines6040102