Microbiota and Radiotherapy: Unlocking the Potential for Improved Gastrointestinal Cancer Treatment
Abstract
:1. Introduction
1.1. Modulation of the Microbiota in Oncological Therapy
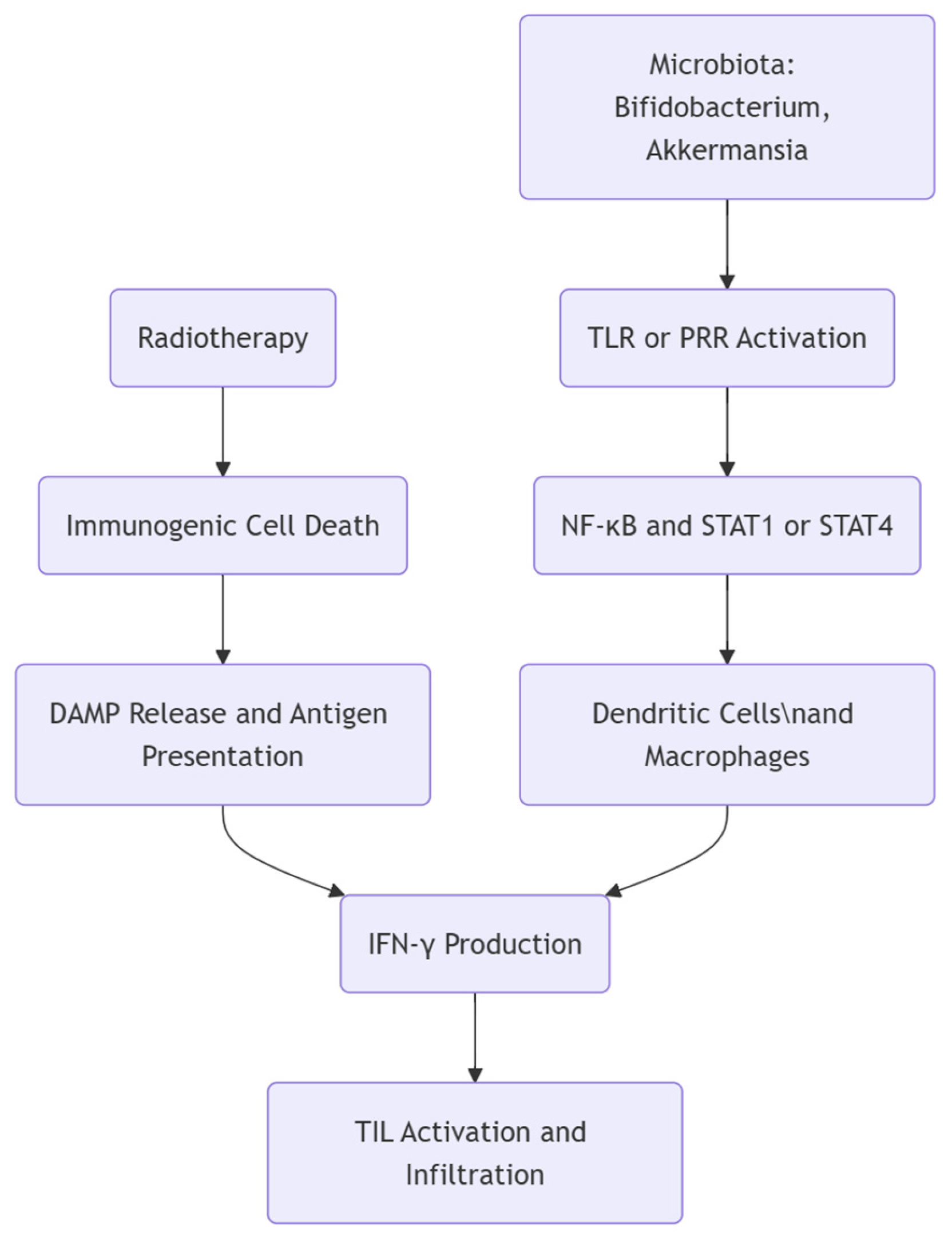
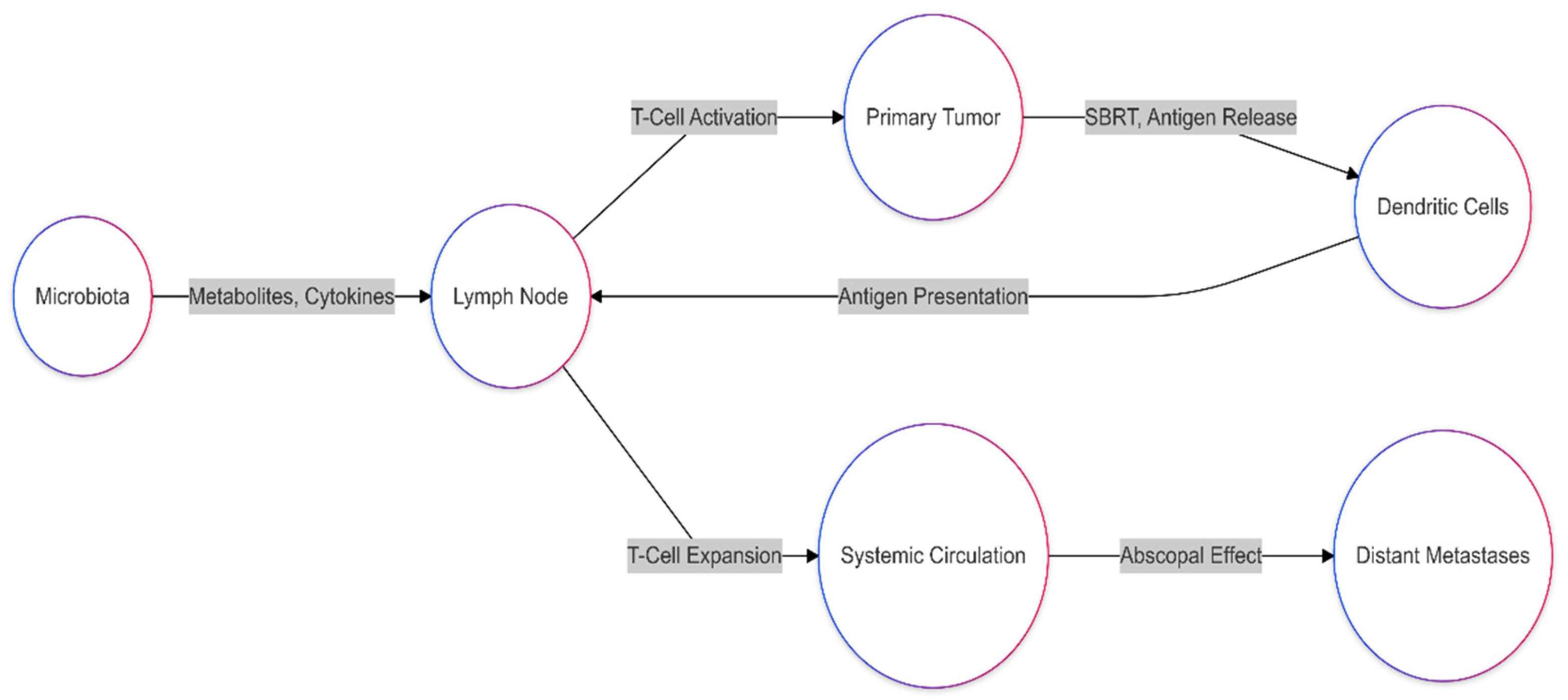
1.2. Interaction Between the Immune System and the Microbiota During Radiotherapy
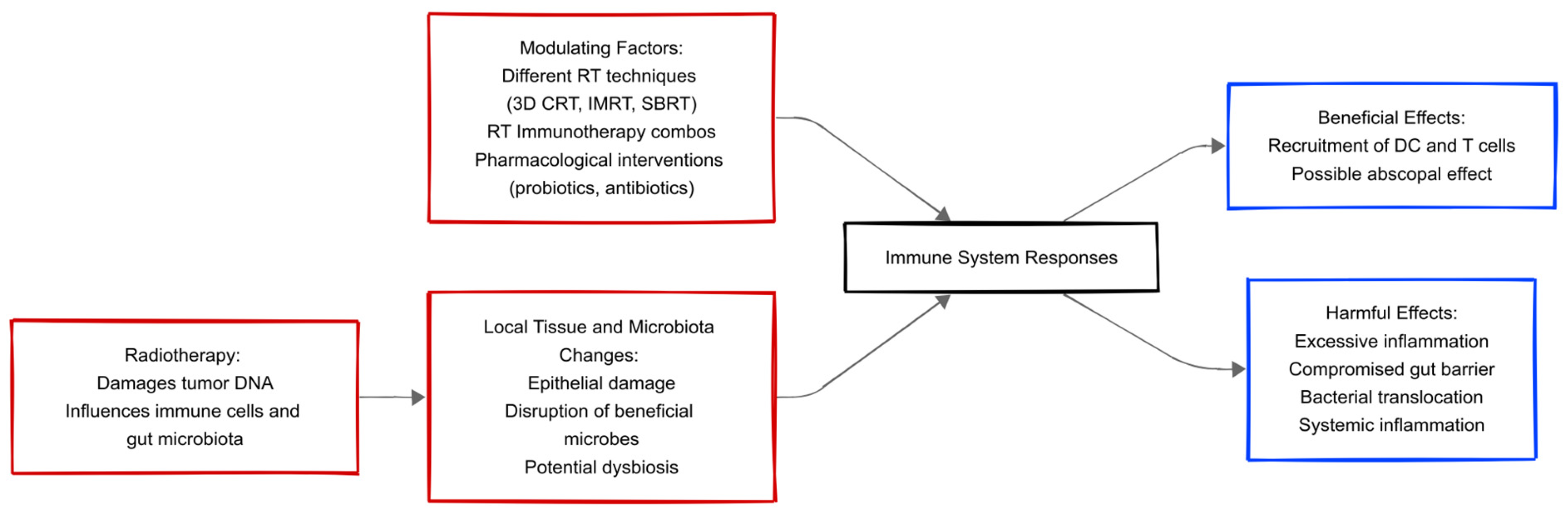
1.3. Effects of Radiotherapy on the Gut Microbiota
2. The Impact of Microbiota Modulation on the Treatment of Gastrointestinal Tract Tumors
2.1. Microbiota Modulation in Radiotherapy for Esophageal Cancer
2.2. Microbiota Modulation in Radiotherapy for Pancreatic–Biliary System Cancer
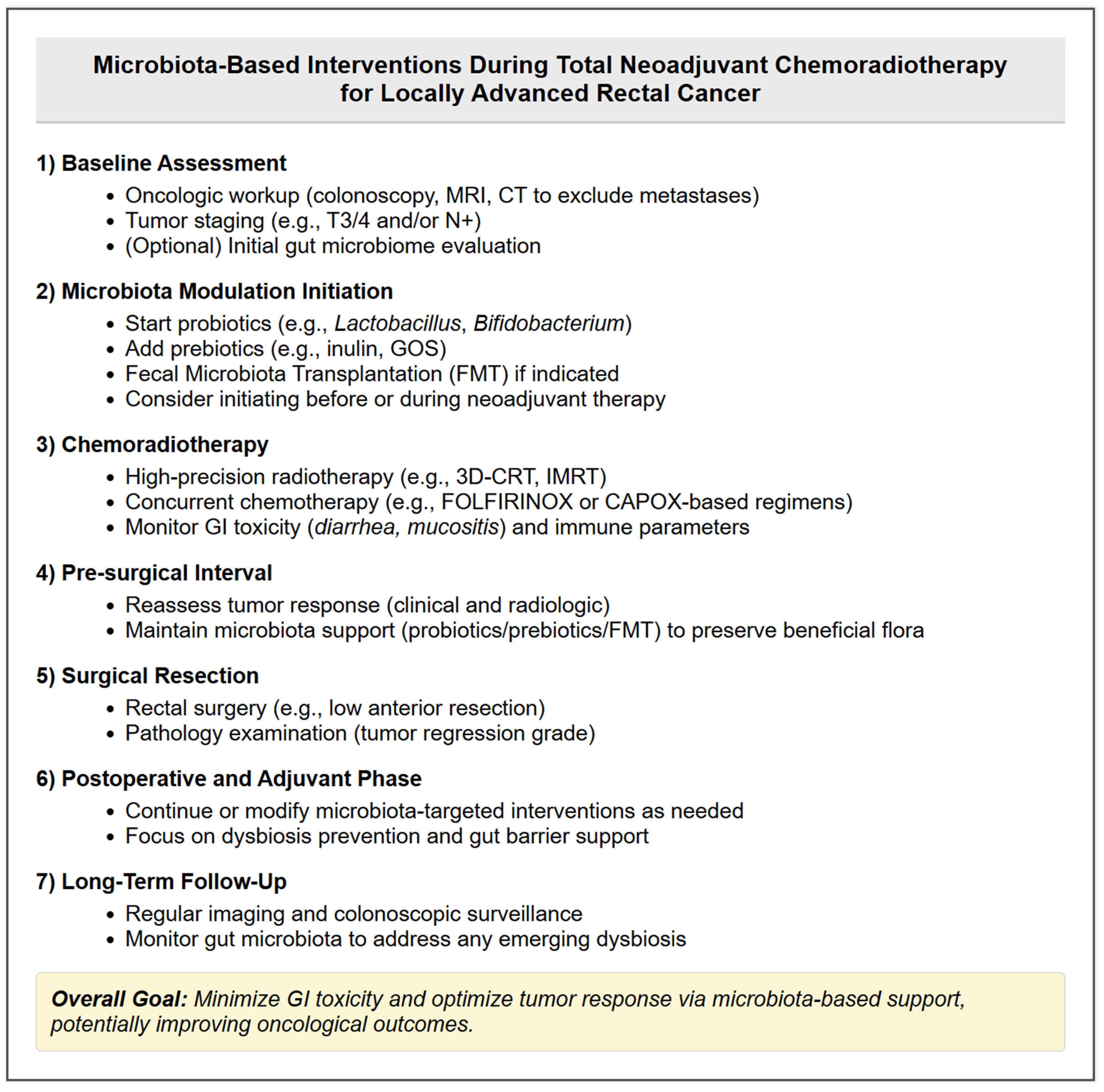
2.3. Microbiota Modulation in Radiotherapy for Rectal Cancer
3. Discussion
Limitations
4. Conclusions
5. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Citrin, D.E. Recent Developments in Radiotherapy. N. Engl. J. Med. 2017, 377, 1065–1075. [Google Scholar] [CrossRef]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Trinchieri, G. Microbiota: A Key Orchestrator of Cancer Therapy. Nat. Rev. Cancer 2017, 17, 271–285. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Hold, G.L.; Flint, H.J. The Gut Microbiota, Bacterial Metabolites and Colorectal Cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Zheng, D.W.; Dong, X.; Pan, P.; Chen, K.W.; Fan, J.X.; Cheng, S.X.; Zhang, X.Z. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nat. Biomed. Eng. 2019, 3, 717–728. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut Microbiome Influences Efficacy of PD-1-Based Immunotherapy Against Epithelial Tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Inohara, N.; Nuñez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal. Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Yue, J. Radiotherapy and the gut microbiome: Facts and fiction. Radiat. Oncol. 2021, 16, 9. [Google Scholar] [CrossRef]
- Tian, T.; Zhao, Y.; Yang, Y.; Wang, T.; Jin, S.; Guo, J.; Liu, Z. The protective role of short-chain fatty acids acting as signal molecules in chemotherapy- or radiation-induced intestinal inflammation. Am. J. Cancer Res. 2020, 10, 3508–3531. [Google Scholar]
- Danis, R.; Mego, M.; Antonova, M.; Stepanova, R.; Svobodnik, A.; Hejnova, R.; Wawruch, M. Orally Administered Probiotics in the Prevention of Chemotherapy (±Radiotherapy)-Induced Gastrointestinal Toxicity: A Systematic Review with Meta-Analysis of Randomized Trials. Integr. Cancer Ther. 2022, 21, 15347354221144309. [Google Scholar] [CrossRef]
- Sanchez-Gimenez, R.; Ahmed-Khodja, W.; Molina, Y.; Peiró, O.M.; Bonet, G.; Carrasquer, A.; Fragkiadakis, G.A.; Bulló, M.; Bardaji, A.; Papandreou, C. Gut Microbiota-Derived Metabolites and Cardiovascular Disease Risk: A Systematic Review of Prospective Cohort Studies. Nutrients 2022, 14, 2654. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cai, X.; Fei, W.; Ye, Y.; Zhao, M.; Zheng, C. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shakhpazyan, N.K.; Mikhaleva, L.M.; Bedzhanyan, A.L.; Gioeva, Z.V.; Mikhalev, A.I.; Midiber, K.Y.; Pechnikova, V.V.; Biryukov, A.E. Exploring the Role of the Gut Microbiota in Modulating Colorectal Cancer Immunity. Cells 2024, 13, 1437. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Hong, W.; Wang, B.; Chen, Y.; Yang, P.; Zhou, J.; Fan, J.; Zeng, Z.; Du, S. Gut microbiota modulate radiotherapy-associated antitumor immune responses against hepatocellular carcinoma Via STING signaling. Gut Microbes 2022, 14, 2119055. [Google Scholar] [CrossRef]
- Lombardo, C.; Fazio, R.; Sinagra, M.; Gattuso, G.; Longo, F.; Lombardo, C.; Salmeri, M.; Zanghì, G.N.; Loreto, C.A.E. Intratumoral Microbiota: Insights from Anatomical, Molecular, and Clinical Perspectives. J. Pers. Med. 2024, 14, 1083. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, G.; Kim, S.; Cho, B.; Kim, S.Y.; Do, E.J.; Bae, D.J.; Kim, S.; Kweon, M.N.; Song, J.S.; et al. Fecal microbiota transplantation improves anti-PD-1 inhibitor efficacy in unresectable or metastatic solid cancers refractory to anti-PD-1 inhibitor. Cell Host Microbe 2024, 32, 1380–1393.e9. [Google Scholar] [CrossRef]
- Uribe-Herranz, M.; Bittinger, K.; Rafail, S.; Guedan, S.; Pierini, S.; Tanes, C.; Ganetsky, A.; Morgan, M.A.; Gill, S.; Tanyi, J.L.; et al. Gut microbiota modulates adoptive cell therapy via CD8α dendritic cells and IL-12. JCI Insight 2018, 3, e94952. [Google Scholar] [CrossRef]
- Li, Z.; Ke, X.; Zuo, D.; Wang, Z.; Fang, F.; Li, B. New Insights into the Relationship between Gut Microbiota and Radiotherapy for Cancer. Nutrients 2022, 15, 48. [Google Scholar] [CrossRef]
- Herrera-Quintana, L.; Vázquez-Lorente, H.; Silva, R.C.M.C.; Olivares-Arancibia, J.; Reyes-Amigo, T.; Pires, B.R.B.; Plaza-Diaz, J. The Role of the Microbiome and of Radiotherapy-Derived Metabolites in Breast Cancer. Cancers 2024, 16, 3671. [Google Scholar] [CrossRef] [PubMed]
- Riehl, T.E.; Foster, L.; Stenson, W.F. Hyaluronic acid is radioprotective in the intestine through a TLR4 and COX-2-mediated mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G309–G316. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Lu, W.; Shen, L.; Wu, Y.; Zhang, Z. The gut microbiota as a booster for radiotherapy: Novel insights into radio-protection and radiation injury. Exp. Hematol. Oncol. 2023, 12, 48. [Google Scholar] [CrossRef]
- Jian, Y.; Zhang, D.; Liu, M.; Wang, Y.; Xu, Z.X. The Impact of Gut Microbiota on Radiation-Induced Enteritis. Front. Cell. Infect. Microbiol. 2021, 11, 586392. [Google Scholar] [CrossRef]
- Peretz, A.; Shlomo, I.B.; Nitzan, O.; Bonavina, L.; Schaffer, P.M.; Schaffer, M. Clostridium difficile Infection: Associations with Chemotherapy, Radiation Therapy, and Targeting Therapy Treatments. Curr. Med. Chem. 2016, 23, 4442–4449. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.; Dong, L.; Chang, P. Potential of Omega-3 Polyunsaturated Fatty Acids in Managing Chemotherapy- or Radiotherapy-Related Intestinal Microbial Dysbiosis. Adv. Nutr. 2019, 10, 133–147. [Google Scholar] [CrossRef]
- van den Ende, T.; de Clercq, N.C.; Davids, M.; Goedegebuure, R.; Doeve, B.H.; Ebrahimi, G.; Buijsen, J.; Hoekstra, R.; Mohammad, N.H.; Bijlsma, M.F.; et al. Fecal, duodenal, and tumor microbiota composition of esophageal carcinoma patients, a longitudinal prospective cohort. J. Natl. Cancer Inst. 2024, 116, 1834–1844. [Google Scholar] [CrossRef]
- Sasaki, T.; Matsumoto, Y.; Murakami, K.; Endo, S.; Toyozumi, T.; Otsuka, R.; Kinoshita, K.; Hu, J.; Iida, S.; Morishita, H.; et al. Gut microbiome can predict chemoradiotherapy efficacy in patients with esophageal squamous cell carcinoma. Esophagus 2023, 20, 691–703. [Google Scholar] [CrossRef]
- Jin, Y.; Dong, H.; Xia, L.; Yang, Y.; Zhu, Y.; Shen, Y.; Zheng, H.; Yao, C.; Wang, Y.; Lu, S. The Diversity of Gut Microbiome is Associated with Favorable Responses to Anti-Programmed Death 1 Immunotherapy in Chinese Patients With NSCLC. J. Thorac. Oncol. 2019, 14, 1378–1389. [Google Scholar] [CrossRef]
- Li, N.; Gao, L.; Ge, Y.; Zhao, L.; Wang, Y.; Bai, C. Impact of the gut microbiome on response and toxicity to chemotherapy in advanced esophageal cancer. Heliyon 2024, 10, e32770. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, J.; Shen, Z.; Zhang, Z.; Wang, S. Characterization of esophageal microbiota in patients with esophagitis and esophageal squamous cell carcinoma. Front. Cell. Infect. Microbiol. 2021, 11, 774330. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, X.; Sun, X.; Li, H.; Wei, J.; Lv, L.; Zhu, L. Investigating the causal role of the gut microbiota in esophageal cancer and its subtypes: A two-sample Mendelian randomization study. BMC Cancer 2024, 24, 416. [Google Scholar] [CrossRef] [PubMed]
- Song, S.T.; Cai, L.Y.; Zeng, X.; Xie, W.F. Gut Microbial Profile in Asymptomatic Gallstones. Front. Microbiol. 2022, 13, 882265. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Kim, Y.; Jeon, J.; Gwak, H.J.; Kim, M.; Kang, K.; Kim, Y.; Jeong, J.; Jung, Y.K.; Lee, K.G.; et al. Association of Microbial Dysbiosis with Gallbladder Diseases Identified by Bile Microbiome Profiling. J. Korean Med. Sci. 2021, 36, e189. [Google Scholar] [CrossRef]
- Li, J.J.; Zhu, M.; Kashyap, P.C.; Chia, N.; Tran, N.H.; McWilliams, R.R.; Bekaii-Saab, T.S.; Ma, W.W. The role of microbiome in pancreatic cancer. Cancer Metastasis Rev. 2021, 40, 777–789. [Google Scholar] [CrossRef]
- Yang, J.; He, Q.; Lu, F.; Chen, K.; Ni, Z.; Wang, H.; Zhou, C.; Zhang, Y.; Chen, B.; Bo, Z.; et al. A distinct microbiota signature precedes the clinical diagnosis of hepatocellular carcinoma. Gut Microbes 2023, 15, 2201159. [Google Scholar] [CrossRef]
- Rao, B.; Ren, T.; Wang, X.; Wang, H.; Zou, Y.; Sun, Y.; Liu, S.; Ren, Z.; Yu, Z. Dysbiosis in the Human Microbiome of Cholangiocarcinoma. Front. Physiol. 2021, 12, 715536. [Google Scholar] [CrossRef]
- Ketpueak, T.; Sriwichaiin, S.; Suparan, K.; Kerdphoo, S.; Charoentum, C.; Suksombooncharoen, T.; Chewaskulyong, B.; Chattipakorn, N.; Chattipakorn, S. Alteration of gut microbiota composition in patients with cholangiocarcinoma with non-responsiveness to first-line chemotherapy: A pilot study. J. Clin. Oncol. 2023, 41, 4104. [Google Scholar] [CrossRef]
- Ren, Z.; Li, A.; Jiang, J.; Zhou, L.; Yu, Z.; Lu, H.; Xie, H.; Chen, X.; Shao, L.; Zhang, R.; et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut 2019, 68, 1014–1023. [Google Scholar] [CrossRef]
- Hou, Y.; Li, J.; Ying, S. Tryptophan Metabolism and Gut Microbiota: A Novel Regulatory Axis Integrating the Microbiome, Immunity, and Cancer. Metabolites 2023, 13, 1166. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Fang, J.Y. Fusobacterium nucleatum, a key pathogenic factor and microbial biomarker for colorectal cancer. Trends Microbiol. 2023, 31, 159–172. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Jin, C.; Yue, K.; Sheng, D.; Zhang, T.; Dou, X.; Liu, J.; Jing, H.; Zhang, L.; et al. Prospective, longitudinal analysis of the gut microbiome in patients with locally advanced rectal cancer predicts response to neoadjuvant concurrent chemoradiotherapy. J. Transl. Med. 2023, 21, 221. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Qu, J.; Ke, X.; Zhang, Y.; Xu, H.; Lv, N.; Leng, J.; Zhang, Y.; Guan, A.; Feng, Y.; et al. Interaction between intratumoral microbiota and tumor mediates the response of neoadjuvant therapy for rectal cancer. Front. Microbiol. 2023, 14, 1229888. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Shen, L.; Shi, W.; Xia, F.; Zhang, H.; Wang, Y.; Zhang, J.; Wang, Y.; Sun, X.; Zhang, Z.; et al. Gut Microbiome Components Predict Response to Neoadjuvant Chemoradiotherapy in Patients with Locally Advanced Rectal Cancer: A Prospective, Longitudinal Study. Clin. Cancer Res. 2021, 27, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cui, B.; Nie, Y.; Sun, L.; Zhang, F. Radiation injury and gut microbiota-based treatment. Protein Cell 2024, 15, 83–97. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, C.J.; Choi, J.H.; Kim, J.-H.; Kim, J.-W.; Kim, J.-Y.; Nam, J.-S. The JAK2/STAT3/CCND2 Axis Promotes Colorectal Cancer Stem Cell Persistence and Radioresistance. J. Exp. Clin. Cancer Res. 2019, 38, 399. [Google Scholar] [CrossRef]
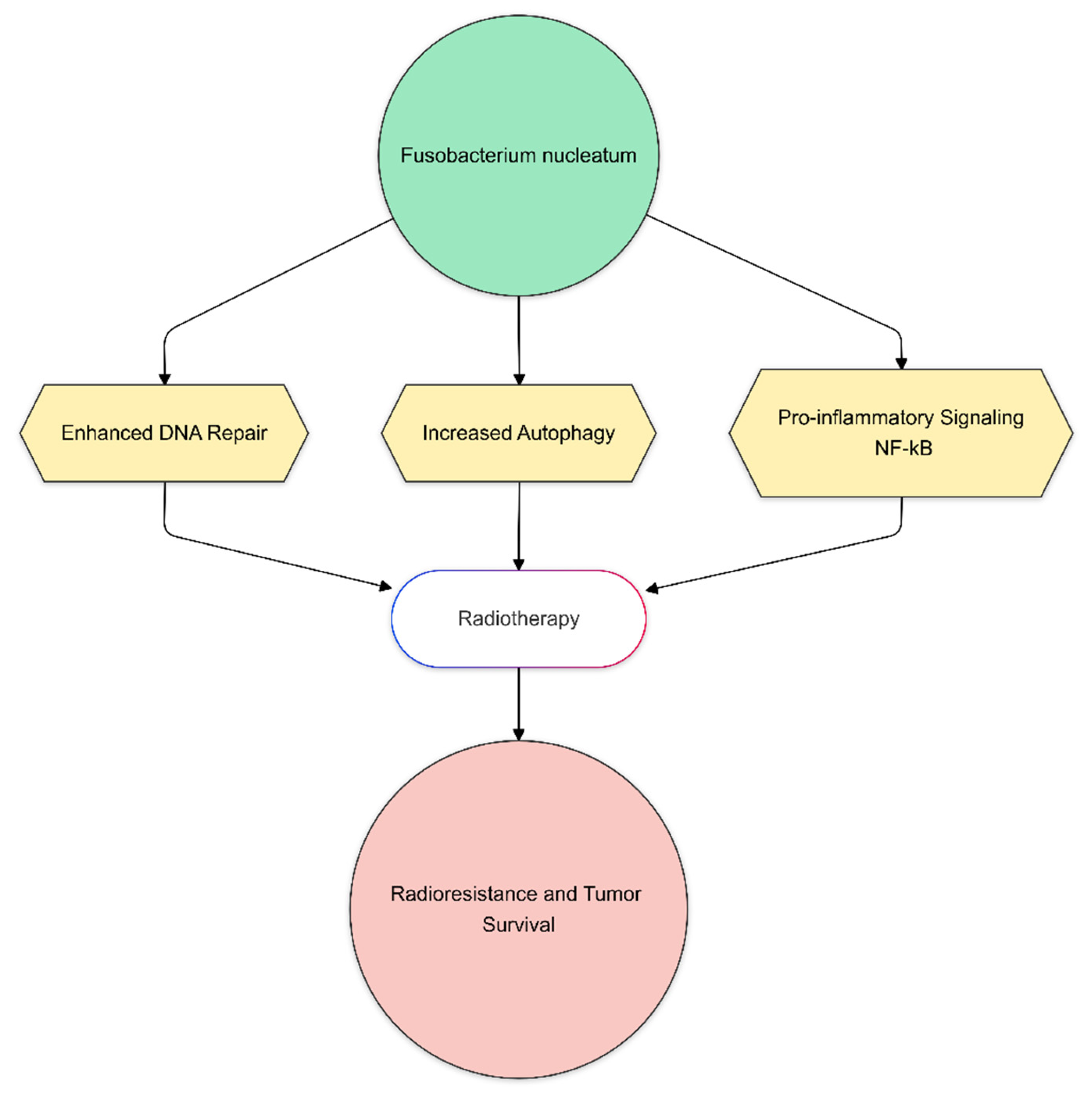
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef]
- Fernandes, A.; Oliveira, A.; Soares, R.; Barata, P. The Effects of Ionizing Radiation on Gut Microbiota, a Systematic Review. Nutrients 2021, 13, 3025. [Google Scholar] [CrossRef]
- Li, Y.; Kundu, P.; Seow, S.W.; de Matos, C.T.; Aronsson, L.; Chin, K.C.; Kärre, K.; Pettersson, S.; Greicius, G. Gut microbiota accelerate tumor growth via c-jun and STAT3 phosphorylation in APCMin/+ mice. Carcinogenesis 2012, 33, 1231–1238. [Google Scholar] [CrossRef]
- Colbert, L.E.; El Alam, M.B.; Wang, R.; Karpinets, T.; Lo, D.; Lynn, E.J.; Harris, T.A.; Elnaggar, J.H.; Yoshida-Court, K.; Tomasic, K.; et al. Tumor-resident Lactobacillus iners confer chemoradiation resistance through lactate-induced metabolic rewiring. Cancer Cell 2023, 41, 1945–1962.e11. [Google Scholar] [CrossRef] [PubMed]
- Pei, B.; Peng, S.; Huang, C.; Zhou, F. Bifidobacterium modulation of tumor immunotherapy and its mechanism. Cancer Immunol. Immunother. 2024, 73, 94. [Google Scholar] [CrossRef] [PubMed]
- Sims, T.T.; El Alam, M.B.; Karpinets, T.V.; Dorta-Estremera, S.; Hegde, V.L.; Nookala, S.; Yoshida-Court, K.; Wu, X.; Biegert, G.W.G.; Delgado Medrano, A.Y.; et al. Gut microbiome diversity is an independent predictor of survival in cervical cancer patients receiving chemoradiation. Commun. Biol. 2021, 4, 237. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, M.; Cardinali, M.; Di Pierro, F.; Zonzini, G.B.; Palazzi, C.M.; Gregoretti, A.; Zerbinati, N.; Guasti, L.; Bertuccioli, A. The Potential Role of Probiotics, Especially Butyrate Producers, in the Management of Gastrointestinal Mucositis Induced by Oncologic Chemo-Radiotherapy. Int. J. Mol. Sci. 2024, 25, 2306. [Google Scholar] [CrossRef]
- Liu, L.; Liang, L.; Luo, Y.; Han, J.; Lu, D.; Cai, R.; Sethi, G.; Mai, S. Unveiling the Power of Gut Microbiome in Predicting Neoadjuvant Immunochemotherapy Responses in Esophageal Squamous Cell Carcinoma. Research 2024, 7, 0529. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Q.; Li, T.; Lu, L.; Wang, F.; Zhang, H.; Liu, Z.; Ma, H.; Zhu, Q.; Wang, J.; et al. Lactobacillus plantarum-derived indole-3-lactic acid ameliorates colorectal tumorigenesis via epigenetic regulation of CD8+ T cell immunity. Cell Metab. 2023, 35, 943–960.e9. [Google Scholar] [CrossRef]
- Tintelnot, J.; Xu, Y.; Lesker, T.R.; Schönlein, M.; Konczalla, L.; Giannou, A.D.; Pelczar, P.; Kylies, D.; Puelles, V.G.; Bielecka, A.A.; et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature 2023, 615, 168–174. [Google Scholar] [CrossRef]
- Shi, W.; Shen, L.; Zou, W.; Wang, J.; Yang, J.; Wang, Y.; Liu, B.; Xie, L.; Zhu, J.; Zhang, Z. The Gut Microbiome Is Associated with Therapeutic Responses and Toxicities of Neoadjuvant Chemoradiotherapy in Rectal Cancer Patients-A Pilot Study. Front. Cell. Infect. Microbiol. 2020, 10, 562463. [Google Scholar] [CrossRef]
- Teng, H.; Wang, Y.; Sui, X.; Fan, J.; Li, S.; Lei, X.; Shi, C.; Sun, W.; Song, M.; Wang, H.; et al. Gut microbiota-mediated nucleotide synthesis attenuates the response to neoadjuvant chemoradiotherapy in rectal cancer. Cancer Cell 2023, 41, 124–138.e6. [Google Scholar] [CrossRef]
- Yang, L.; Li, A.; Wang, Y.; Zhang, Y. Intratumoral microbiota: Roles in cancer initiation, development and therapeutic efficacy. Signal Transduct. Target. Ther. 2023, 8, 35. [Google Scholar] [CrossRef]
- Ohsawa, M.; Nishi, H.; Emi, M.; Yoshikawa, T.; Hamai, Y.; Ibuki, Y.; Kurokawa, T.; Hirohata, R.; Kitasaki, N.; Kawada-Matsuo, M.; et al. Impact of Fusobacterium nucleatum in the treatment of cancer, including radiotherapy and its future potential in esophageal cancer. J. Radiat. Res. 2024, 65 (Suppl. S1), i126–i134. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, C.Y.; Lau, H.C.; Huang, Q.; Gong, H.; Sun, J.; Cao, P.; Hu, C.; Zhang, M.; Tao, L.; Zhou, L. Fusobacterium nucleatum impairs DNA mismatch repair and stability in patients with squamous cell carcinoma of the head and neck. Cancer 2022, 128, 3170–3184. [Google Scholar] [CrossRef] [PubMed]
- Hickey, A.; Stamou, P.; Udayan, S.; Ramón-Vázquez, A.; Esteban-Torres, M.; Bottacini, F.; Woznicki, J.A.; Hughes, O.; Melgar, S.; Ventura, M.; et al. Bifidobacterium breve Exopolysaccharide Blocks Dendritic Cell Maturation and Activation of CD4+ T Cells. Front. Microbiol. 2021, 12, 653587. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, F.; Gao, Y.; Kang, S.; Li, J.; Guo, J. Microbiome in radiotherapy: An emerging approach to enhance treatment efficacy and reduce tissue injury. Mol. Med. 2024, 30, 105. [Google Scholar] [CrossRef] [PubMed]
| Study | Participants (n) | Cancer Type | Treatment | Diversity Impact | Key Bacteria |
|---|---|---|---|---|---|
| Van den Ende et al. (2024) [27] | 172 | Predominantly adenocarcinoma | Neoadjuvant chemoradiotherapy | Lower diversity = poorer survival | Fusobacterium (poor outcomes) |
| Sasaki et al. (2023) [28] | 51 | Squamous cell carcinoma | Chemoradiotherapy | Higher diversity = better outcomes | Lactobacillaceae (favorable response), Fusobacteriaceae (advanced stages) |
| Li et al. (2024) [30] | 31 | Squamous cell carcinoma | Chemoradiotherapy | Higher diversity = better outcomes | Not specified |
| Jiang et al. (2021) [31] | 32 | Not explicitly stated | Chemoradiotherapy | Higher diversity = better outcomes | Bacteroides, Faecalibacterium (favorable response) |
| Bacterial Species | Mechanism of Action | Key Virulence (or Virulence-Associated) Genes | Impact on Radiotherapy/Chemoradiotherapy |
|---|---|---|---|
| Bifidobacterium spp. | Produces short-chain fatty acids (e.g., butyrate); enhances dendritic cell activation and T-cell priming |
| Boosts anti-tumor immune responses; improves treatment efficacy; and reduces toxicity |
| Faecalibacterium spp. | Produces butyrate; promotes regulatory T-cell differentiation and exerts anti-inflammatory effects |
| Associated with better clinical outcomes and diminished RT-induced toxicity |
| Lactobacillus spp. | Supports gut barrier integrity; produces anti-inflammatory metabolites; and modulates immune responses |
| Mitigates RT-induced inflammation and mucosal injury; enhances chemoradiotherapy responses |
| Fusobacterium nucleatum | Activates pro-inflammatory pathways (NF-κB); promotes DNA repair and autophagy |
| Contributes to radioresistance, potentially leading to poorer treatment responses |
| Akkermansia muciniphila | Stimulates Toll-like receptor signaling to boost IFN-γ production and antigen presentation |
| May enhance immune-mediated responses during RT/chemoradiotherapy |
| Clostridium sensu stricto 1 | May produce beneficial metabolites that support immune homeostasis and modulate local inflammatory responses |
| Correlated with favorable responses to neoadjuvant chemoradiotherapy in rectal cancer |
| Intestinimonas | Produces short-chain fatty acids; supports mucosal and immune homeostasis |
| Linked to improved response in patients undergoing neoadjuvant chemoradiotherapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vučinić, D.; Redžović, A.; Hauser, G.; Mikolašević, I. Microbiota and Radiotherapy: Unlocking the Potential for Improved Gastrointestinal Cancer Treatment. Biomedicines 2025, 13, 526. https://doi.org/10.3390/biomedicines13020526
Vučinić D, Redžović A, Hauser G, Mikolašević I. Microbiota and Radiotherapy: Unlocking the Potential for Improved Gastrointestinal Cancer Treatment. Biomedicines. 2025; 13(2):526. https://doi.org/10.3390/biomedicines13020526
Chicago/Turabian StyleVučinić, Damir, Arnela Redžović, Goran Hauser, and Ivana Mikolašević. 2025. "Microbiota and Radiotherapy: Unlocking the Potential for Improved Gastrointestinal Cancer Treatment" Biomedicines 13, no. 2: 526. https://doi.org/10.3390/biomedicines13020526
APA StyleVučinić, D., Redžović, A., Hauser, G., & Mikolašević, I. (2025). Microbiota and Radiotherapy: Unlocking the Potential for Improved Gastrointestinal Cancer Treatment. Biomedicines, 13(2), 526. https://doi.org/10.3390/biomedicines13020526







