Cetuximab–Toxin Conjugate and NPe6 with Light Enhanced Cytotoxic Effects in Head and Neck Squamous Cell Carcinoma In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Database Analysis
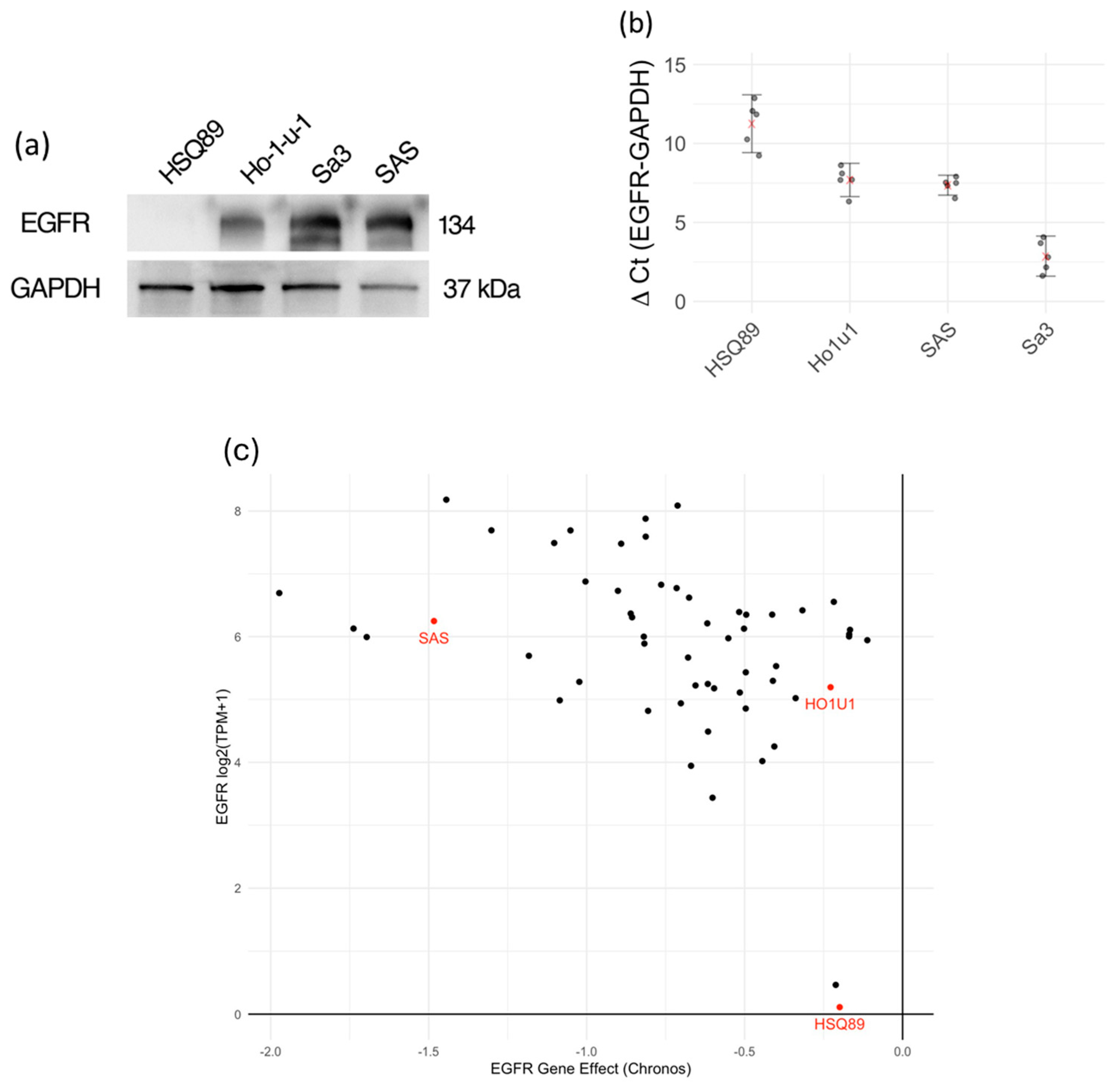
2.3. Reverse Transcription Real-Time PCR
2.4. Western Blot Analysis
2.5. Immunotoxin
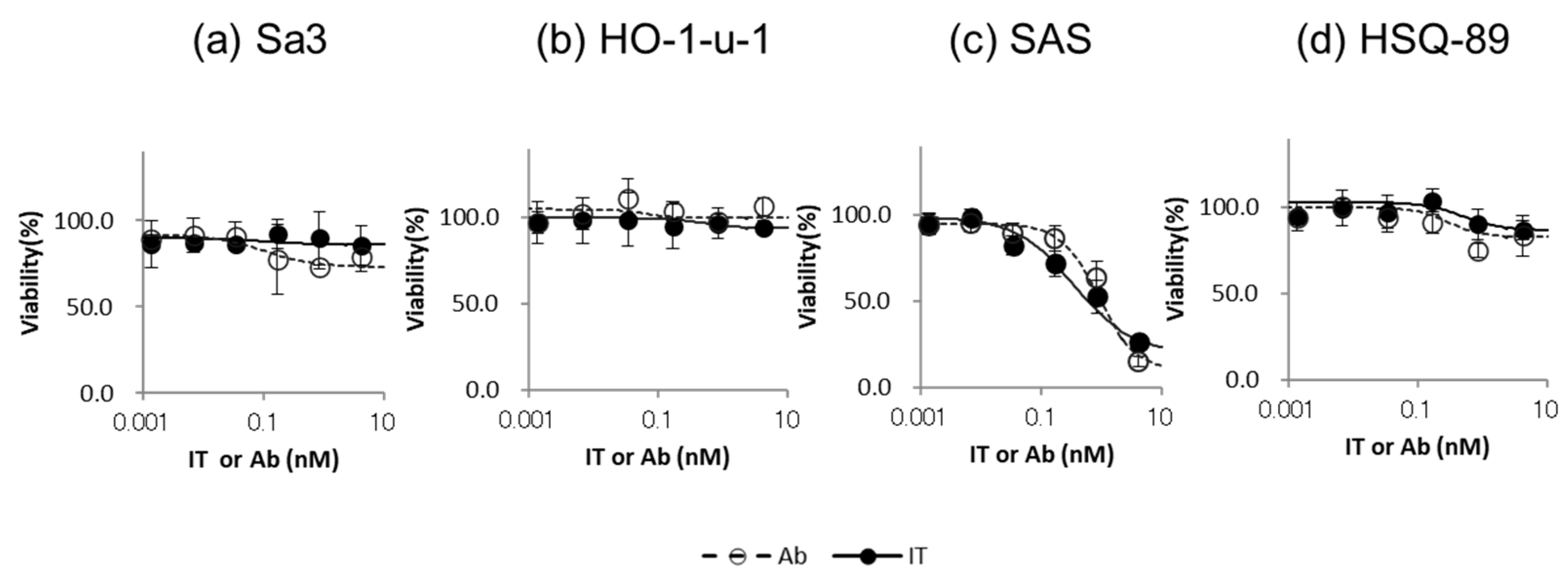
2.6. Cytotoxicity Assay of IT-Cmab and Cmab
2.7. Light Sources
2.8. Photosensitizer
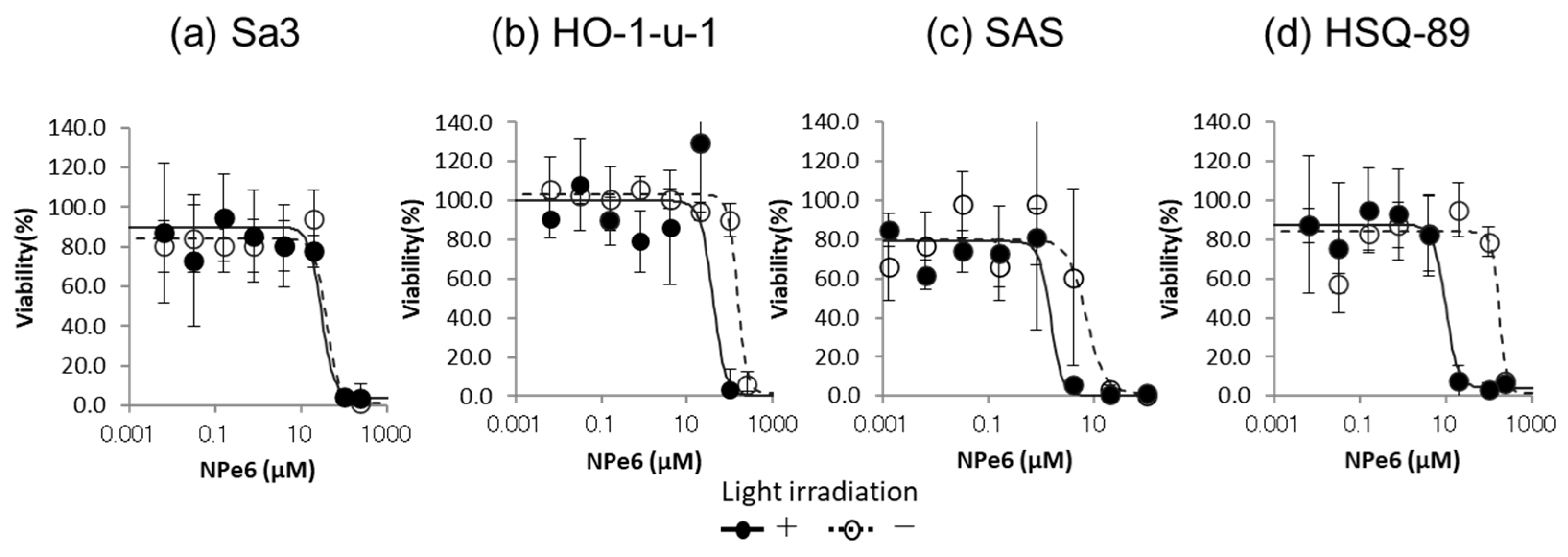
2.9. Cytotoxicity Assay of NPe6
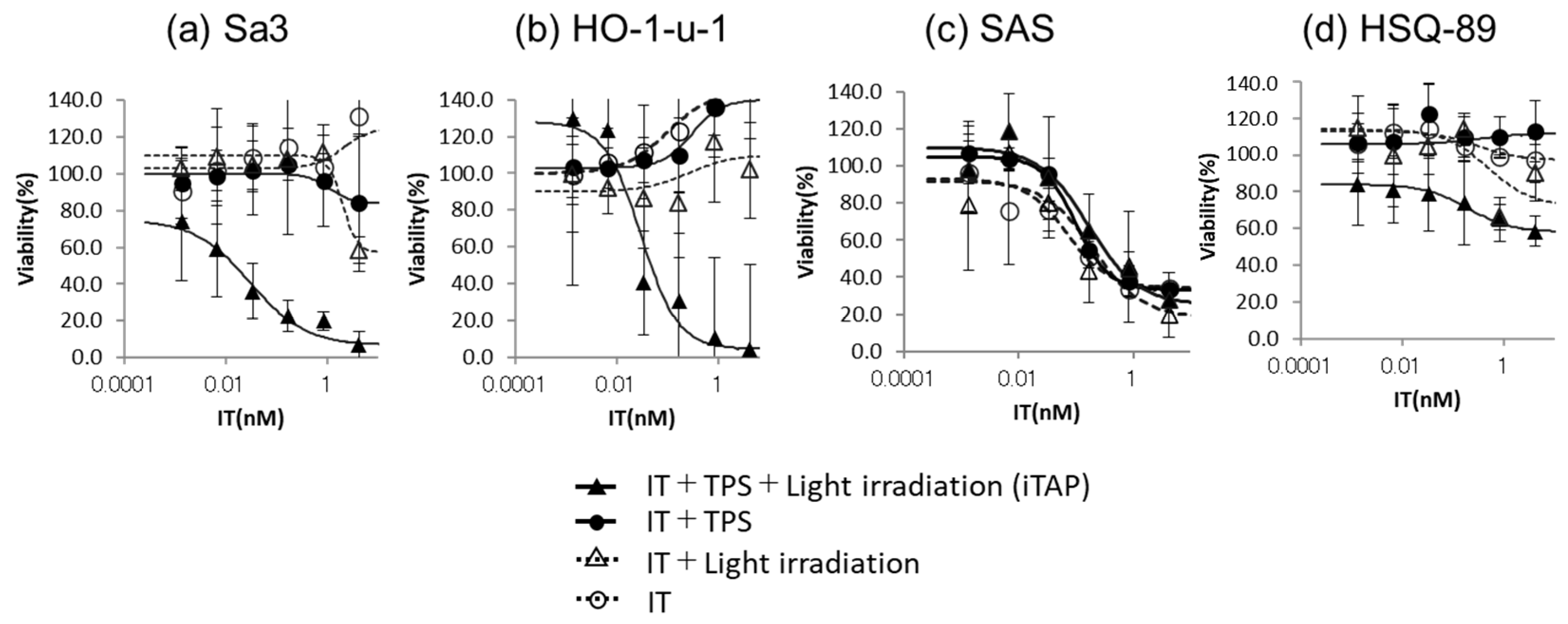
2.10. Cytotoxicity Assay of iTAP Using IT-Cmab, NPe6, and the Exciting Wavelength
2.11. Comparison with Cmab with PDT and IT-Cmab with PDT (iTAP) about Cytotoxicity Assay
2.12. Statistical Analysis
3. Results
3.1. Expression of EGFR in Various HNSCC Cells
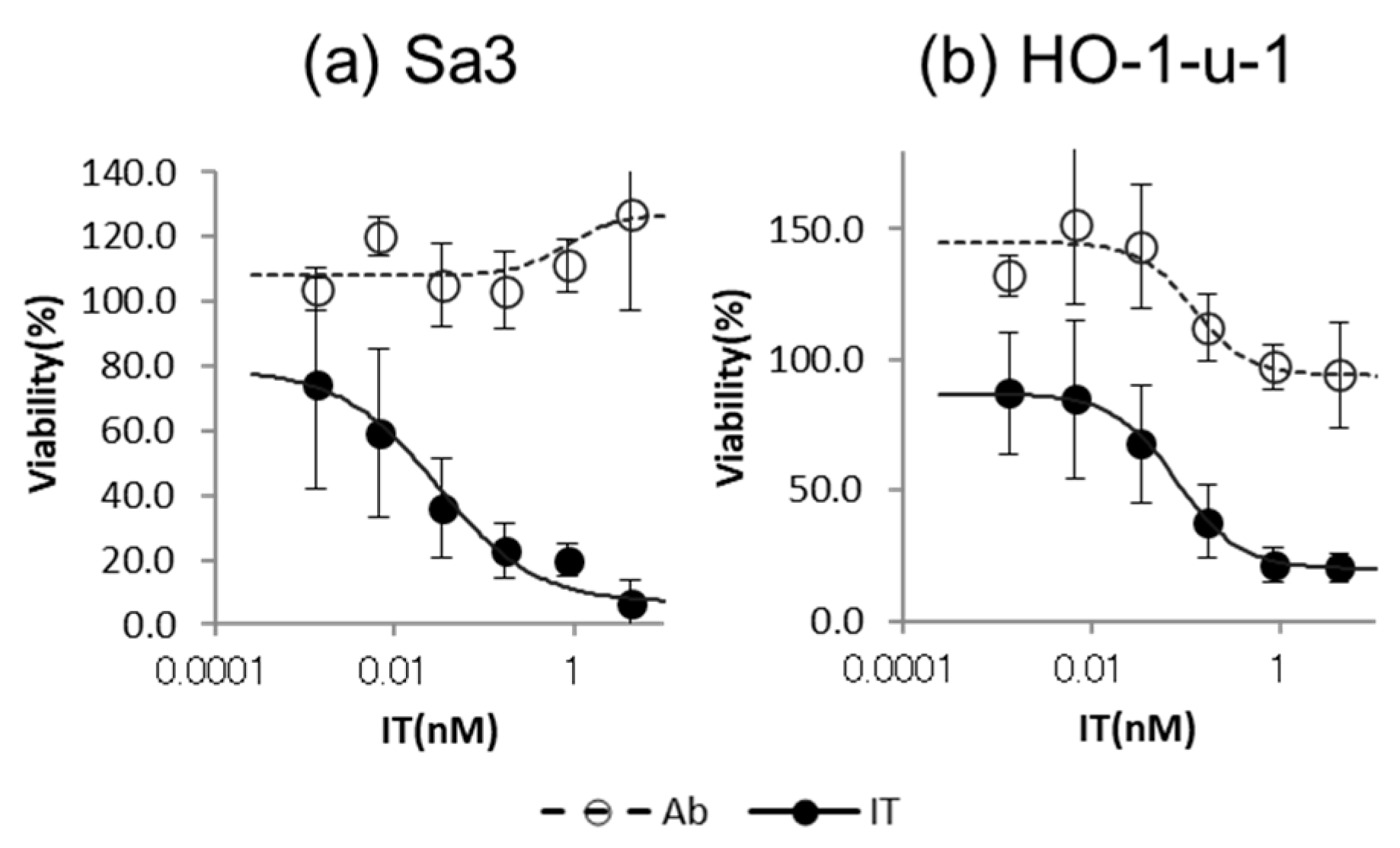
3.2. Cytotoxicity Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Riechelmann, H.; Dejaco, D.; Steinbichler, T.B.; Lettenbichler-Haug, A.; Anegg, M.; Ganswindt, U.; Gamerith, G.; Riedl, D. Functional Outcomes in Head and Neck Cancer Patients. Cancers 2022, 14, 2135. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Choyke, P.L. Near-infrared photoimmunotherapy of cancer. Acc. Chem. Res. 2019, 52, 2332–2339. [Google Scholar] [CrossRef]
- Mitsunaga, M.; Ogawa, M.; Kosaka, N.; Rosenblum, L.T.; Choyke, P.L.; Kobayashi, H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat. Med. 2011, 17, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Ando, K.; Okuyama, S.; Moriguchi, S.; Ogura, T.; Totoki, S.; Hanaoka, H.; Nagaya, T.; Kokawa, R.; Takakura, H.; et al. Photoinduced ligand release from a silicon phthalocyanine dye conjugated with monoclonal antibodies: A mechanism of cancer cell cytotoxicity after near-infrared photoimmunotherapy. ACS Cent. Sci. 2018, 4, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Tomita, Y.; Nakamura, Y.; Lee, M.J.; Lee, S.; Tomita, S.; Nagaya, T.; Sato, K.; Yamauchi, T.; Iwai, H.; et al. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget 2017, 8, 10425–10436. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, N.; Mitsui, K.; Kusano, A.O.; Iwanari, H.; Hoshi, K.; Takato, T.; Abe, T.; Hamakubo, T. Enhancement of anti-Robo1 immunotoxin cytotoxicity to head and neck squamous cell carcinoma via photochemical internalization. Arch. Cancer Res. 2017, 5, 157. [Google Scholar] [CrossRef]
- Komatsu, N.; Komatsu, M.; Ohashi, R.; Hori, A.; Hoshi, K.; Takato, T.; Abe, T.; Hamakubo, T. Photosensitizer with Illumination Enhances In Vivo Antitumor Effect of Anti-ROBO1 Immunotoxin on Maxillary Sinus Squamous Cell Carcinoma. Anticancer Res. 2020, 40, 3793–3799. [Google Scholar] [CrossRef]
- Sonokawa, T.; Obi, N.; Usuda, J.; Sudo, Y.; Hamakubo, T. Development of a new minimally invasive phototherapy for lung cancer using antibody–toxin conjugate. Thorac. Cancer 2023, 14, 645–653. [Google Scholar] [CrossRef]
- Komatsu, N.; Komatsu, M.; Ohashi, R.; Horii, A.; Hoshi, K.; Takato, T.; Abe, T.; Hamakubo, T. Saponin facilitates anti-Robo1 immunotoxin cytotoxic effects on maxillary sinus squamous cell carcinoma. J. Oncol. 2020, 2020, 9593516. [Google Scholar] [CrossRef]
- Giansanti, F.; Flavell, D.J.; Angelucci, F.; Fabbrini, M.S.; Ippoliti, R. Strategies to improve the clinical utility of Saporin-based targeted toxins. Toxins 2018, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Penget, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–902. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Usuda, J.; Ichinose, S.; Ishizumi, T.; Hayashi, H.; Ohtani, K.; Maehara, S.; Ono, S.; Honda, H.; Kajiwara, N.; Uchida, O.; et al. Outcome of photodynamic therapy using NPe6 for bronchogenic carcinoma in central airways > 1.0 cm in diameter. Clin. Cancer Res. 2010, 16, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kesselet, H.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Weyergang, A.; Prasmickaite, L.; Bonsted, A.; Hogset, A.; Strand, M.T.; Wagner, E.; Selbo, P.K. Photochemical internalization (PCI): A Technology for Drug Delivery. Methods Mol. Biol. 2010, 635, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Shafirstein, G.; Battoo, A.; Harris, K.; Baumann, H.; Gollnick, S.O.; Lindenmann, J.; Nwogu, C.E. Photodynamic Therapy of Non–Small Cell Lung Cancer. Narrative Review and Future Directions. Ann. Am. Thorac. Soc. 2016, 13, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Kasai, H.; Horimatsu, T.; Yoshimura, K.; Teramukai, S.; Morita, S.; Tada, H.; Yamamoto, Y.; Kataoka, H.; Kakushima, N.; et al. A multicenter phase II study of salvage photodynamic therapy using talaporfin sodium (ME2906) and a diode laser (PNL6405EPG) for local failure after chemoradiotherapy or radiotherapy for esophageal cancer. Oncotarget 2017, 8, 22135–22144. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, J. Photodynamic therapy for malignant brain tumors. Neurol. Med. Chir. 2016, 56, 151–157. [Google Scholar] [CrossRef]
- Harada, M.; Miyata, K.; Wada, T.; Ohmata, T.; Morita, N.; Sakamoto, T. Establishment and characterization of a cell line (Sa3) from squamous cell carcinoma of the human gingiva. Jpn. J. Oral Maxillofac. Surg. 1993, 39, 965–971. [Google Scholar] [CrossRef]
- Miyauchi, S.; Moroyama, T.; Sakamoto, T.; Okamoto, T.; Takada, K. Establishment of human tumor cell line (Ueda-1) derived from squamous cell carcinoma of the floor of the mouth. Jpn. J. Oral Maxillofac. Surg. 1985, 31, 1347–1351. [Google Scholar] [CrossRef]
- Fujinaga, T.; Kumamaru, W.; Sugiura, T.; Kobayashi, Y.; Ohyama, Y.; Ikari, T.; Onimaru, M.; Akimoto, N.; Jogo, L.; Mori, Y. Biological characterization and analysis of metastasis-related genes in cell lines derived from the primary lesion and lymph node metastasis of a squamous cell carcinoma arising in the mandibular gingiva. Int. J. Oncol. 2014, 44, 1614–1624. [Google Scholar] [CrossRef] [PubMed]
- Iwamatsu, Y.; Nakayama, K.; Nakagawa, K.; Ikeda, M.; Numata, M.; Hiromatsu, K.; Hasegawa, K. Influence of Verapamil on Effectivenes of Cisplation to the Cell-Line Derived from Human Squamous Carcinoma. Nihon. Univ. Dent. J. 1991, 65, 1070–1077. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Burnette, W.N. “Western blotting”: Electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose radiographic detection with antibody radioiodinated protein, A. Anal. Biochem. 1981, 112, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, H.; Ishiyama, M.; Ohseto, F.; Sasamoto, K.; Hamamoto, T.; Suzuki, K.; Watanabe, M. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal. Commun. 1999, 36, 47–50. [Google Scholar] [CrossRef]
- Voelkel, R.; Weible, K.J. Laser Beam Homogenizing: Limitations and Constraints. Proc. Spie. 2008, 71020, 222–233. [Google Scholar] [CrossRef]
- Miyashita, T.; Shinada, N. Illumination Apparatus for Microscope. U.S. Patent 6,507,434 B2, 10 January 2006. [Google Scholar]
- Maehara, S.; Usuda, J.; Ishizumi, T.; Ichinose, S.; Ohtani, K.; Inoue, T.; Imai, K.; Furumoto, H.; Kudo, Y.; Kajiwara, N.; et al. Combination effect of photodynamic therapy using NPe6 with pemetrexed for human malignant pleural mesothelioma cells. Int. J. Oncol. 2015, 46, 741–749. [Google Scholar] [CrossRef]
- Ceci, C.; Lacal, P.M.; Graziani, G. Antibody-drug conjugates: Resurgent anticancer agents with multi-targeted therapeutic potential. Pharmacol. Ther. 2022, 236, 108106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komatsu, N.; Kosai, A.; Kuroda, M.; Hamakubo, T.; Abe, T. Cetuximab–Toxin Conjugate and NPe6 with Light Enhanced Cytotoxic Effects in Head and Neck Squamous Cell Carcinoma In Vitro. Biomedicines 2024, 12, 973. https://doi.org/10.3390/biomedicines12050973
Komatsu N, Kosai A, Kuroda M, Hamakubo T, Abe T. Cetuximab–Toxin Conjugate and NPe6 with Light Enhanced Cytotoxic Effects in Head and Neck Squamous Cell Carcinoma In Vitro. Biomedicines. 2024; 12(5):973. https://doi.org/10.3390/biomedicines12050973
Chicago/Turabian StyleKomatsu, Noriko, Azuma Kosai, Mikako Kuroda, Takao Hamakubo, and Takahiro Abe. 2024. "Cetuximab–Toxin Conjugate and NPe6 with Light Enhanced Cytotoxic Effects in Head and Neck Squamous Cell Carcinoma In Vitro" Biomedicines 12, no. 5: 973. https://doi.org/10.3390/biomedicines12050973



