HIV-Related Oral Mucosa Lesions: A Cross-Sectional Study on a Cohort of Italian Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Clinical Oral Examination, Specimen Collection and Cyto/Histopathological Analyses
2.3. Statistical Analysis
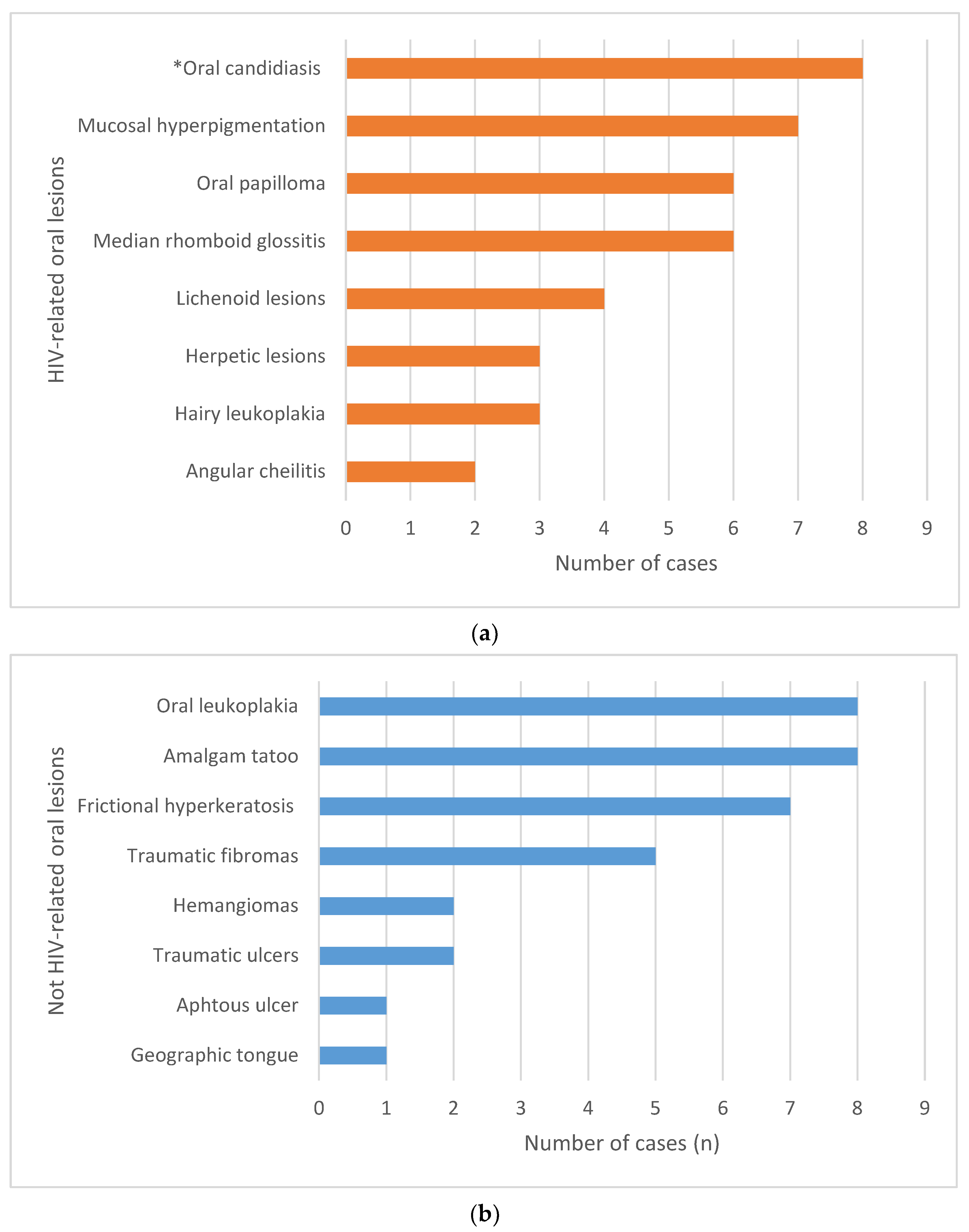
3. Results
3.1. Oral HPV Infection
3.2. Oral Mucosal Lesions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization HIV. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 31 March 2023).
- Melhuish, A.; Lewthwaite, P. Natural History of HIV and AIDS. Medicine 2022, 50, 298–303. [Google Scholar] [CrossRef]
- Bour, S.; Geleziunas, R.; Wainberg, M.A. The Human Immunodeficiency Virus Type 1 (HIV-1) CD4 Receptor and Its Central Role in Promotion of HIV-1 Infection. Microbiol. Rev. 1995, 59, 63–93. [Google Scholar] [CrossRef]
- Langford, S.E.; Ananworanich, J.; Cooper, D.A. Predictors of Disease Progression in HIV Infection: A Review. AIDS Res. Ther. 2007, 4, 11. [Google Scholar] [CrossRef]
- McBride, J.A.; Striker, R. Imbalance in the Game of T Cells: What Can the CD4/CD8 T-Cell Ratio Tell Us about HIV and Health? PLoS Pathog. 2017, 13, e1006624. [Google Scholar] [CrossRef] [PubMed]
- Okoye, A.A.; Picker, L.J. CD4+ T Cell Depletion in HIV Infection: Mechanisms of Immunological Failure. Immunol. Rev. 2013, 254, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Iamaroon, A. Oral Manifestations of Monkeypox: Brief Review. Dent. Med. Probl. 2022, 59, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Paradowska-Stolarz, A.M. Oral Manifestations of COVID-19: Brief Review. Dent. Med. Probl. 2021, 58, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Ebara, Y.; Nomura, S.; Yamada, Y. Chronological Changes in Strawberry Tongue in Toxic Shock Syndrome Toxin-1-Mediated Exanthematous Disease. J. Gen. Fam. Med. 2020, 21, 280–281. [Google Scholar] [CrossRef]
- Gouk, T.; Nissanka-Jayasuriya, E.; Anushan Hiranya Jayasinghe, L.; Withanage, S.; Doumas, S. Syphilitic Ulcer Mimicking Oral Cancer. Br. Dent. J. 2023, 235, 957–958. [Google Scholar] [CrossRef]
- Pecci-Lloret, M.P.; Ramirez-Santisteban, E.; Hergueta-Castillo, A.; Guerrero-Gironés, J.; Oñate-Sánchez, R.E. Oral Manifestations of Crohn’s Disease: A Systematic Review. J. Clin. Med. 2023, 12, 6450. [Google Scholar] [CrossRef]
- Li, C.; Wu, Y.; Xie, Y.; Zhang, Y.; Jiang, S.; Wang, J.; Luo, X.; Chen, Q. Oral Manifestations Serve as Potential Signs of Ulcerative Colitis: A Review. Front. Immunol. 2022, 13, 1013900. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Amador, V.; Ponce-de-León, S.; Anaya-Saavedra, G.; Ramírez, B.C.; Sierra-Madero, J. Oral Lesions as Clinical Markers of Highly Active Antiretroviral Therapy Failure: A Nested Case-Control Study in Mexico City. Clin. Infect. Dis. 2007, 45, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Lomelí-Martínez, S.M.; González-Hernández, L.A.; Ruiz-Anaya, A.d.J.; Lomelí-Martínez, M.A.; Martínez-Salazar, S.Y.; Mercado González, A.E.; Andrade-Villanueva, J.F.; Varela-Hernández, J.J. Oral Manifestations Associated with HIV/AIDS Patients. Medicina 2022, 58, 1214. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, F.; Fei, Y.; Dong, G.; Cao, P.; Liu, Y. Immune Indices and Oral Health in Patients Infected with the Human Immunodeficiency Virus. BMC Oral Health 2023, 23, 1009. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Westhausen, A.M.; Priepke, F.; Bergmann, F.J.; Reichart, P.A. Decline in the Rate of Oral Opportunistic Infections Following Introduction of Highly Active Antiretroviral Therapy. J. Oral Pathol. Med. 2000, 29, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Amador, V.; Esquivel-Pedraza, L.; Sierra-Madero, J.; Anaya-Saavedra, G.; González-Ramírez, I.; Ponce-de-León, S. The Changing Clinical Spectrum of Human Immunodeficiency Virus (HIV)-Related Oral Lesions in 1,000 Consecutive Patients: A 12-Year Study in a Referral Center in Mexico. Medicine 2003, 82, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, T.A.; Greenspan, D.; Greenspan, J.S. Oral Lesions of HIV Disease and HAART in Industrialized Countries. Adv. Dent. Res. 2006, 19, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, O.O.; Hassan, Z. The Impact of Highly Active Antiretroviral Therapy (HAART) on the Clinical Features of HIV—Related Oral Lesions in Nigeria. AIDS Res. Ther. 2010, 7, 19. [Google Scholar] [CrossRef]
- de Almeida, V.L.; Lima, I.F.P.; Ziegelmann, P.K.; Paranhos, L.R.; de Matos, F.R. Impact of Highly Active Antiretroviral Therapy on the Prevalence of Oral Lesions in HIV-Positive Patients: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Surg. 2017, 46, 1497–1504. [Google Scholar] [CrossRef]
- Ottria, L.; Lauritano, D.; Oberti, L.; Candotto, V.; Cura, F.; Tagliabue, A.; Tettamanti, L. Prevalence of HIV-Related Oral Manifestations and Their Association with HAART and CD4+ T Cell Count: A Review. J. Biol. Regul. Homeost. Agents 2018, 32, 51–59. [Google Scholar]
- Patil, N.; Chaurasia, V.R.; Babaji, P.; Ramesh, D.; Jhamb, K.; Sharma, A.M. The Effect of Highly Active Antiretroviral Therapy on the Prevalence of Oral Manifestation in Human Immunodeficiency Virus-Infected Patients in Karnataka, India. Eur. J. Dent. 2015, 9, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Perla, N.; Kumar, S.; Jadhav, A.; Bhalinge, P.; Dadpe, M.; Acharya, S. Quantification of Oral Candidal Carriage Rate and Prevalence of Oral Candidal Species in HIV Patients with and Without Highly Active Antiretroviral Therapy. J. Microsc. Ultrastruct. 2021, 9, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Subramanyam, G.B.; Kumar, G.; Bhushan, V. Assessment of Oral Mucosal Lesions among HIV Positive Transgenders Residing in Odisha with and without Antiretroviral Therapy. J. Fam. Med. Prim. Care 2022, 11, 7106–7112. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Saavedra, G.; Flores-Moreno, B.; García-Carrancá, A.; Irigoyen-Camacho, E.; Guido-Jiménez, M.; Ramírez-Amador, V. HPV Oral Lesions in HIV-Infected Patients: The Impact of Long-Term HAART. J. Oral Pathol. Med. 2013, 42, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Vergori, A.; Garbuglia, A.R.; Piselli, P.; Del Nonno, F.; Sias, C.; Lupi, F.; Lapa, D.; Baiocchini, A.; Cimaglia, C.; Gentile, M.; et al. Oral Human Papillomavirus DNA Detection in HIV-Positive Men: Prevalence, Predictors, and Co-Occurrence at Anal Site. BMC Infect. Dis. 2018, 18, 25. [Google Scholar] [CrossRef]
- Ucciferri, C.; Tamburro, M.; Falasca, K.; Sammarco, M.L.; Ripabelli, G.; Vecchiet, J. Prevalence of Anal, Oral, Penile and Urethral Human Papillomavirus in HIV Infected and HIV Uninfected Men Who Have Sex with Men. J. Med. Virol. 2018, 90, 358–366. [Google Scholar] [CrossRef]
- Marchetti, G.; Comi, L.; Bini, T.; Rovati, M.; Bai, F.; Cassani, B.; Ravizza, M.; Tarozzi, M.; Pandolfo, A.; Dalzero, S.; et al. HPV Infection in a Cohort of HIV-Positive Men and Women: Prevalence of Oncogenic Genotypes and Predictors of Mucosal Damage at Genital and Oral Sites. J. Sex. Transm. Dis. 2013, 2013, 915169. [Google Scholar] [CrossRef]
- Parisi, S.G.; Cruciani, M.; Scaggiante, R.; Boldrin, C.; Andreis, S.; Dal Bello, F.; Pagni, S.; Barelli, A.; Sattin, A.; Mengoli, C.; et al. Anal and Oral Human Papillomavirus (HPV) Infection in HIV-Infected Subjects in Northern Italy: A Longitudinal Cohort Study among Men Who Have Sex with Men. BMC Infect. Dis. 2011, 11, 150. [Google Scholar] [CrossRef]
- Vani, N.V.; Madhanagopal, R.; Swaminathan, R.; Ganesan, T.S. Dynamics of Oral Human Papillomavirus Infection in Healthy Population and Head and Neck Cancer. Cancer Med. 2023, 12, 11731–11745. [Google Scholar] [CrossRef]
- Gheit, T.; Rollo, F.; Brancaccio, R.N.; Robitaille, A.; Galati, L.; Giuliani, M.; Latini, A.; Pichi, B.; Benevolo, M.; Cuenin, C.; et al. Oral Infection by Mucosal and Cutaneous Human Papillomaviruses in the Men Who Have Sex with Men from the OHMAR Study. Viruses 2020, 12, 899. [Google Scholar] [CrossRef]
- Radwan-Oczko, M.; Owczarek-Drabińska, J.; Szczygielska, A.; Szczepaniak, M.; Duś-Ilnicka, I. Salivary HPV Infection in Healthy People. Postępy Hig. Med. Dośw. 2022, 76, 143–148. [Google Scholar] [CrossRef]
- Campisi, G.; Pizzo, G.; Mancuso, S.; Margiotta, V. Gender Differences in Human Immunodeficiency Virus-Related Oral Lesions: An Italian Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 91, 546–551. [Google Scholar] [CrossRef]
- Margiotta, V.; Campisi, G.; Mancuso, S.; Accurso, V.; Abbadessa, V. HIV Infection: Oral Lesions, CD4+ Cell Count and Viral Load in an Italian Study Population. J. Oral Pathol. Med. 1999, 28, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.; Lajolo, C.; Sartorio, A.; Ammassari, A.; Lacaita, M.G.; Scivetti, M.; Tamburrini, E.; Tumbarello, M. Oral Lesions in HIV and HCV Co-Infected Individuals in HAART Era. J. Oral Pathol. Med. 2008, 37, 468–474. [Google Scholar] [CrossRef]
- Bravo, I.M.; Correnti, M.; Escalona, L.; Perrone, M.; Brito, A.; Tovar, V.; Rivera, H. Prevalence of Oral Lesions in HIV Patients Related to CD4 Cell Count and Viral Load in a Venezuelan Population. Med. Oral Patol. Oral Cirugia Bucal 2006, 11, E33–E39. [Google Scholar]
- Sharma, G.; Pai, K.M.; Setty, S.; Ramapuram, J.T.; Nagpal, A. Oral Manifestations as Predictors of Immune Suppression in a HIV-/AIDS-Infected Population in South India. Clin. Oral Investig. 2009, 13, 141–148. [Google Scholar] [CrossRef]
- Sud, N.; Shanker, V.; Sharma, A.; Sharma, N.L.; Gupta, M. Mucocutaneous Manifestations in 150 HIV-Infected Indian Patients and Their Relationship with CD4 Lymphocyte Counts. Int. J. STD AIDS 2009, 20, 771–774. [Google Scholar] [CrossRef]
- Bodhade, A.S.; Ganvir, S.M.; Hazarey, V.K. Oral Manifestations of HIV Infection and Their Correlation with CD4 Count. J. Oral Sci. 2011, 53, 203–211. [Google Scholar] [CrossRef] [PubMed][Green Version]
- INSIGHT START Study Group; Lundgren, J.D.; Babiker, A.G.; Godin, F.; Emery, S.; Grund, B.; Sharma, S.; Avihingsanon, A.; Cooper, D.A.; Fätkenheuer, G.; et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N. Engl. J. Med. 2015, 373, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Berberi, A.; Aoun, G. Oral Lesions Associated with Human Immunodeficiency Virus in 75 Adult Patients: A Clinical Study. J. Korean Assoc. Oral Maxillofac. Surg. 2017, 43, 388–394. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Umadevi, K.M.R.; Ranganathan, K.; Pavithra, S.; Hemalatha, R.; Saraswathi, T.R.; Kumarasamy, N.; Solomon, S.; Greenspan, J.S. Oral Lesions among Persons with HIV Disease with and without Highly Active Antiretroviral Therapy in Southern India. J. Oral Pathol. Med. 2007, 36, 136–141. [Google Scholar] [CrossRef]
- Hegde, V.; Shetty, P.J.; Alva, S.; Chengappa, S.K. Assessment of Dental Caries Experience, Periodontal Status, and Oral Mucosal Lesions among Human Immunodeficiency Virus Seropositives with and without Antiretroviral Therapy: A Cross-Sectional Study. J. Indian Assoc. Public Health Dent. 2016, 14, 46. [Google Scholar] [CrossRef]
- Radithia, D.; Subarnbhesaj, A.; Ayuningtyas, N.-F.; Bakti, R.-K.; Mahdani, F.-Y.; Pratiwi, A.-S.; Ayunnisa, N.; Putri, S.-F.; Pramitha, S.-R. Oral Hyperpigmentation as an Adverse Effect of Highly Active Antiretroviral Therapy in HIV Patients: A Systematic Review and Pooled Prevalence. J. Clin. Exp. Dent. 2023, 15, e561–e570. [Google Scholar] [CrossRef]
- Maloth, S.; Shrinivas, T.; Krishna Pramod, B.; Nagarathna, P.J. Prevalence of Oromucosal Lesions in HIV Positive Patients Receiving Haart-A Prospective Clinical Study. J. Fam. Med. Prim. Care 2020, 9, 4821–4825. [Google Scholar] [CrossRef]
- Pakfetrat, A.; Falaki, F.; Delavarian, Z.; Dalirsani, Z.; Sanatkhani, M.; Zabihi Marani, M. Oral Manifestations of Human Immunodeficiency Virus-Infected Patients. Iran. J. Otorhinolaryngol. 2015, 27, 43–54. [Google Scholar]
- Cameron, J.E.; Mercante, D.; O’Brien, M.; Gaffga, A.M.; Leigh, J.E.; Fidel, P.L.; Hagensee, M.E. The Impact of Highly Active Antiretroviral Therapy and Immunodeficiency on Human Papillomavirus Infection of the Oral Cavity of Human Immunodeficiency Virus-Seropositive Adults. Sex. Transm. Dis. 2005, 32, 703–709. [Google Scholar] [CrossRef]
- D’Souza, G.; Agrawal, Y.; Halpern, J.; Bodison, S.; Gillison, M.L. Oral Sexual Behaviors Associated with Prevalent Oral Human Papillomavirus Infection. J. Infect. Dis. 2009, 199, 1263–1269. [Google Scholar] [CrossRef]
- Marais, D.J.; Passmore, J.-A.S.; Denny, L.; Sampson, C.; Allan, B.R.; Williamson, A.-L. Cervical and Oral Human Papillomavirus Types in HIV-1 Positive and Negative Women with Cervical Disease in South Africa. J. Med. Virol. 2008, 80, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.L.; van Rensburg, E.J.; van Heerden, W.F.P.; Boy, S.C. Human Papilloma Virus Types in the Oral and Cervical Mucosa of HIV-Positive South African Women Prior to Antiretroviral Therapy. J. Oral Pathol. Med. 2008, 37, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, A.; Wieland, U. Human Papillomavirus-Associated Diseases in HIV-Infected Men Who Have Sex with Men. Curr. Opin. Infect. Dis. 2009, 22, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Palefsky, J. Biology of HPV in HIV Infection. Adv. Dent. Res. 2006, 19, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Paradowska-Stolarz, A.; Wieckiewicz, M.; Owczarek, A.; Wezgowiec, J. Natural Polymers for the Maintenance of Oral Health: Review of Recent Advances and Perspectives. Int. J. Mol. Sci. 2021, 22, 10337. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Ndokaj, A.; Bietolini, S.; Nisii, V.; Duś-Ilnicka, I.; Ottolenghi, L. Green Dentistry: Organic Toothpaste Formulations. A Literature Review. Dent. Med. Probl. 2022, 59, 461–474. [Google Scholar] [CrossRef] [PubMed]
| Demographic Characteristics | n (%) |
|---|---|
| Sex | |
| Males | 132 (74.6%) |
| Females | 45 (25.4%) |
| Age | |
| Under 35 | 43 (24.3%) |
| 35–42 | 48 (27.1%) |
| 43–50 | 52 (29.4%) |
| Over 51 | 34 (19.2%) |
| cART | |
| Yes | 140 (79%) |
| No | 37 (21%) |
| CD4+ (cells/µL) | |
| Under 200 | 12 (6.8%) |
| 200–500 | 80 (45.2%) |
| Over 500 | 85 (48%) |
| Route of transmission * | |
| MSM | 100 (56.5%) |
| MSW or WSM | 52 (29.38%) |
| IDUs | 23 (13%) |
| Vertical trasmission | 1 (0.56%) |
| Transfusion | 1 (0.56%) |
| Demographic Characteristics | n | |||
|---|---|---|---|---|
| HPV− | HPV+ | LR-HPV | HR-HPV | |
| Sex | ||||
| Males | 104 | 28 | 16 | 12 |
| Females | 38 | 7 | 3 | 4 |
| Age | ||||
| Under 35 | 35 | 8 | 4 | 4 |
| 36–42 | 38 | 10 | 7 | 3 |
| 43–50 | 44 | 8 | 4 | 4 |
| Over 51 | 25 | 9 | 4 | 5 |
| cART | ||||
| Yes | 110 | 30 | 16 | 14 |
| No | 32 | 5 | 3 | 2 |
| CD4+ (cells/µL) | ||||
| Under 200 | 11 | 1 | 0 | 1 |
| 200–500 | 66 | 14 | 4 | 10 |
| Over 500 | 65 | 20 | 12 | 8 |
| Total | 142 | 35 | 19 | 16 |
| Demographic and Clinical Characteristics | Number of Patients (%) |
|---|---|
| Sex | |
| Males | 22 (73.3%) |
| Females | 8 (26.7%) |
| Age | |
| Under 35 | 5 (16.7%) |
| 35–42 | 2 (6.6) |
| 43–50 | 8 (26.7%) |
| Over 51 | 15 (50%) |
| cART | |
| Yes | 26 (86.7%) |
| No | 4 (13.3%) |
| CD4+ (cells/µL) | |
| Under 200 | 4 (13.3%) |
| 200–500 | 14 (46.7%) |
| Over 500 | 12 (40%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarozzi, M.; Baruzzi, E.; Decani, S.; Tincati, C.; Santoro, A.; Moneghini, L.; Lodi, G.; Sardella, A.; Carrassi, A.; Varoni, E.M. HIV-Related Oral Mucosa Lesions: A Cross-Sectional Study on a Cohort of Italian Patients. Biomedicines 2024, 12, 436. https://doi.org/10.3390/biomedicines12020436
Tarozzi M, Baruzzi E, Decani S, Tincati C, Santoro A, Moneghini L, Lodi G, Sardella A, Carrassi A, Varoni EM. HIV-Related Oral Mucosa Lesions: A Cross-Sectional Study on a Cohort of Italian Patients. Biomedicines. 2024; 12(2):436. https://doi.org/10.3390/biomedicines12020436
Chicago/Turabian StyleTarozzi, Marco, Elisa Baruzzi, Sem Decani, Camilla Tincati, Andrea Santoro, Laura Moneghini, Giovanni Lodi, Andrea Sardella, Antonio Carrassi, and Elena Maria Varoni. 2024. "HIV-Related Oral Mucosa Lesions: A Cross-Sectional Study on a Cohort of Italian Patients" Biomedicines 12, no. 2: 436. https://doi.org/10.3390/biomedicines12020436
APA StyleTarozzi, M., Baruzzi, E., Decani, S., Tincati, C., Santoro, A., Moneghini, L., Lodi, G., Sardella, A., Carrassi, A., & Varoni, E. M. (2024). HIV-Related Oral Mucosa Lesions: A Cross-Sectional Study on a Cohort of Italian Patients. Biomedicines, 12(2), 436. https://doi.org/10.3390/biomedicines12020436









