Sirtuins Affect Cancer Stem Cells via Epigenetic Regulation of Autophagy
Abstract
:1. Introduction
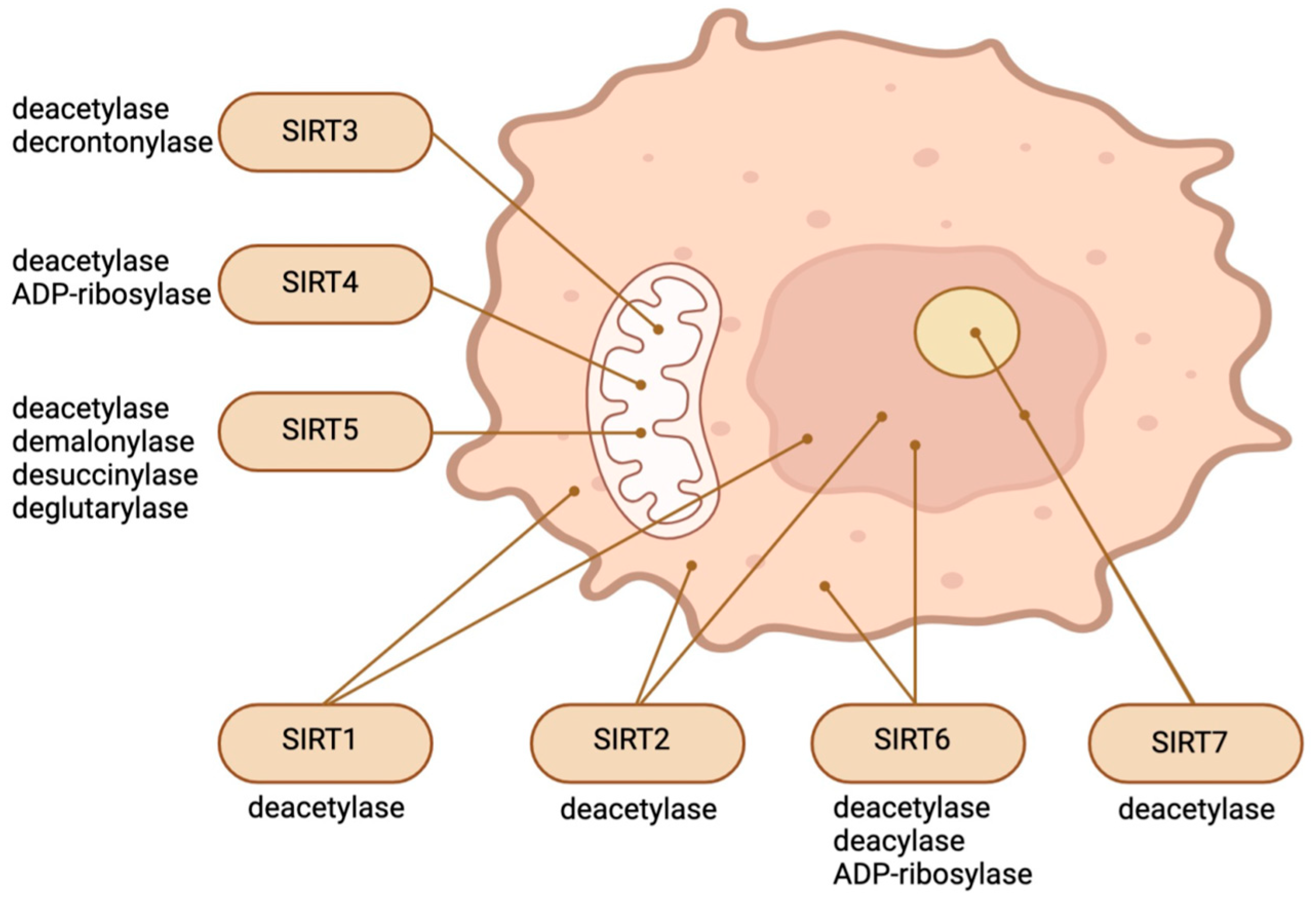
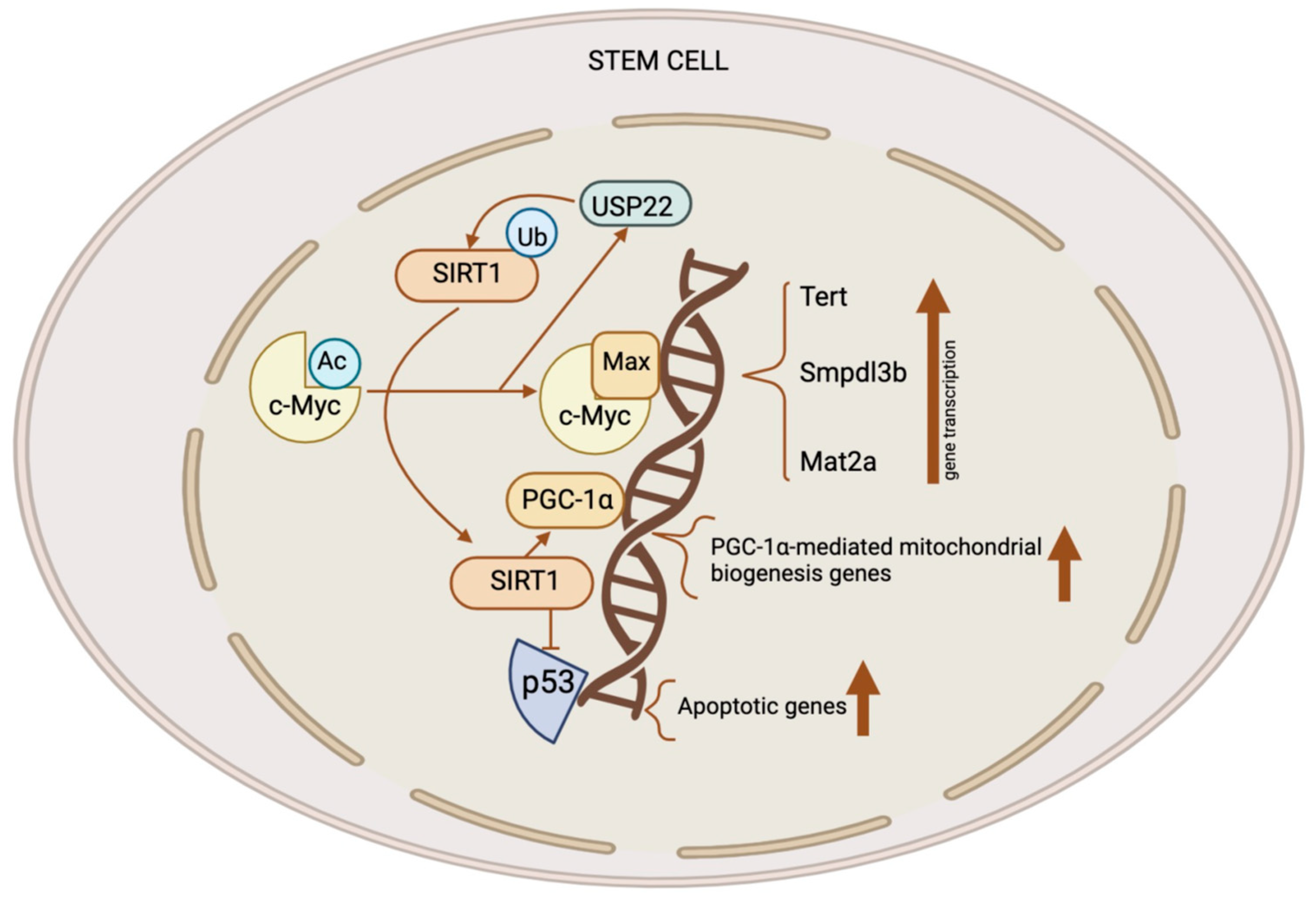
2. SIRTs in the Epigenetic Regulation of Stem Cells
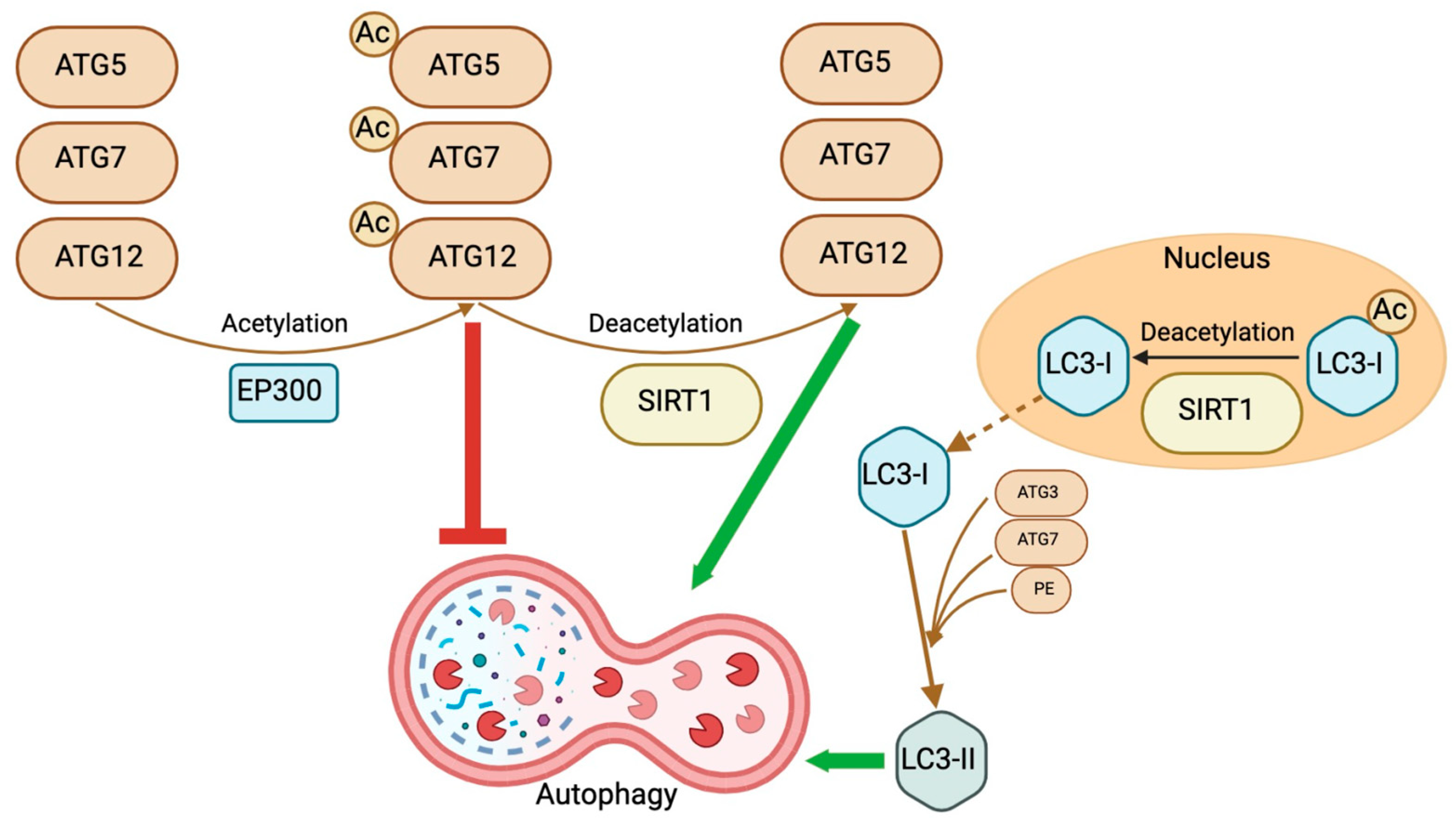
3. SIRTs and Autophagy
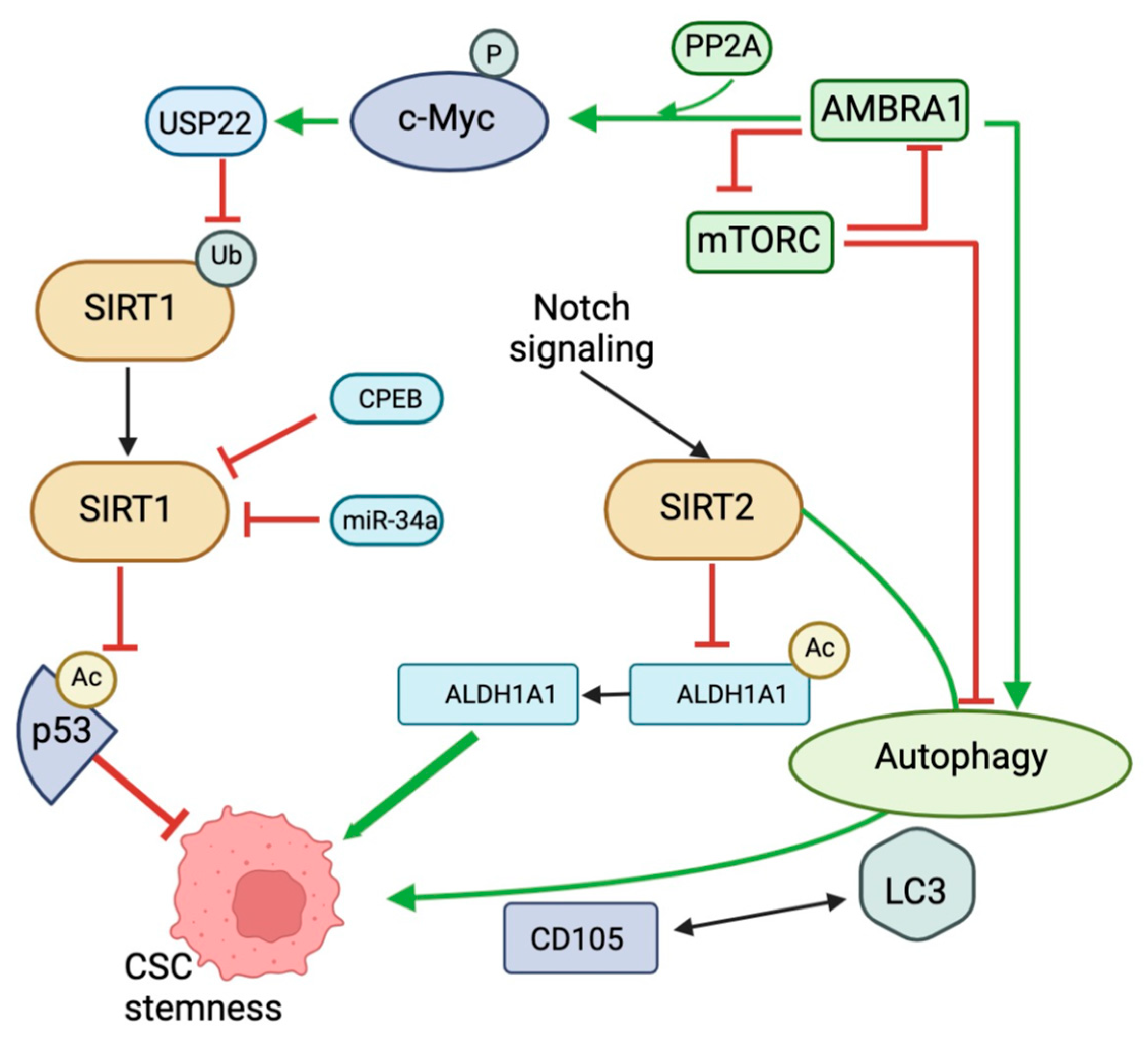
4. SIRT Regulation of Autophagy in Cancer Stem Cells
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fang, Y.; Táng, S.; Li, X. Sirtuins in Metabolic and Epigenetic Regulation of Stem Cells. Trends Endocrinol. Metab. 2019, 30, 177–188. [Google Scholar] [CrossRef]
- Pande, S.; Raisuddin, S. Molecular and Cellular Regulatory Roles of Sirtuin Protein. Crit. Rev. Food Sci. Nutr. 2023, 63, 9895–9913. [Google Scholar] [CrossRef]
- He, W.; Newman, J.C.; Wang, M.; Ho, L.; Verdin, E. Mitochondrial Sirtuins: Regulators of Protein Acylation and Metabolism. Trends Endocrinol. Metab. 2012, 23, 467–476. [Google Scholar] [CrossRef]
- Choudhary, C.; Weinert, B.T.; Nishida, Y.; Verdin, E.; Mann, M. The Growing Landscape of Lysine Acetylation Links Metabolism and Cell Signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 536–550. [Google Scholar] [CrossRef]
- Wagner, G.R.; Hirschey, M.D. Nonenzymatic Protein Acylation as a Carbon Stress Regulated by Sirtuin Deacylases. Mol. Cell 2014, 54, 5–16. [Google Scholar] [CrossRef]
- Imai, S.; Guarente, L. It Takes Two to Tango: NAD+ and Sirtuins in Aging/Longevity Control. NPJ Aging Mech. Dis. 2016, 2, 16017. [Google Scholar] [CrossRef]
- Frye, R.A. Phylogenetic Classification of Prokaryotic and Eukaryotic SIR2-like Proteins. Biochem. Biophys. Res. Commun. 2000, 273, 793–798. [Google Scholar] [CrossRef]
- Tanno, M.; Sakamoto, J.; Miura, T.; Shimamoto, K.; Horio, Y. Nucleocytoplasmic Shuttling of the NAD+-Dependent Histone Deacetylase SIRT1. J. Biol. Chem. 2007, 282, 6823–6832. [Google Scholar] [CrossRef]
- Ford, E.; Voit, R.; Liszt, G.; Magin, C.; Grummt, I.; Guarente, L. Mammalian Sir2 Homolog SIRT7 Is an Activator of RNA Polymerase I Transcription. Genes Dev. 2006, 20, 1075–1080. [Google Scholar] [CrossRef]
- Mostoslavsky, R.; Chua, K.F.; Lombard, D.B.; Pang, W.W.; Fischer, M.; Gellon, L.; Liu, P.; Mostoslavsky, G.; Franco, S.; Murphy, M.M.; et al. Genomic Instability and Aging-like Phenotype in the Absence of Mammalian SIRT6. Cell 2006, 124, 315–329. [Google Scholar] [CrossRef]
- Verdin, E.; Hirschey, M.D.; Finley, L.W.S.; Haigis, M.C. Sirtuin Regulation of Mitochondria: Energy Production, Apoptosis, and Signaling. Trends Biochem. Sci. 2010, 35, 669–675. [Google Scholar] [CrossRef] [PubMed]
- van de Ven, R.A.H.; Santos, D.; Haigis, M.C. Mitochondrial Sirtuins and Molecular Mechanisms of Aging. Trends Mol. Med. 2017, 23, 320–331. [Google Scholar] [CrossRef]
- Chang, H.-C.; Guarente, L. SIRT1 and Other Sirtuins in Metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef]
- Satoh, A.; Imai, S.; Guarente, L. The Brain, Sirtuins, and Ageing. Nat. Rev. Neurosci. 2017, 18, 362–374. [Google Scholar] [CrossRef]
- Flick, F.; Lüscher, B. Regulation of Sirtuin Function by Posttranslational Modifications. Front. Pharmacol. 2012, 3, 29. [Google Scholar] [CrossRef]
- Buler, M.; Andersson, U.; Hakkola, J. Who Watches the Watchmen? Regulation of the Expression and Activity of Sirtuins. FASEB J. 2016, 30, 3942–3960. [Google Scholar] [CrossRef] [PubMed]
- Łanoszka, K.; Vlčková, N. Natural Sirtuin1 Activators and Atherosclerosis: An Overview. Curr. Atheroscler. Rep. 2023, 25, 979–994. [Google Scholar] [CrossRef]
- Táng, S.; Fang, Y.; Huang, G.; Xu, X.; Padilla-Banks, E.; Fan, W.; Xu, Q.; Sanderson, S.M.; Foley, J.F.; Dowdy, S.; et al. Methionine Metabolism Is Essential for SIRT 1-regulated Mouse Embryonic Stem Cell Maintenance and Embryonic Development. EMBO J. 2017, 36, 3175–3193. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Li, X. The SIRT1-c-Myc Axis in Regulation of Stem Cells. Front. Cell Dev. Biol. 2023, 11, 1236968. [Google Scholar] [CrossRef]
- Táng, S.; Huang, G.; Fan, W.; Chen, Y.; Ward, J.M.; Xu, X.; Xu, Q.; Kang, A.; McBurney, M.W.; Fargo, D.C.; et al. SIRT1-Mediated Deacetylation of CRABPII Regulates Cellular Retinoic Acid Signaling and Modulates Embryonic Stem Cell Differentiation. Mol. Cell 2014, 55, 843–855. [Google Scholar] [CrossRef]
- Liu, C.; Song, Z.; Wang, L.; Yu, H.; Liu, W.; Shang, Y.; Xu, Z.; Zhao, H.; Gao, F.; Wen, J.K.; et al. Sirt1 Regulates Acrosome Biogenesis by Modulating Autophagic Flux during Spermiogenesis in Mice. Development 2016, 144, 441–451. [Google Scholar] [CrossRef]
- Rodriguez, R.M.; Fernandez, A.F.; Fraga, M.F. Role of Sirtuins in Stem Cell Differentiation. Genes Cancer 2013, 4, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Lim, J.; Lee, S.; Jeong, J.; Kang, H.-J.; Kim, Y.H.; Kang, J.-W.; Yu, H.Y.; Jeong, E.M.; Kim, K.; et al. SIRT1 Regulates DNA Methylation and Differentiation Potential of Embryonic Stem Cells by Antagonizing DNMT3L. Cell Rep. 2017, 18, 1930–1945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chung, S.; Xu, Z.; Xu, Y. Oct4 Maintains the Pluripotency of Human Embryonic Stem Cells by Inactivating P53 Through Sirt1-Mediated Deacetylation. Stem Cells 2014, 32, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.O.; Taylor, A.C.; Bell, E.L.; Lim, R.; Kim, D.M.; Guarente, L. Sirtuin 1 Promotes Deacetylation of Oct4 and Maintenance of Naive Pluripotency. Cell Rep. 2016, 17, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Calvanese, V.; Lara, E.; Suárez-Álvarez, B.; Dawud, R.A.; Vázquez-Chantada, M.; Martínez-Chantar, M.L.; Embade, N.; López-Nieva, P.; Horrillo, A.; Hmadcha, A.; et al. Sirtuin 1 Regulation of Developmental Genes during Differentiation of Stem Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 13736–13741. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Song, E.K.; Guo, Y.; Ou, X.; Mantel, C.; Broxmeyer, H.E. SIRT1 Regulates Apoptosis and Nanog Expression in Mouse Embryonic Stem Cells by Controlling P53 Subcellular Localization. Cell Stem Cell 2008, 2, 241–251. [Google Scholar] [CrossRef]
- Shiraki, N.; Shiraki, Y.; Tsuyama, T.; Obata, F.; Miura, M.; Nagae, G.; Aburatani, H.; Kume, K.; Endo, F.; Kume, S. Methionine Metabolism Regulates Maintenance and Differentiation of Human Pluripotent Stem Cells. Cell Metab. 2014, 19, 780–794. [Google Scholar] [CrossRef] [PubMed]
- De Bonis, M.L.; Ortega, S.; Blasco, M.A. SIRT1 Is Necessary for Proficient Telomere Elongation and Genomic Stability of Induced Pluripotent Stem Cells. Stem Cell Rep. 2014, 2, 690–706. [Google Scholar] [CrossRef]
- Kang, E.; Wang, X.; Tippner-Hedges, R.; Ma, H.; Folmes, C.D.L.; Gutiérrez, N.M.; Lee, Y.; Van Dyken, C.; Ahmed, R.; Liu, Y.; et al. Age-Related Accumulation of Somatic Mitochondrial DNA Mutations in Adult-Derived Human IPSCs. Cell Stem Cell 2016, 18, 625–636. [Google Scholar] [CrossRef]
- Luo, H.; Mu, W.; Karki, R.; Chiang, H.-H.; Mohrin, M.; Shin, J.; Ohkubo, R.; Ito, K.; Kanneganti, T.; Chen, D. Mitochondrial Stress-Initiated Aberrant Activation of the NLRP3 Inflammasome Regulates the Functional Deterioration of Hematopoietic Stem Cell Aging. Cell Rep. 2019, 26, 945–954.e4. [Google Scholar] [CrossRef]
- He, M.; Chiang, H.-H.; Luo, H.; Zheng, Z.; Qiao, Q.; Wang, L.; Tan, M.; Ohkubo, R.; Mu, W.; Zhao, S.; et al. An Acetylation Switch of the NLRP3 Inflammasome Regulates Aging-Associated Chronic Inflammation and Insulin Resistance. Cell Metab. 2020, 31, 580–591.e5. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.; Han, M.; Cha, H.; Zoldan, J.; Burkart, A.; Jung, J.H.; Jang, Y.; Kim, C.-H.; Jeong, H.-C.; Kim, B.-G.; et al. Metabolic Control of Primed Human Pluripotent Stem Cell Fate and Function by the miR-200c–SIRT2 Axis. Nat. Cell Biol. 2017, 19, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Ohkubo, R.; Widjaja, A.; Chen, D. The Mitochondrial Metabolic Checkpoint in Stem Cell Aging and Rejuvenation. Mech. Ageing Dev. 2020, 188, 111254. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Barthez, M.; Chen, D. Mitochondrial Regulation in Stem Cells. Trends Cell Biol. 2023, S0962-8924, 00207-6. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Brown, K.; Hirschey, M.D.; Verdin, E.; Chen, D. Calorie Restriction Reduces Oxidative Stress by SIRT3-Mediated SOD2 Activation. Cell Metab. 2010, 12, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Xie, S.Z.; Qiu, X.; Mohrin, M.; Shin, J.; Liu, Y.; Zhang, D.; Scadden, D.T.; Chen, D. SIRT3 Reverses Aging-Associated Degeneration. Cell Rep. 2013, 3, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.M.; Shaw, C.A.; Gatza, C.E.; Fisk, C.J.; Donehower, L.A.; Goodell, M.A. Aging Hematopoietic Stem Cells Decline in Function and Exhibit Epigenetic Dysregulation. PLoS Biol. 2007, 5, e201. [Google Scholar] [CrossRef]
- Li, S.; Zheng, W. Mammalian Sirtuins SIRT4 and SIRT7. Prog. Mol. Biol. Transl. Sci. 2018, 154, 147–168. [Google Scholar] [CrossRef]
- He, L.; Liu, Q.; Cheng, J.; Cao, M.; Zhang, S.; Wan, X.; Li, J.; Tu, H. SIRT4 in Ageing. Biogerontology 2023, 24, 347–362. [Google Scholar] [CrossRef]
- Liu, B.; Qu, J.; Zhang, W.; Belmonte, J.C.I.; Liu, G. A Stem Cell Aging Framework, from Mechanisms to Interventions. Cell Rep. 2022, 41, 111451. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Shin, M.H.; Kim, M.K.; Kim, Y.K.; Shin, H.S.; Lee, D.H.; Chung, J.H. Increased Histone Acetylation and Decreased Expression of Specific Histone Deacetylases in Ultraviolet-Irradiated and Intrinsically Aged Human Skin in vivo. Int. J. Mol. Sci. 2021, 22, 2032. [Google Scholar] [CrossRef]
- Kofman, A.E.; Huszar, J.M.; Payne, C. Transcriptional Analysis of Histone Deacetylase Family Members Reveal Similarities between Differentiating and Aging Spermatogonial Stem Cells. Stem Cell Rev. Rep. 2012, 9, 59–64. [Google Scholar] [CrossRef]
- Castex, J.; Willmann, D.; Kanouni, T.; Arrigoni, L.; Li, Y.; Friedrich, M.; Schleicher, M.; Wöhrle, S.; Pearson, M.; Kraut, N.; et al. Inactivation of Lsd1 Triggers Senescence in Trophoblast Stem Cells by Induction of Sirt4. Cell Death Dis. 2017, 8, e2631. [Google Scholar] [CrossRef]
- Su, S.; Ndiaye, M.A.; Singh, C.K.; Ahmad, N. Mitochondrial Sirtuins in Skin and Skin Cancers. Photochem. Photobiol. 2020, 96, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Benavente, C.A.; Schnell, S.A.; Jacobson, E.L. Effects of Niacin Restriction on Sirtuin and PARP Responses to Photodamage in Human Skin. PLoS ONE 2012, 7, e42276. [Google Scholar] [CrossRef]
- Wang, H.F.; Li, Q.; Feng, R.; Wen, T. Transcription Levels of Sirtuin Family in Neural Stem Cells and Brain Tissues of Adult Mice. Cell. Mol. Biol. 2012, 58, OL1737-43. [Google Scholar]
- Wang, Y.; Yue, J.; Xiao, M.; Lü, X.; Chin, Y.E. SIRT4-Catalyzed Deacetylation of Axin1 Modulates the WNT/Β-Catenin Signaling Pathway. Front. Oncol. 2022, 12, 872444. [Google Scholar] [CrossRef]
- Wang, F.; Wang, K.; Xu, W.; Zhao, S.; Ye, D.; Wang, Y.; Xu, Y.; Zhou, L.; Chu, Y.; Zhang, C.; et al. SIRT5 Desuccinylates and Activates Pyruvate Kinase M2 to Block Macrophage IL-1Β Production and to Prevent DSS-Induced Colitis in Mice. Cell Rep. 2017, 19, 2331–2344. [Google Scholar] [CrossRef]
- Ye, X.; Niu, X.; Gu, L.; Xu, Y.; Li, Z.; Ye, Y.; Chen, Z.; Lu, S. Desuccinylation of Pyruvate Kinase M2 by SIRT5 Contributes to Antioxidant Response and Tumor Growth. Oncotarget 2016, 8, 6984–6993. [Google Scholar] [CrossRef]
- Nishida, Y.; Rardin, M.J.; Carrico, C.; He, W.; Sahu, A.K.; Gut, P.; Najjar, R.; Fitch, M.; Hellerstein, M.K.; Gibson, B.W.; et al. SIRT5 Regulates Both Cytosolic and Mitochondrial Protein Malonylation with Glycolysis as a Major Target. Mol. Cell 2015, 59, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Chen, Y.; Tishkoff, D.X.; Peng, C.; Tan, M.; Dai, L.; Xie, Z.; Zhang, Y.; Zwaans, B.; Skinner, M.; et al. SIRT5-Mediated Lysine Desuccinylation Impacts Diverse Metabolic Pathways. Mol. Cell 2013, 50, 919–930. [Google Scholar] [CrossRef]
- Zhang, Y.; Bharathi, S.S.; Rardin, M.J.; Lu, J.; Maringer, K.; Sims-Lucas, S.; Prochownik, E.V.; Gibson, B.W.; Goetzman, E.S. Lysine Desuccinylase SIRT5 Binds to Cardiolipin and Regulates the Electron Transport Chain. J. Biol. Chem. 2017, 292, 10239–10249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bharathi, S.S.; Rardin, M.J.; Uppala, R.; Verdin, E.; Gibson, B.W.; Goetzman, E.S. SIRT3 and SIRT5 Regulate the Enzyme Activity and Cardiolipin Binding of Very Long-Chain Acyl-COA Dehydrogenase. PLoS ONE 2015, 10, e0122297. [Google Scholar] [CrossRef]
- Sadhukhan, S.; Liu, X.; Ryu, D.; Nelson, O.D.; Stupinski, J.A.; Li, Z.; Chen, W.; Zhang, S.; Weiss, R.S.; Locasale, J.W.; et al. Metabolomics-Assisted Proteomics Identifies Succinylation and SIRT5 as Important Regulators of Cardiac Function. Proc. Natl. Acad. Sci. USA 2016, 113, 4320–4325. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Liu, G.; Qu, J. Mitochondrial Sirtuins, Metabolism, and Aging. J. Genet. Genom. 2022, 49, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Chopra, H.; Hung, M.; Kwong, D.L.W.; Zhang, C.F. Insights into Endothelial Progenitor Cells: Origin, Classification, Potentials, and Prospects. Stem Cells Int. 2018, 2018, 9847015. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhi, H.; Zhang, X.; Liang, J.; He, J.; Su, C.; Xia, W.; Zhang, G.; Tao, J. Mitochondrial Dysfunction-Mediated Decline in Angiogenic Capacity of Endothelial Progenitor Cells Is Associated with Capillary Rarefaction in Patients with Hypertension via Downregulation of CXCR4/JAK2/SIRT5 Signaling. EBioMedicine 2019, 42, 64–75. [Google Scholar] [CrossRef]
- Hsu, Y.; Wu, Y.; Yu, T.-H.; Wei, Y. Mitochondria in Mesenchymal Stem Cell Biology and Cell Therapy: From Cellular Differentiation to Mitochondrial Transfer. Semin. Cell Dev. Biol. 2016, 52, 119–131. [Google Scholar] [CrossRef]
- Ou, T.; Yang, W.; Li, W.; Lu, Y.; Dong, Z.; Zhu, H.; Sun, X.; Dong, Z.; Weng, X.; Chang, S.; et al. SIRT5 Deficiency Enhances the Proliferative and Therapeutic Capacities of Adipose-derived Mesenchymal Stem Cells via Metabolic Switching. Clin. Transl. Med. 2020, 10, e172. [Google Scholar] [CrossRef]
- Tasselli, L.; Zheng, W.; Chua, K.F. SIRT6: Novel Mechanisms and Links to Aging and Disease. Trends Endocrinol. Metab. 2017, 28, 168–185. [Google Scholar] [CrossRef]
- Orkin, S.H.; Hochedlinger, K. Chromatin Connections to Pluripotency and Cellular Reprogramming. Cell 2011, 145, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.P.; Yabuuchi, A.; Rao, S.; Huang, Y.; Cunniff, K.; Nardone, J.; Laiho, A.; Tahiliani, M.; Sommer, C.; Mostoslavsky, G.; et al. TET1 and TET2 Regulate 5-Hydroxymethylcytosine Production and Cell Lineage Specification in Mouse Embryonic Stem Cells. Cell Stem Cell 2011, 8, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Etchegaray, J.; Chávez, L.; Huang, Y.; Ross, K.N.; Choi, J.; Martínez-Pastor, B.; Walsh, R.; Sommer, C.; Lienhard, M.; Gladden, A.D.; et al. The Histone Deacetylase SIRT6 Controls Embryonic Stem Cell Fate via TET-Mediated Production of 5-Hydroxymethylcytosine. Nat. Cell Biol. 2015, 17, 545–557. [Google Scholar] [CrossRef]
- Wang, H.; Diao, D.; Shi, Z.; Zhu, X.; Gao, Y.; Gao, S.; Liu, X.; Wu, Y.; Rudolph, K.L.; Liu, G.; et al. SIRT6 Controls Hematopoietic Stem Cell Homeostasis through Epigenetic Regulation of Wnt Signaling. Cell Stem Cell 2016, 18, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Guan, D.; Liu, X.; Li, J.; Wang, L.; Wu, J.; Zhou, J.; Zhang, W.; Ren, R.; Zhang, W.; et al. SIRT6 Safeguards Human Mesenchymal Stem Cells from Oxidative Stress by Coactivating NRF2. Cell Res. 2016, 26, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Yan, P.; Zhang, J.; Song, H.; Wu, Y.; Gong, H.; Li, H.; Wu, J.; Xie, J.; Li, R. Knockdown of SIRT6 Enables Human Bone Marrow Mesenchymal Stem Cell Senescence. Rejuvenation Res. 2016, 19, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, Y.; Fu, D.; Liu, Y.; Huang, C. SIRT6 Regulates Osteogenic Differentiation of Rat Bone Marrow Mesenchymal Stem Cells Partially via Suppressing the Nuclear Factor-ΚB Signaling Pathway. Stem Cells 2014, 32, 1943–1955. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Diecke, S.; Zhang, W.Y.; Lan, F.; He, C.; Mordwinkin, N.M.; Chua, K.F.; Wu, J.C. The Role of SIRT6 Protein in Aging and Reprogramming of Human Induced Pluripotent Stem Cells. J. Biol. Chem. 2013, 288, 18439–18447. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A. SIRT7 in the Aging Process. Cell. Mol. Life Sci. 2022, 79, 297. [Google Scholar] [CrossRef]
- Vázquez, B.N.; Thackray, J.K.; Simonet, N.G.; Kane-Goldsmith, N.; Martínez-Redondo, P.; Nguyen, T.; Bunting, S.F.; Vaquero, A.; Tischfield, J.A.; Serrano, L. SIRT 7 Promotes Genome Integrity and Modulates Non-homologous End Joining DNA Repair. EMBO J. 2016, 35, 1488–1503. [Google Scholar] [CrossRef]
- Mohrin, M.; Shin, J.; Liu, Y.; Brown, K.; Luo, H.; Xi, Y.; Haynes, C.M.; Chen, D. A Mitochondrial UPR-Mediated Metabolic Checkpoint Regulates Hematopoietic Stem Cell Aging. Science 2015, 347, 1374–1377. [Google Scholar] [CrossRef]
- Mohrin, M.; Widjaja, A.; Liu, Y.; Luo, H.; Chen, D. The Mitochondrial Unfolded Protein Response Is Activated upon Hematopoietic Stem Cell Exit from Quiescence. Aging Cell 2018, 17, e12756. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tang, X.; Zhang, S.; Jin, M.; Wang, M.; Deng, Z.; Liu, Z.; Qian, M.; Shi, W.; Wang, Z.; et al. SIRT 7 Activates Quiescent Hair Follicle Stem Cells to Ensure Hair Growth in Mice. EMBO J. 2020, 39, e104365. [Google Scholar] [CrossRef]
- Bi, S.; Liu, Z.; Wu, Z.; Wang, Z.; Liu, X.; Wang, S.; Ren, J.; Yao, Y.; Zhang, W.; Song, M.; et al. SIRT7 Antagonizes Human Stem Cell Aging as a Heterochromatin Stabilizer. Protein Cell 2020, 11, 483–504. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Li, Q.; Chang, H.-C.; Tang, Y. SIRT7 Facilitates CENP-A Nucleosome Assembly and Suppresses Intestinal Tumorigenesis. iScience 2020, 23, 101461. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Ran, S.; Liu, B.; Ji, L. miR-152 Induces Human Dental Pulp Stem Cell Senescence by Inhibiting SIRT7 Expression. FEBS Lett. 2016, 590, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.E.M.; Zhang, W.; Ye, C.C.Y.; Gao, X.; Jiang, L.; Zhao, T.; Pan, Z.; Xue, D. Knockdown of SIRT7 Enhances the Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells Partly via Activation of the Wnt/β-Catenin Signaling Pathway. Cell Death Dis. 2017, 8, e3042. [Google Scholar] [CrossRef]
- Liu, H.; Hu, L.; Yu, G.; Yang, H.; Cao, Y.; Wang, S. LNCRNA, PLXDC2-OT Promoted the Osteogenesis Potentials of MSCs by Inhibiting the Deacetylation Function of RBM6/SIRT7 Complex and OSX Specific Isoform. Stem Cells 2021, 39, 1049–1066. [Google Scholar] [CrossRef]
- Petrini, S.; Borghi, R.; D’Oria, V.; Restaldi, F.; Moreno, S.; Novelli, A.; Bertini, E.; Compagnucci, C. Aged Induced Pluripotent Stem Cell (iPSCs) as a New Cellular Model for Studying Premature Aging. Aging 2017, 9, 1453–1469. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Ding, X.; Li, Q.; Qiu, F.; Wang, M.; Shen, Z.; Zheng, H.; Fu, G. Bone Marrow Mesenchymal Stem Cell-Secreted Exosomes Carrying microRNA-125b Protect against Myocardial Ischemia Reperfusion Injury via Targeting SIRT7. Mol. Cell. Biochem. 2019, 465, 103–114. [Google Scholar] [CrossRef]
- Ding, C.; Zhu, L.; Shen, H.; Lu, J.; Zou, Q.; Huang, C.; Li, H.; Huang, B. Exosomal miRNA-17-5p Derived from Human Umbilical Cord Mesenchymal Stem Cells Improves Ovarian Function in Premature Ovarian Insufficiency by Regulating SIRT7. Stem Cells 2020, 38, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Vakhrusheva, O.; Braeuer, D.; Liu, Z.; Braun, T.; Bober, E. Sirt7-Dependent Inhibition of Cell Growth and Proliferation Might Be Instrumental to Mediate Tissue Integrity during Aging. J. Physiol. Pharmacol. 2008, 59 (Suppl. S9), 201–212. [Google Scholar] [PubMed]
- Wątroba, M.; Dudek, I.; Skoda, M.; Stangret, A.; Rzodkiewicz, P.; Szukiewicz, D. Sirtuins, Epigenetics and Longevity. Ageing Res. Rev. 2017, 40, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, M.; Di Rienzo, M.; Piacentini, M.; Fimia, G.M. Emerging Mechanisms in Initiating and Terminating Autophagy. Trends Biochem. Sci. 2017, 42, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yang, C.; Tooze, S.A. Autophagy Pathway: Cellular and Molecular Mechanisms. Autophagy 2017, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Cao, L.; Mostoslavsky, R.; Lombard, D.B.; Liu, J.; Bruns, N.E.; Tsokos, M.; Alt, F.W.; Finkel, T. A Role for the NAD-Dependent Deacetylase Sirt1 in the Regulation of Autophagy. Proc. Natl. Acad. Sci. USA 2008, 105, 3374–3379. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Xu, Y.; Wan, W.; Shou, X.; Qian, J.; You, Z.; Liu, B.; Chang, C.; Zhou, T.; Lippincott-Schwartz, J.; et al. Deacetylation of Nuclear LC3 Drives Autophagy Initiation under Starvation. Mol. Cell 2015, 57, 456–466. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Li, T.Y.; Liu, Q.; Zhang, C.; Li, X.; Chen, Y.; Zhang, S.; Lian, G.; Liu, Q.; Ruan, K.; et al. GSK3-TIP60-ULK1 Signaling Pathway Links Growth Factor Deprivation to Autophagy. Science 2012, 336, 477–481. [Google Scholar] [CrossRef]
- Shen, Q.; Shi, Y.; Liu, J.; Su, H.; Huang, J.; Zhang, Y.; Peng, C.; Zhou, T.; Sun, Q.; Wan, W.; et al. Acetylation of STX17 (Syntaxin 17) Controls Autophagosome Maturation. Autophagy 2020, 17, 1157–1169. [Google Scholar] [CrossRef]
- Su, H.; Yang, F.; Wang, Q.; Shen, Q.; Huang, J.; Peng, C.; Zhang, Y.; Wan, W.; Wong, C.C.L.; Sun, Q.; et al. VPS34 Acetylation Controls Its Lipid Kinase Activity and the Initiation of Canonical and Non-Canonical Autophagy. Mol. Cell 2017, 67, 907–921.e7. [Google Scholar] [CrossRef]
- Yi, C.; Ma, M.; Ran, L.; Zheng, J.; Tong, J.; Zhu, J.; Ma, C.; Sun, Y.; Zhang, S.; Feng, W.; et al. Function and Molecular Mechanism of Acetylation in Autophagy Regulation. Science 2012, 336, 474–477. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Jiang, W.-X.; Qin, L.-Y.; Gong, Z.; Wan, W.; Li, J.; Wang, Y.; Zhang, H.; Peng, C.; Zhou, T.; et al. Requirement for P62 Acetylation in the Aggregation of Ubiquitylated Proteins under Nutrient Stress. Nat. Commun. 2019, 10, 5792. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Zheng, L.; Qi, Z.; Li, X.; Zhang, X.; Li, Z.; Bai, X.; Zhang, Z.; Huo, W.; Zhao, X.; et al. Deacetylation of TFEB Promotes Fibrillar Aβ Degradation by Upregulating Lysosomal Biogenesis in Microglia. Protein Cell 2016, 7, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y.; Liu, J.; Zhang, J.; Xu, M.; You, Z.; Peng, C.; Gong, Z.; Liu, W. Acetyltransferase GCN5 Regulates Autophagy and Lysosome Biogenesis by Targeting TFEB. EMBO Rep. 2019, 21, e48335. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zhou, Z.; Park, J.-E.; Wang, L.; Wu, S.; Sun, X.; Lu, L.; Wang, T.; Lin, Q.; et al. Importance of TFEB Acetylation in Control of Its Transcriptional Activity and Lysosomal Function in Response to Histone Deacetylase Inhibitors. Autophagy 2018, 14, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wan, W. Acetylation in the Regulation of Autophagy. Autophagy 2022, 19, 379–387. [Google Scholar] [CrossRef]
- Matsuura, A.; Tsukada, M.; Wada, Y.; Ohsumi, Y. Apg1p, a Novel Protein Kinase Required for the Autophagic Process in Saccharomyces Cerevisiae. Gene 1997, 192, 245–250. [Google Scholar] [CrossRef]
- Hosokawa, N.; Sasaki, T.; Iemura, S.I.; Natsume, T.; Hara, T.; Mizushima, N. Atg101, a Novel Mammalian Autophagy Protein Interacting with Atg13. Autophagy 2009, 5, 973–979. [Google Scholar] [CrossRef]
- Chang, Y.; Neufeld, T.P. An Atg1/Atg13 Complex with Multiple Roles in TOR-Mediated Autophagy Regulation. Mol. Biol. Cell 2009, 20, 2004–2014. [Google Scholar] [CrossRef]
- Hosokawa, N.; Hara, T.; Kaizuka, T.; Kishi, C.; Takamura, A.; Miura, Y.; Iemura, S.I.; Natsume, T.; Takehana, K.; Yamada, N.; et al. Nutrient-Dependent mTORC1 Association with the ULK1–Atg13–FIP200 Complex Required for Autophagy. Mol. Biol. Cell 2009, 20, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Ganley, I.G.; Lam, D.; Wang, J.; Ding, X.; Chen, S.; Jiang, X. ULK1·ATG13·FIP200 Complex Mediates MTOR Signaling and Is Essential for Autophagy. J. Biol. Chem. 2009, 284, 12297–12305. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Jun, C.; Ro, S.H.; Kim, Y.M.; Otto, N.; Cao, J.; Kundu, M.; Kim, D.-H. ULK-ATG13-FIP200 Complexes Mediate MTOR Signaling to the Autophagy Machinery. Mol. Biol. Cell 2009, 20, 1992–2003. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K. AMPK and mTOR Regulate Autophagy through Direct Phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.C.; Tian, Y.; Yuan, H.; Park, H.W.; Chang, Y.; Kim, J.; Kim, H.; Neufeld, T.P.; Dillin, A.; Guan, K.L. ULK1 Induces Autophagy by Phosphorylating Beclin-1 and Activating VPS34 Lipid Kinase. Nat. Cell Biol. 2013, 15, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, X.; Zhang, P.; Chen, W.D.; Zhang, H.L.; Li, D.D.; Deng, R.; Qian, X.; Jiao, L.; Ji, J.; et al. Acetylation of Beclin 1 Inhibits Autophagosome Maturation and Promotes Tumour Growth. Nat. Commun. 2015, 6, 7215. [Google Scholar] [CrossRef] [PubMed]
- Itakura, E.; Kishi, C.; Inoue, K.; Mizushima, N. Beclin 1 Forms Two Distinct Phosphatidylinositol 3-Kinase Complexes with Mammalian Atg14 and UVRAG. Mol. Biol. Cell 2008, 19, 5360–5372. [Google Scholar] [CrossRef] [PubMed]
- Kihara, A.; Noda, T.; Ishihara, N.; Ohsumi, Y. Two Distinct Vps34 Phosphatidylinositol 3–Kinase Complexes Function in Autophagy and Carboxypeptidase Y Sorting inSaccharomyces Cerevisiae. J. Cell Biol. 2001, 152, 519–530. [Google Scholar] [CrossRef]
- Sun, Q.; Fan, W.; Chen, K.; Ding, X.; Chen, S.; Zhong, Q. Identification of Barkor as a Mammalian Autophagy-Specific Factor for Beclin 1 and Class III Phosphatidylinositol 3-Kinase. Proc. Natl. Acad. Sci. USA 2008, 105, 19211–19216. [Google Scholar] [CrossRef]
- Funderburk, S.F.; Wang, Q.J.; Yue, Z. The Beclin 1–VPS34 Complex—At the Crossroads of Autophagy and Beyond. Trends Cell Biol. 2010, 20, 355–362. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, J.; Liao, W.; Liu, X.; Zhang, H.; Wang, S.; Wang, D.; Feng, J.; Yu, L.; Zhu, W. Cytosolic FoxO1 Is Essential for the Induction of Autophagy and Tumour Suppressor Activity. Nat. Cell Biol. 2010, 12, 665–675. [Google Scholar] [CrossRef]
- Zheng, M.; Hu, C.; Wu, M.; Chin, Y.E. Emerging Role of SIRT2 in Non-Small Cell Lung Cancer (Review). Oncol. Lett. 2021, 22, 731. [Google Scholar] [CrossRef]
- Tang, H.; Wang, M.-Y.; Xiao, W.; Wen, J.-W. SIRT2-Reverses Drug-Resistance of HL-60/A through Autophagy Mechanism. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2019, 27, 409–414. [Google Scholar]
- Kumar, S.; Jain, A.; Farzam, F.; Jia, J.; Gu, Y.; Choi, S.-M.; Mudd, M.; Claude-Taupin, A.; Wester, M.J.; Lidke, K.A.; et al. Mechanism of Stx17 Recruitment to Autophagosomes via IRGM and Mammalian Atg8 Proteins. J. Cell Biol. 2018, 217, 997–1013. [Google Scholar] [CrossRef]
- Nakatogawa, H.; Ichimura, Y.; Ohsumi, Y. ATG8, a Ubiquitin-like Protein Required for Autophagosome Formation, Mediates Membrane Tethering and Hemifusion. Cell 2007, 130, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.; Outzen, H.; Øvervatn, A.; Bjørkøy, G.; Johansen, T. P62/SQSTM1 Binds Directly to Atg8/LC3 to Facilitate Degradation of Ubiquitinated Protein Aggregates by Autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef] [PubMed]
- Dooley, H.C.; Razi, M.; Polson, H.E.J.; Girardin, S.E.; Wilson, M.; Tooze, S.A. WIPI2 Links LC3 Conjugation with PI3P, Autophagosome Formation, and Pathogen Clearance by Recruiting Atg12–5-16L1. Mol. Cell 2014, 55, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, Y.; Kirisako, T.; Takao, T.; Satomi, Y.; Shimonishi, Y.; Ishihara, N.; Mizushima, N.; Tanida, I.; Kominami, E.; Ohsumi, M.; et al. A Ubiquitin-like System Mediates Protein Lipidation. Nature 2000, 408, 488–492. [Google Scholar] [CrossRef]
- Seok, S.; Fu, T.; Choi, S.E.; Yang, L.; Zhu, R.; Kumar, S.; Sun, X.; Yoon, G.; Kang, Y.; Zhong, W.; et al. Transcriptional Regulation of Autophagy by an FXR–CREB Axis. Nature 2014, 516, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Garcia-Arencibia, M.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.L.; Colella, P.; et al. TFEB Links Autophagy to Lysosomal Biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef]
- Ferron, M.; Settembre, C.; Shimazu, J.; Lacombe, J.; Kato, S.; Rawlings, D.J.; Ballabio, A.; Karsenty, G. A RANKL–PKCβ–TFEB Signaling Cascade Is Necessary for Lysosomal Biogenesis in Osteoclasts. Genes Dev. 2013, 27, 955–969. [Google Scholar] [CrossRef]
- Palmieri, M.; Pal, R.; Sardiello, M. AKT Modulates the Autophagy-Lysosome Pathway via TFEB. Cell Cycle 2017, 16, 1237–1238. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Zoncu, R.; Medina, D.L.; Vetrini, F.; Erdin, S.; Erdin, S.; Huynh, T.; Ferron, M.; Karsenty, G.; Vellard, M.; et al. A Lysosome-to-Nucleus Signalling Mechanism Senses and Regulates the Lysosome via mTOR and TFEB. EMBO J. 2012, 31, 1095–1108. [Google Scholar] [CrossRef]
- Füllgrabe, J.; Lynch-Day, M.A.; Heldring, N.; Li, W.; Struijk, R.B.; Ma, Q.; Hermanson, O.; Rosenfeld, M.G.; Klionsky, D.J.; Joseph, B. The Histone H4 Lysine 16 Acetyltransferase hMOF Regulates the Outcome of Autophagy. Nature 2013, 500, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.S.; Shin, H.; Kim, D.; Baek, S.A.; Choi, S.A.; Ahn, H.; Shamim, A.; Kim, J.; Kim, I.S.; Kim, K.K.; et al. Pontin Arginine Methylation by CARM1 Is Crucial for Epigenetic Regulation of Autophagy. Nat. Commun. 2020, 11, 6297. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Su, H.; Zhang, D.; Wang, Y.; Shen, Q.; Liu, B.; Huang, R.; Zhou, T.; Peng, C.; Wong, C.C.L.; et al. AMPK-Dependent Phosphorylation of GAPDH Triggers Sirt1 Activation and Is Necessary for Autophagy upon Glucose Starvation. Mol. Cell 2015, 60, 930–940. [Google Scholar] [CrossRef]
- Kim, J.E.; Chen, J.; Lou, Z. DBC1 Is a Negative Regulator of SIRT1. Nature 2008, 451, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Kruse, J.-P.; Tang, Y.; Jung, S.Y.; Qin, J.; Gu, W. Negative Regulation of the Deacetylase SIRT1 by DBC1. Nature 2008, 451, 587–590. [Google Scholar] [CrossRef]
- Seo, A.Y.; Lau, P.-W.; Feliciano, D.; Sengupta, P.; Gros, M.A.L.; Cinquin, B.; Larabell, C.A.; Lippincott-Schwartz, J. AMPK and Vacuole-Associated Atg14p Orchestrate μ-Lipophagy for Energy Production and Long-Term Survival under Glucose Starvation. eLife 2017, 6, e21690. [Google Scholar] [CrossRef]
- Roberts, P.M.; Moshitch-Moshkovitz, S.; Kvam, E.; O’Toole, E.; Winey, M.; Goldfarb, D.S. Piecemeal Microautophagy of Nucleus inSaccharomyces Cerevisiae. Mol. Biol. Cell 2003, 14, 129–141. [Google Scholar] [CrossRef]
- Lemasters, J.J. Variants of Mitochondrial Autophagy: Types 1 and 2 Mitophagy and Micromitophagy (Type 3). Redox Biol. 2014, 2, 749–754. [Google Scholar] [CrossRef]
- Filali-Mouncef, Y.; Hunter, C.J.; Roccio, F.; Zagkou, S.; Dupont, N.; Primard, C.; Proikas-Cezanne, T.; Reggiori, F. The Ménage à Trois of Autophagy, Lipid Droplets and Liver Disease. Autophagy 2021, 18, 50–72. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Jing, X.; Guo, J.; Yao, X.; Guo, F. Mitophagy in Degenerative Joint Diseases. Autophagy 2020, 17, 2082–2092. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The Biogenesis, Biology and Characterization of Circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Huang, W.; Fang, Z.; Ding, F.; Zou, F.; Ma, X.; Tao, J.; Guo, J.; Xia, X.; Wang, H.; et al. CircERCC2 Ameliorated Intervertebral Disc Degeneration by Regulating Mitophagy and Apoptosis through miR-182-5p/SIRT1 Axis. Cell Death Dis. 2019, 10, 751. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-N.; Yang, R.; Zheng, H.; Yu, W.; Zheng, X.; Li, B.; Jiang, S.; Jiang, L. PGC-1α Acts as an Mediator of Sirtuin2 to Protect Annulus Fibrosus from Apoptosis Induced by Oxidative Stress through Restraining Mitophagy. Int. J. Biol. Macromol. 2019, 136, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.B.; Samant, S.; Sundaresan, N.R.; Raghuraman, H.; Kim, G.; Bonner, M.Y.; Arbiser, J.L.; Walker, D.I.; Jones, D.P.; Gius, D.; et al. Honokiol Blocks and Reverses Cardiac Hypertrophy in Mice by Activating Mitochondrial Sirt3. Nat. Commun. 2015, 6, 6656. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nisar, M.; Huang, C.; Pan, X.; Lin, D.; Zheng, G.; Jin, H.; Chen, D.; Tian, N.; Huang, Q.; et al. Small Molecule Natural Compound Agonist of SIRT3 as a Therapeutic Target for the Treatment of Intervertebral Disc Degeneration. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy Regulates Lipid Metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef]
- Van Zutphen, T.; Todde, V.; De Boer, R.; Kreim, M.; Hofbauer, H.F.; Wolinski, H.; Veenhuis, M.; Van Der Klei, I.J.; Kohlwein, S.D. Lipid Droplet Autophagy in the yeastSaccharomyces Cerevisiae. Mol. Biol. Cell 2014, 25, 290–301. [Google Scholar] [CrossRef]
- Sathyanarayan, A.; Mashek, M.T.; Mashek, D.G. ATGL Promotes Autophagy/Lipophagy via SIRT1 to Control Hepatic Lipid Droplet Catabolism. Cell Rep. 2017, 19, 1–9. [Google Scholar] [CrossRef]
- Martínez-López, N.; Garcia-Macía, M.; Sahu, S.; Athonvarangkul, D.; Liebling, E.J.; Merlo, P.; Cecconi, F.; Schwartz, G.J.; Singh, R. Autophagy in the CNS and Periphery Coordinate Lipophagy and Lipolysis in the Brown Adipose Tissue and Liver. Cell Metab. 2016, 23, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.C.; Fang, C.; Russell, R.C.; Kim, J.H.; Fan, W.; Liu, R.; Zhong, Q.; Guan, K.L. Differential Regulation of Distinct VPS34 Complexes by AMPK in Nutrient Stress and Autophagy. Cell 2013, 152, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.G.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK Regulates Energy Expenditure by Modulating NAD+ Metabolism and SIRT1 Activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, T.-N.; Chen, H.; Yu, X.; Lv, J.-L.; Liu, Y.; Zheng, G.; Zhao, J.-Q.; Wei, Y.; Guo, J.; et al. The Sirtuin Family in Health and Disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [CrossRef] [PubMed]
- Cantor, J.R.; Sabatini, D.M. Cancer Cell Metabolism: One Hallmark, Many Faces. Cancer Discov. 2012, 2, 881–898. [Google Scholar] [CrossRef]
- Műzes, G.; Sipos, F. Autoimmunity and Carcinogenesis: Their Relationship under the Umbrella of Autophagy. Biomedicines 2023, 11, 1130. [Google Scholar] [CrossRef] [PubMed]
- Műzes, G.; Sipos, F. Tumorigenesis: Interplay of Pattern Recognition Receptors and Autophagy. Magy. Onkol. 2016, 60, 55–63. [Google Scholar]
- Műzes, G.; Constantinovits, M.; Fűri, I.; Tulassay, Z.; Sipos, F. Interaction of Autophagy and Toll-Like Receptors: A Regulatory Cross- Talk—Even in Cancer Cells? Curr. Drug Targets 2014, 15, 743–752. [Google Scholar] [CrossRef]
- Sipos, F.; Székely, H.; Kis, I.D.; Tulassay, Z.; Műzes, G. Relation of the IGF/IGF1R System to Autophagy in Colitis and Colorectal Cancer. World J. Gastroenterol. 2017, 23, 8109–8119. [Google Scholar] [CrossRef]
- Műzes, G.; Sipos, F. Anti-Tumor Immunity, Autophagy and Chemotherapy. World J. Gastroenterol. 2012, 18, 6537. [Google Scholar] [CrossRef]
- Műzes, G.; Sipos, F. Metastatic Cell Dormancy and Re-Activation: An Overview on Series of Molecular Events Critical for Cancer Relapse. Anti-Cancer Agents Med. Chem. 2017, 17, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Aventaggiato, M.; Vernucci, E.; Barreca, F.; Russo, M.A.; Tafani, M. Sirtuins’ Control of Autophagy and Mitophagy in Cancer. Pharmacol. Ther. 2021, 221, 107748. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E.; Lindeman, G.J. Cancer Stem Cells in Solid Tumours: Accumulating Evidence and Unresolved Questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Van Seuningen, I. On the Epigenetic Origin of Cancer Stem Cells. Biochim. Et Biophys. Acta (BBA)—Rev. Cancer 2012, 1826, 83–88. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, C.; Vassilopoulos, A. Sirtuins at the Crossroads of Stemness, Aging, and Cancer. Aging Cell 2017, 16, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Bhatia, R. Role of SIRT1 in the Growth and Regulation of Normal Hematopoietic and Leukemia Stem Cells. Curr. Opin. Hematol. 2015, 22, 324–329. [Google Scholar] [CrossRef]
- Shi, L.; Tang, X.; Qian, M.; Liu, Z.; Meng, F.; Fu, L.; Wang, Z.; Zhu, W.; Huang, J.; Zhou, Z.; et al. A SIRT1-Centered Circuitry Regulates Breast Cancer Stemness and Metastasis. Oncogene 2018, 37, 6299–6315. [Google Scholar] [CrossRef]
- Ma, W.; Xiao, G.G.; Murata, J.; Lü, Y.; Song, B.; Wang, L.; Fan, S.; Fan, P.; Hou, Z.; Li, J.; et al. Dysregulation of the miR-34a-SIRT1 Axis Inhibits Breast Cancer Stemness. Oncotarget 2015, 6, 10432–10444. [Google Scholar] [CrossRef]
- Deng, C. SIRT1, Is It a Tumor Promoter or Tumor Suppressor? Int. J. Biol. Sci. 2009, 5, 147–152. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.-R.; Kwon, O.; Lee, T.H.; Nakano, I.; Miyoshi, H.; Chun, K.; Park, J.B.; Lee, H.J.; Kim, S.U.; et al. SIRT1 Is Required for Oncogenic Transformation of Neural Stem Cells and for the Survival of “Cancer Cells with Neural Stemness” in a P53-Dependent Manner. Neuro-Oncology 2014, 17, 95–106. [Google Scholar] [CrossRef]
- Chen, X.; Sun, K.; Jiao, S.; Cai, N.; Zhao, X.; Zou, H.; Xie, Y.; Wang, Z.; Zhong, M.; Wei, L. High Levels of SIRT1 Expression Enhance Tumorigenesis and Associate with a Poor Prognosis of Colorectal Carcinoma Patients. Sci. Rep. 2014, 4, 7481. [Google Scholar] [CrossRef] [PubMed]
- Nalls, D.; Tang, S.-N.; Rodova, M.; Srivastava, R.K.; Shankar, S. Targeting Epigenetic Regulation of MIR-34A for Treatment of Pancreatic Cancer by Inhibition of Pancreatic Cancer Stem Cells. PLoS ONE 2011, 6, e24099. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, L.; Li, L.; Wang, Z.; Ho, Y.; McDonald, T.; Holyoake, T.L.; Chen, W.; Bhatia, S. Activation of P53 by SIRT1 Inhibition Enhances Elimination of CML Leukemia Stem Cells in Combination with Imatinib. Cancer Cell 2012, 21, 266–281. [Google Scholar] [CrossRef]
- Li, L.; Osdal, T.; Ho, Y.; Chun, S.; McDonald, T.; Agarwal, P.; Lin, A.; Chu, S.; Qi, J.; Li, L.; et al. SIRT1 Activation by a C-MYC Oncogenic Network Promotes the Maintenance and Drug Resistance of Human FLT3-ITD Acute Myeloid Leukemia Stem Cells. Cell Stem Cell 2014, 15, 431–446. [Google Scholar] [CrossRef]
- Bosch-Presegué, L.; Vaquero, A. The Dual Role of Sirtuins in Cancer. Genes Cancer 2011, 2, 648–662. [Google Scholar] [CrossRef]
- Carafa, V.; Altucci, L.; Nebbioso, A. Dual Tumor Suppressor and Tumor Promoter Action of Sirtuins in Determining Malignant Phenotype. Front. Pharmacol. 2019, 10, 38. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, Z.; Li, L.; Zhang, H.; Modi, H.; Horne, D.; Bhatia, R.; Chen, W. Activation of Stress Response Gene SIRT1 by BCR-ABL Promotes Leukemogenesis. Blood 2012, 119, 1904–1914. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Hsu, C.C.; Yung, M.C.; Chen, K.Y.; Tzao, C.; Wu, W.; Chou, H.; Lee, Y.Y.; Lü, K.; Chiou, S.H.; et al. Enhanced Radiosensitivity and Radiation-Induced Apoptosis in Glioma CD133-Positive Cells by Knockdown of SirT1 Expression. Biochem. Biophys. Res. Commun. 2009, 380, 236–242. [Google Scholar] [CrossRef]
- Yin, J.; Park, G.; Lee, J.E.; Park, J.Y.; Kim, T.-H.; Kim, Y.; Lee, S.; Yoo, H.; Kim, J.H.; Park, J.B. CPEB1 Modulates Differentiation of Glioma Stem Cells via Downregulation of HES1 and SIRT1 Expression. Oncotarget 2014, 5, 6756–6769. [Google Scholar] [CrossRef]
- Izumi, H.; Kaneko, Y.; Nakagawara, A. Molecular Regulation of Autophagy and Asymmetric Cell Division by Cancer Stem Cell Marker CD133. Cells 2023, 12, 819. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Kim, D.J.; Lee, H.S.; Jeong, C.H.; Cho, E.J.; Kim, M.O.; Byun, S.; Lee, K.Y.; Yao, K.; Carper, A.; et al. Autophagy and Cellular Senescence Mediated by SOX2 Suppress Malignancy of Cancer Cells. PLoS ONE 2013, 8, e57172. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Peng, D.; Zhang, S.; Zhang, Y.; Wang, Q.; Guan, L. Buyang Huanwu Decoction Promotes Neurogenesis via Sirtuin 1/Autophagy Pathway in a Cerebral Ischemia Model. Mol. Med. Rep. 2021, 24, 791. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Yang, Y.; Yu, Q.; Shi, S.; Zhou, S. Electroacupuncture Alleviates Ischaemic Brain Injury by Regulating the miRNA-34/Wnt/Autophagy Axis. Brain Res. Bull. 2021, 170, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Kaller, M.; Hermeking, H. CRISPR/Cas9-Mediated Inactivation of miR-34a and miR-34b/c in HCT116 Colorectal Cancer Cells: Comprehensive Characterization after Exposure to 5-FU Reveals EMT and Autophagy as Key Processes Regulated by miR-34. Cell Death Differ. 2023, 30, 2017–2034. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, P.; Chen, J.; Chen, Z.; Liu, Z.; Feng, G.; Sha, F.; Li, Z.; Xu, Z.-D.; Huang, Y.; et al. CD44 Connects Autophagy Decline and Ageing in the Vascular Endothelium. Nat. Commun. 2023, 14, 5524. [Google Scholar] [CrossRef]
- Hasmim, M.; Janji, B.; Khaled, M.; Noman, M.Z.; Louache, F.; Bordereaux, D.; Abderamane, A.; Baud, V.; Mami-Chouaib, F.; ChouaiB, S. Cutting Edge: NANOG Activates Autophagy under Hypoxic Stress by Binding to BNIP3L Promoter. J. Immunol. 2017, 198, 1423–1428. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; García-Cañaveras, J.C.; Guo, L.; Kan, M.; Yu, S.; Blair, I.A.; Rabinowitz, J.D.; Yang, X. Chaperone-Mediated Autophagy Regulates the Pluripotency of Embryonic Stem Cells. Science 2020, 369, 397–403. [Google Scholar] [CrossRef]
- Dobbin, Z.C.; Landen, C.N. The Importance of the PI3K/AKT/MTOR Pathway in the Progression of Ovarian Cancer. Int. J. Mol. Sci. 2013, 14, 8213–8227. [Google Scholar] [CrossRef]
- Beyer, A.; Toro, L.E.N.; Hughes, W.E.; Young, M.; Clough, A.V.; Gao, F.; Medhora, M.; Audi, S.H.; Jacobs, E.R. Autophagy, TERT, and Mitochondrial Dysfunction in Hyperoxia. Am. J. Physiol.-Heart Circ. Physiol. 2021, 321, H985–H1003. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Shen, J.; Chen, Z.; Yang, J.; Xie, B.; Jia, Y.; Jayasinghe, U.; Wang, J.; Zhao, W.; Xie, S.; et al. H19/Let-7/Lin28 ceRNA Network Mediates Autophagy Inhibiting Epithelial-mesenchymal Transition in Breast Cancer. Int. J. Oncol. 2020, 56, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, C.-C.; Chen, W. CD150−Side Population Defines Leukemia Stem Cells in a BALB/c Mouse Model of CML and Is Depleted by Genetic Loss of SIRT1. Stem Cells 2015, 33, 3437–3451. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z. Function of Sirtuins in Cancer Stem Cells. Int. J. Stem Cell Res. Ther. 2016, 3, 024. [Google Scholar] [CrossRef]
- Eppert, K.; Takenaka, K.; Lechman, E.R.; Waldron, L.; Nilsson, B.; van Galen, P.; Metzeler, K.H.; Poeppl, A.; Ling, V.; Beyene, J.; et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat. Med. 2011, 17, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, G.J. Emerging roles of Myc in stem cell biology and novel tumor therapies. J. Exp. Clin. Cancer Res. 2018, 37, 173. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, S.; Kumar, P.; Garg, N. Cellular experiments to study the inhibition of c-Myc/MAX heterodimerization. Integr. Methods Protein Biochem. Part A 2022, 675, 193–205. [Google Scholar]
- Adhikary, S.; Marinoni, F.; Hock, A.; Hulleman, E.; Popov, N.; Beier, R.; Bernard, S.; Quarto, M.; Capra, M.; Goettig, S.; et al. The ubiquitin ligase HectH9 regulates transcriptional activation by Myc and is essential for tumor cell proliferation. Cell 2005, 123, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Menssen, A.; Hydbring, P.; Kapelle, K.; Vervoorts, J.; Diebold, J.; Luscher, B.; Larsson, L.G.; Hermeking, H. The c-MYC oncoprotein, the NAMPT enzyme, the SIRT1-inhibitor DBC1, and the SIRT1 deacetylase form a positive feedback loop. Proc. Natl. Acad. Sci. USA 2012, 109, E187–E196. [Google Scholar] [CrossRef]
- Yuan, J.; Minter-Dykhouse, K.; Lou, Z.K. A c-Myc-SIRT1 feedback loop regulates cell growth and transformation. J. Cell Biol. 2009, 185, 203–211. [Google Scholar] [CrossRef]
- Sipos, F.; Firneisz, G.; Műzes, G. Therapeutic Aspects of C-MYC Signaling in Inflammatory and Cancerous Colonic Diseases. World J. Gastroenterol. 2016, 22, 7938. [Google Scholar] [CrossRef]
- Fimia, G.M.; Stoykova, A.; Romagnoli, A.; Giunta, L.; Di Bartolomeo, S.; Nardacci, R.; Corazzari, M.; Fuoco, C.; Ucar, A.; Schwartz, P.J.; et al. Ambra1 Regulates Autophagy and Development of the Nervous System. Nature 2007, 447, 1121–1125. [Google Scholar] [CrossRef]
- Gu, W.; Wan, D.; Qian, Q.; Yi, B.; He, Z.; Gu, Y.; Wang, L.; He, S. Ambra1 Is an Essential Regulator of Autophagy and Apoptosis in SW620 Cells: Pro-Survival Role of AmBra1. PLoS ONE 2014, 9, e90151. [Google Scholar] [CrossRef] [PubMed]
- Cianfanelli, V.; Fuoco, C.; Lorente, M.; Salazar, M.; Quondamatteo, F.; Gherardini, P.F.; De Zio, D.; Nazio, F.; Antonioli, M.; D’Orazio, M.; et al. Erratum: Corrigendum: AMBRA1 Links Autophagy to Cell Proliferation and Tumorigenesis by Promoting c-Myc Dephosphorylation and Degradation. Nat. Cell Biol. 2015, 17, 706. [Google Scholar] [CrossRef] [PubMed]
- Nazio, F.; Strappazzon, F.; Antonioli, M.; Bielli, P.; Cianfanelli, V.; Bordi, M.; Gretzmeier, C.; Dengjel, J.; Piacentini, M.; Fimia, G.M.; et al. mTOR Inhibits Autophagy by Controlling ULK1 Ubiquitylation, Self-Association and Function through AMBRA1 and TRAF6. Nat. Cell Biol. 2013, 15, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Ks, S.; Jh, P.; Heo, J.Y.; Jing, K.; Han, J.-T.; Min, K.; Kim, C.; Koh, G.-Y.; Lim, K.; Kang, G.; et al. SIRT2 Regulates Tumour Hypoxia Response by Promoting HIF-1α Hydroxylation. Oncogene 2014, 34, 1354–1362. [Google Scholar] [CrossRef]
- Si, X.; Chen, W.; Guo, X.; Chen, L.; Wang, G.; Xu, Y. Activation of GSK3Β by SIRT2 Is Required for Early Lineage Commitment of Mouse Embryonic Stem Cell. PLoS ONE 2013, 8, e76699. [Google Scholar] [CrossRef]
- Zhao, D.; Mo, Y.; Li, M.T.; Zou, S.W.; Cheng, Z.L.; Sun, Y.; Xiong, Y.; Guan, K.L.; Lei, Q.-Y. NOTCH-Induced Aldehyde Dehydrogenase 1A1 Deacetylation Promotes Breast Cancer Stem Cells. J. Clin. Investig. 2014, 124, 5453–5465. [Google Scholar] [CrossRef]
- Sayd, S.; Thirant, C.; El-Habr, E.A.; Lipecka, J.; Dubois, L.G.; Bogeas, A.; Tahiri-Jouti, N.; Chneiweiss, H.; Junier, M. Sirtuin-2 Activity Is Required for Glioma Stem Cell Proliferation Arrest but not Necrosis Induced by Resveratrol. Stem Cell Rev. Rep. 2013, 10, 103–113. [Google Scholar] [CrossRef]
- Ming, M.; Qiang, L.; Zhao, B.; He, Y. Mammalian SIRT2 Inhibits Keratin 19 Expression and Is a Tumor Suppressor in Skin. Exp. Dermatol. 2014, 23, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Jia, J.; Heng, G.; Xu, H.; Shan, J.; Wang, G.; Liu, C.; Xia, J.; Zhou, H.; Wu, M.; et al. Sirtuin-1/Mitochondrial Ribosomal Protein S5 Axis Enhances the Metabolic Flexibility of Liver Cancer Stem Cells. Hepatology 2019, 70, 1197–1213. [Google Scholar] [CrossRef]
- Zanjani, L.S.; Madjd, Z.; Abolhasani, M.; Shariftabrizi, A.; Rasti, A.; Asgari, M. Expression of CD105 Cancer Stem Cell Marker in Three Subtypes of Renal Cell Carcinoma. Cancer Biomark. 2018, 21, 821–837. [Google Scholar] [CrossRef]
- Peired, A.J.; Sisti, A.; Romagnani, P. Renal Cancer Stem Cells: Characterization and Targeted Therapies. Stem Cells Int. 2016, 2016, 8342625. [Google Scholar] [CrossRef]
- Sakurai, T.; Okumura, H.; Matsumoto, M.; Uchikado, Y.; Setoyama, T.; Omoto, I.; Owaki, T.; Maemura, K.; Ishigami, S.; Natsugoe, S. The Expression of LC-3 Is Related to Tumor Suppression through Angiogenesis in Esophageal Cancer. Med. Oncol. 2013, 30, 701. [Google Scholar] [CrossRef]
- Han, Z.; Chang, C.; Zhu, W.; Zhang, Y.; Zheng, J.; Kang, X.; Jin, G.; Gong, Z. Role of SIRT2 in Regulating the Dexamethasone-Activated Autophagy Pathway in Skeletal Muscle Atrophy. Biochem. Cell Biol. 2021, 99, 562–569. [Google Scholar] [CrossRef]
- Nowicki, M.; Wierzbowska, A.; Stec-Martyna, E.; Kulczycka-Wojdala, D.; Nowicki, G.; Szmigielska-Kapłon, A. SIRT1-SIRT7 Expression in Patients with Lymphoproliferative Disorders Undergoing Hematopoietic Stem Cell Mobilization. Cancers 2022, 14, 1213. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, W.; Chen, X.; Wang, S.; Qian, W. Oxidative Stress Response Induced by Chemotherapy in Leukemia Treatment. Mol. Clin. Oncol. 2018, 8, 391–399. [Google Scholar] [CrossRef]
- Zheng, J.; Shi, L.; Liang, F.; Xu, W.; Li, T.; Gao, L.; Sun, Z.; Yu, J.; Zhang, J. SIRT3 Ameliorates Oxidative Stress and Mitochondrial Dysfunction after Intracerebral Hemorrhage in Diabetic Rats. Front. Neurosci. 2018, 12, 414. [Google Scholar] [CrossRef]
- Zhang, T.; Ge, C.; Shen, S.; Tong, Q.; Ma, X.; Lin, L. SIRT3 Promotes Lipophagy and Chaperon-Mediated Autophagy to Protect Hepatocytes against Lipotoxicity. Cell Death Differ. 2019, 27, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Fang, C.; Xu, S.; Wang, B.; Li, D.; Liu, X.; Mi, Y.; Guo, H.; Jiang, J. SIRT3 Attenuates Doxorubicin-Induced Cardiotoxicity by Inhibiting NLRP3 Inflammasome via Autophagy. Biochem. Pharmacol. 2023, 207, 115354. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; He, Y.; Moqbel, S.A.A.; Zhou, X.; Wu, L.; Bao, J. SIRT3 Ameliorates Osteoarthritis via Regulating Chondrocyte Autophagy and Apoptosis through the PI3K/Akt/mTOR Pathway. Int. J. Biol. Macromol. 2021, 175, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Barreca, F.; Aventaggiato, M.; Vitiello, L.; Sansone, L.; Russo, M.A.; Mai, A.; Valente, S.; Tafani, M. SIRT5 Activation and Inorganic Phosphate Binding Reduce Cancer Cell Vitality by Modulating Autophagy/Mitophagy and ROS. Antioxidants 2023, 12, 1635. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yan, H.; An, S.; Shen, M.; Jia, W.; Zhang, R.; Zhao, L.; Huang, G.; Li, J. SIRT5-mediated Deacetylation of LDHB Promotes Autophagy and Tumorigenesis in Colorectal Cancer. Mol. Oncol. 2018, 13, 358–375. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Qian, Q.; Xu, Y.; Xu, X.; Zhang, L.; He, S.; Li, D. SIRT5 Regulates Autophagy and Apoptosis in Gastric Cancer Cells. J. Int. Med. Res. 2021, 49, 030006052098635. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, X.; Ren, Y.; Li, J.; Li, P.; Jiao, Q.; Meng, P.; Wang, F.; Wang, Y.; Wang, Y.; et al. Loss of SIRT4 Promotes the Self-Renewal of Breast Cancer Stem Cells. Theranostics 2020, 10, 9458–9476. [Google Scholar] [CrossRef]
- He, L.; Wang, J.; Yang, Y.; Zou, P.; Xia, Z.; Li, J. SIRT4 Suppresses Doxorubicin-Induced Cardiotoxicity by Regulating the AKT/MTOR/Autophagy Pathway. Toxicology 2022, 469, 153119. [Google Scholar] [CrossRef]
- Li, J.; Zhan, H.; Ren, Y.; Feng, M.; Wang, Q.; Jiao, Q.; Wang, Y.; Liu, X.; Zhang, S.; Wang, C.; et al. Sirtuin 4 Activates Autophagy and Inhibits Tumorigenesis by Upregulating the P53 Signaling Pathway. Cell Death Differ. 2022, 30, 313–326. [Google Scholar] [CrossRef]
- Yin, J.; Cai, G.; Wang, H.; Chen, W.; Liu, S.; Huang, G. SIRT4 Is an Independent Prognostic Factor in Bladder Cancer and Inhibits Bladder Cancer Growth by Suppressing Autophagy. Cell Div. 2023, 18, 9. [Google Scholar] [CrossRef]
- Ioris, R.M.; Galiè, M.; Ramadori, G.; Anderson, J.D.; Charollais, A.; Konstantinidou, G.; Brénachot, X.; Aras, E.; Goga, A.; Ceglia, N.; et al. SIRT6 Suppresses Cancer Stem-like Capacity in Tumors with PI3K Activation Independently of Its Deacetylase Activity. Cell Rep. 2017, 18, 1858–1868. [Google Scholar] [CrossRef]
- Huang, N.; Liu, Z.; Zhu, J.; Cui, Z.; Li, Y.; Yu, Y.; Sun, F.; Pan, Q.; Yang, Q. Sirtuin 6 Plays an Oncogenic Role and Induces Cell Autophagy in Esophageal Cancer Cells. Tumor Biol. 2017, 39, 101042831770853. [Google Scholar] [CrossRef]
- Sun, D.; Luo, M.; Jeong, M.; Rodriguez, B.; Xia, Z.; Hannah, R.; Wang, H.; Le, T.T.; Faull, K.F.; Chen, R.; et al. Epigenomic Profiling of Young and Aged HSCs Reveals Concerted Changes during Aging That Reinforce Self-Renewal. Cell Stem Cell 2014, 14, 673–688. [Google Scholar] [CrossRef]
- Zhao, E.; Hou, J.; Ke, X.; Abbas, M.N.; Kausar, S.; Zhang, L.; Cui, H. The Roles of Sirtuin Family Proteins in Cancer Progression. Cancers 2019, 11, 1949. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Zhang, N.; Sun, H.; Su, L.; Zhang, C.; Xu, H.; Feng, J.; Wang, M.; Chen, J.; Liu, L.; et al. Disruption of SIRT7 Increases the Efficacy of Checkpoint Inhibitor via MEF2D Regulation of Programmed Cell Death 1 Ligand 1 in Hepatocellular Carcinoma Cells. Gastroenterology 2020, 158, 664–678.e24. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.; Hsieh, T.C.; Halicka, H.D.; Darzynkiewicz, Z.; Wu, J.M. Upregulation of PD-L1 Expression by Resveratrol and Piceatannol in Breast and Colorectal Cancer Cells Occurs via HDAC3/p300-mediated NF-κB Signaling. Int. J. Oncol. 2018, 53, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Cao, N.; Li, Z.; Han, J.; Li, L. Resveratrol, an Activator of SIRT1, Induces Protective Autophagy in Non-small-cell Lung Cancer via Inhibiting Akt/mTOR and Activating p38-MAPK. OncoTargets Ther. 2018, 11, 7777–7786. [Google Scholar] [CrossRef] [PubMed]
- Iachettini, S.; Trisciuoglio, D.; Rotili, D.; Lucidi, A.; Salvati, E.; Zizza, P.; Di Leo, L.; Del Bufalo, D.; Ciriolo, M.R.; Leonetti, C.; et al. Pharmacological Activation of SIRT6 Triggers Lethal Autophagy in Human Cancer Cells. Cell Death Dis. 2018, 9, 996. [Google Scholar] [CrossRef] [PubMed]
- Mandhair, H.; Novak, U.; Radpour, R. Epigenetic Regulation of Autophagy: A Key Modification in Cancer Cells and Cancer Stem Cells. World J. Stem Cells 2021, 13, 542–567. [Google Scholar] [CrossRef]
- Powell, M.J.; Casimiro, M.C.; Cordon-Cardo, C.; He, X.; Yeow, W.-S.; Wang, C.; McCue, P.A.; McBurney, M.W.; Pestell, R.G. Disruption of a SIRT1-Dependent Autophagy Checkpoint in the Prostate Results in Prostatic Intraepithelial Neoplasia Lesion Formation. Cancer Res. 2011, 71, 964–975. [Google Scholar] [CrossRef]
- Wang, X.; Golino, J.L.; Xie, C. Autophagy Regulation on Cancer Stem Cell Maintenance, Metastasis, and Therapy Resistance. Cancers 2022, 14, 381. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sipos, F.; Műzes, G. Sirtuins Affect Cancer Stem Cells via Epigenetic Regulation of Autophagy. Biomedicines 2024, 12, 386. https://doi.org/10.3390/biomedicines12020386
Sipos F, Műzes G. Sirtuins Affect Cancer Stem Cells via Epigenetic Regulation of Autophagy. Biomedicines. 2024; 12(2):386. https://doi.org/10.3390/biomedicines12020386
Chicago/Turabian StyleSipos, Ferenc, and Györgyi Műzes. 2024. "Sirtuins Affect Cancer Stem Cells via Epigenetic Regulation of Autophagy" Biomedicines 12, no. 2: 386. https://doi.org/10.3390/biomedicines12020386





