Catfish Egg Lectin Enhances the Cytotoxicity of Sunitinib on Gb3-Expressing Renal Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lectin and Cell Lines
2.2. Flow-Cytometric Analysis of Cellular Propidium Iodide (PI) Uptake to Measure Cell Permeability
2.3. Cell Viability Assay
2.4. Flow-Cytometric Analysis of Cell Surface Gb3 Expression
2.5. Thin-Layer Chromatography (TLC) for Glycolipid Expression Analysis
2.6. Analysis of the Effect of a Combination of SU and SAL
2.7. Analysis of the Effect of a Combination of Other Molecular-Targeted Agents and SAL
2.8. Efflux of SU from SAL-Treated TOS1 Cells
2.9. Time-Series Measurements of Intracellular SU Contents
2.10. Reverse Transcription-Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.11. Statistical Analysis
3. Results
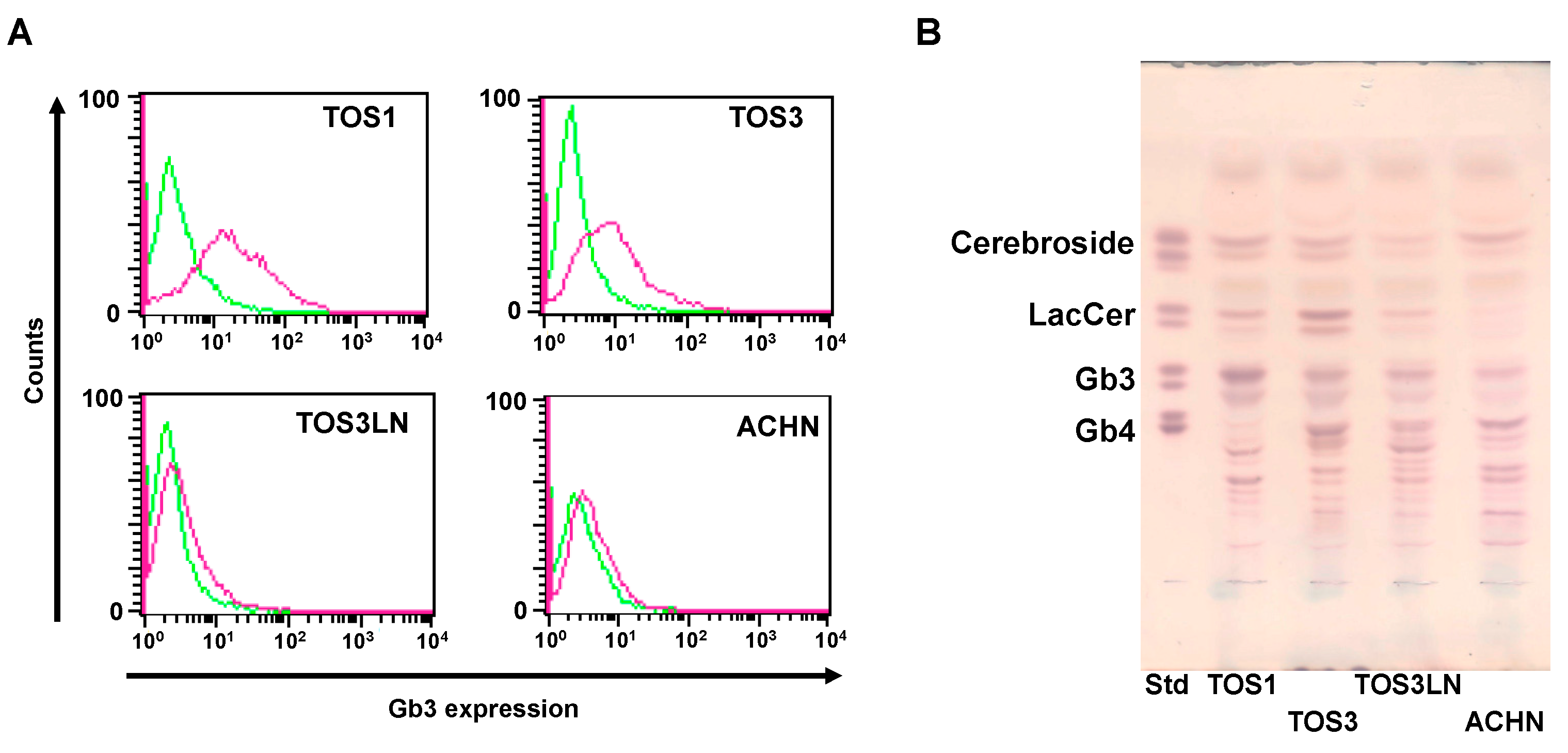
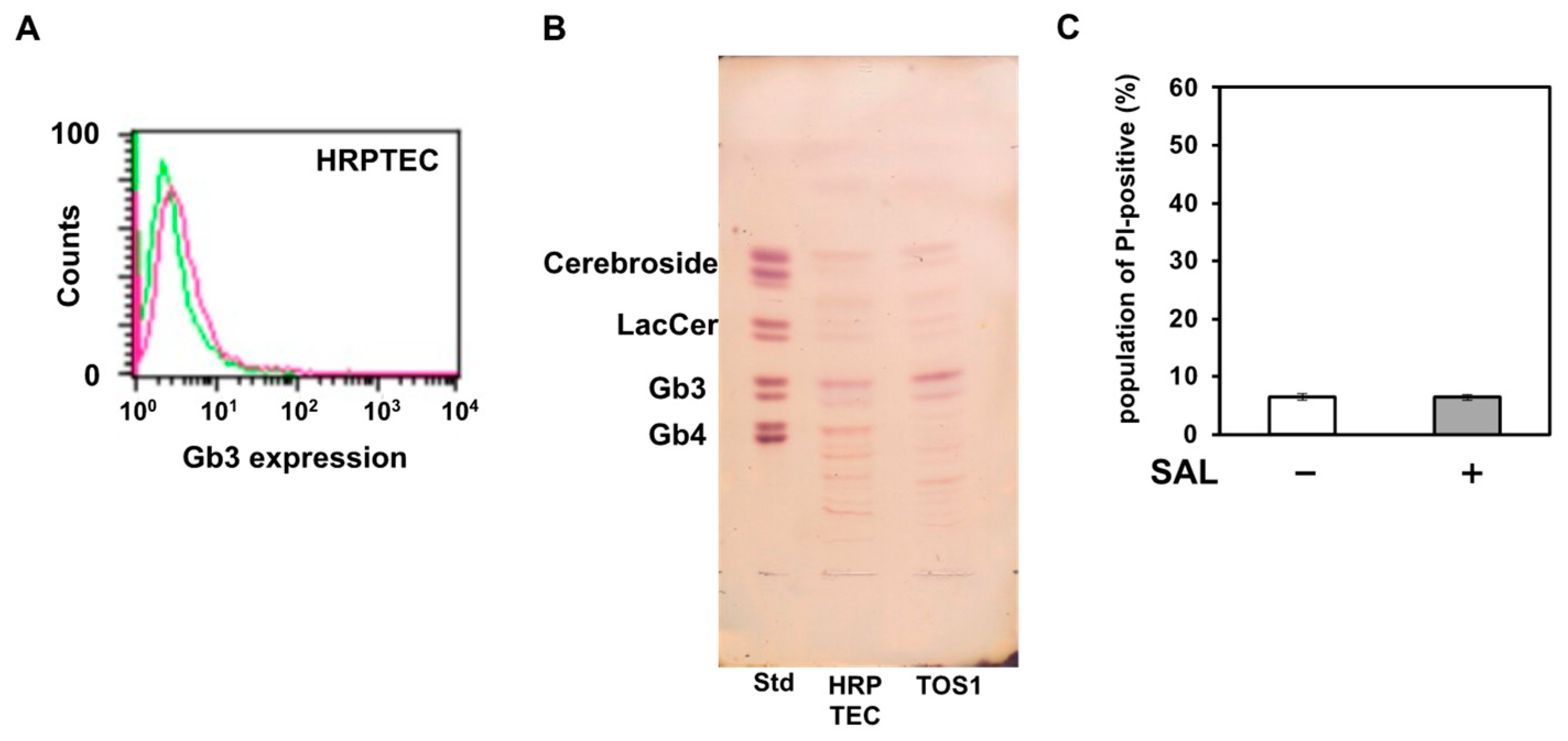
3.1. Expression of Gb3 on RCC Cell Lines
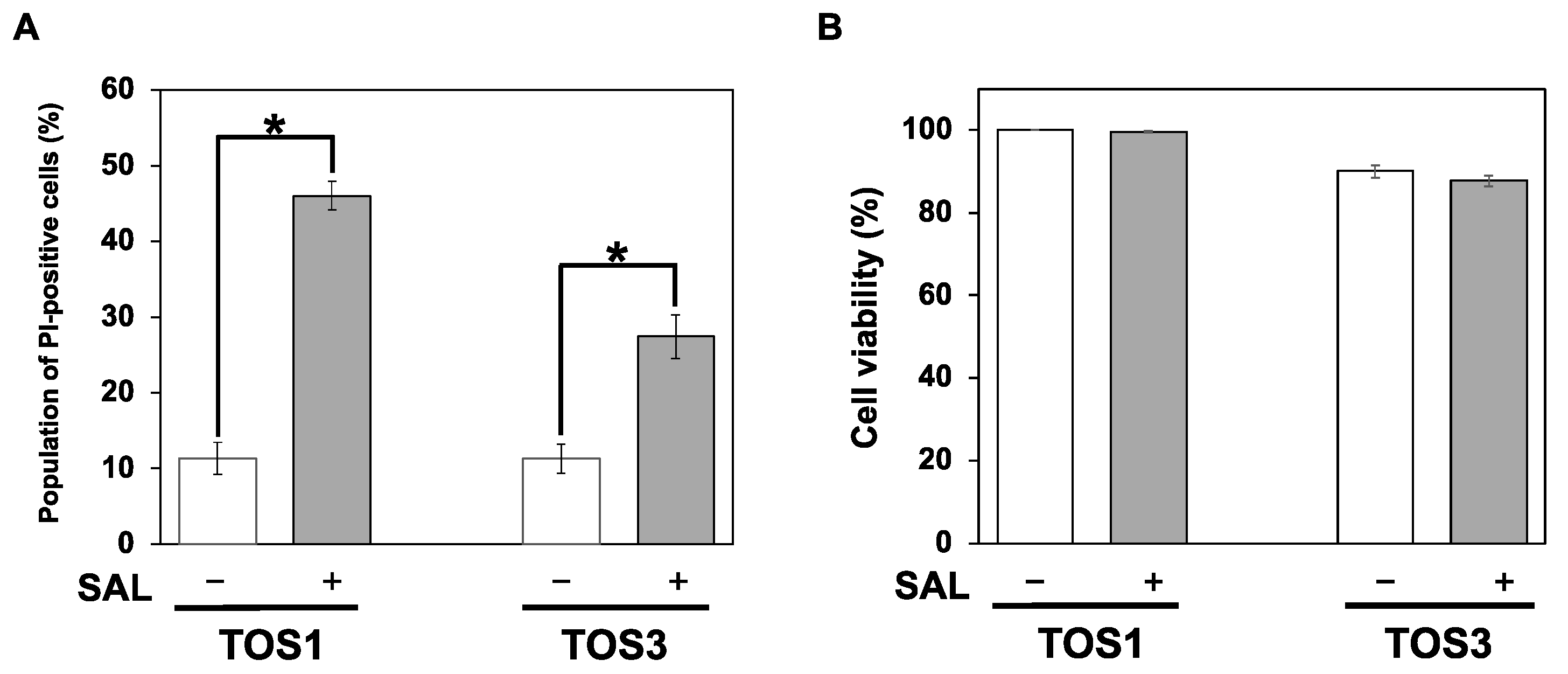
3.2. PI Uptake by and Viability of SAL-Treated TOS1 and TOS3 Cells
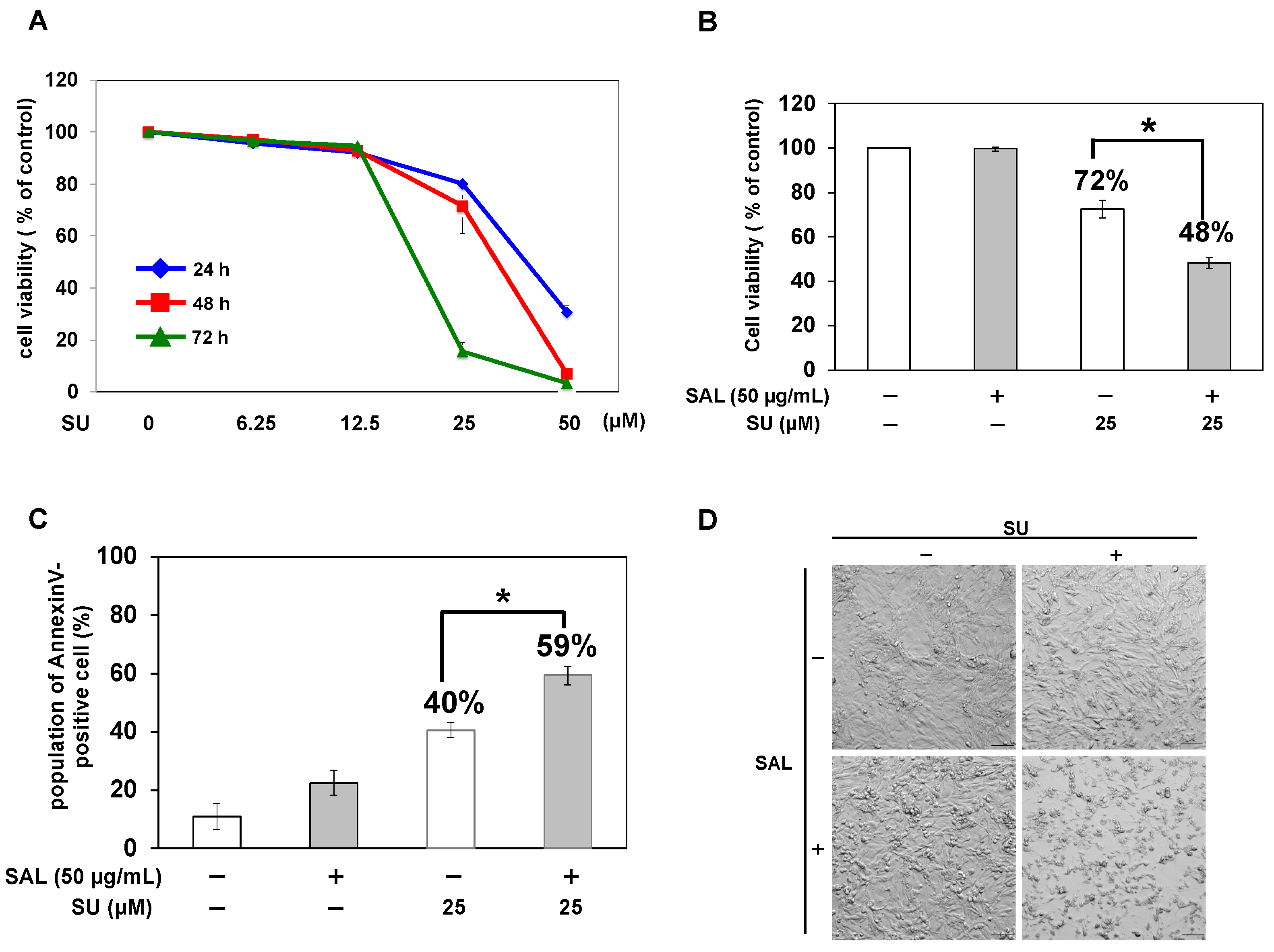
3.3. Combined Effects of SAL and SU in TOS1 Cells
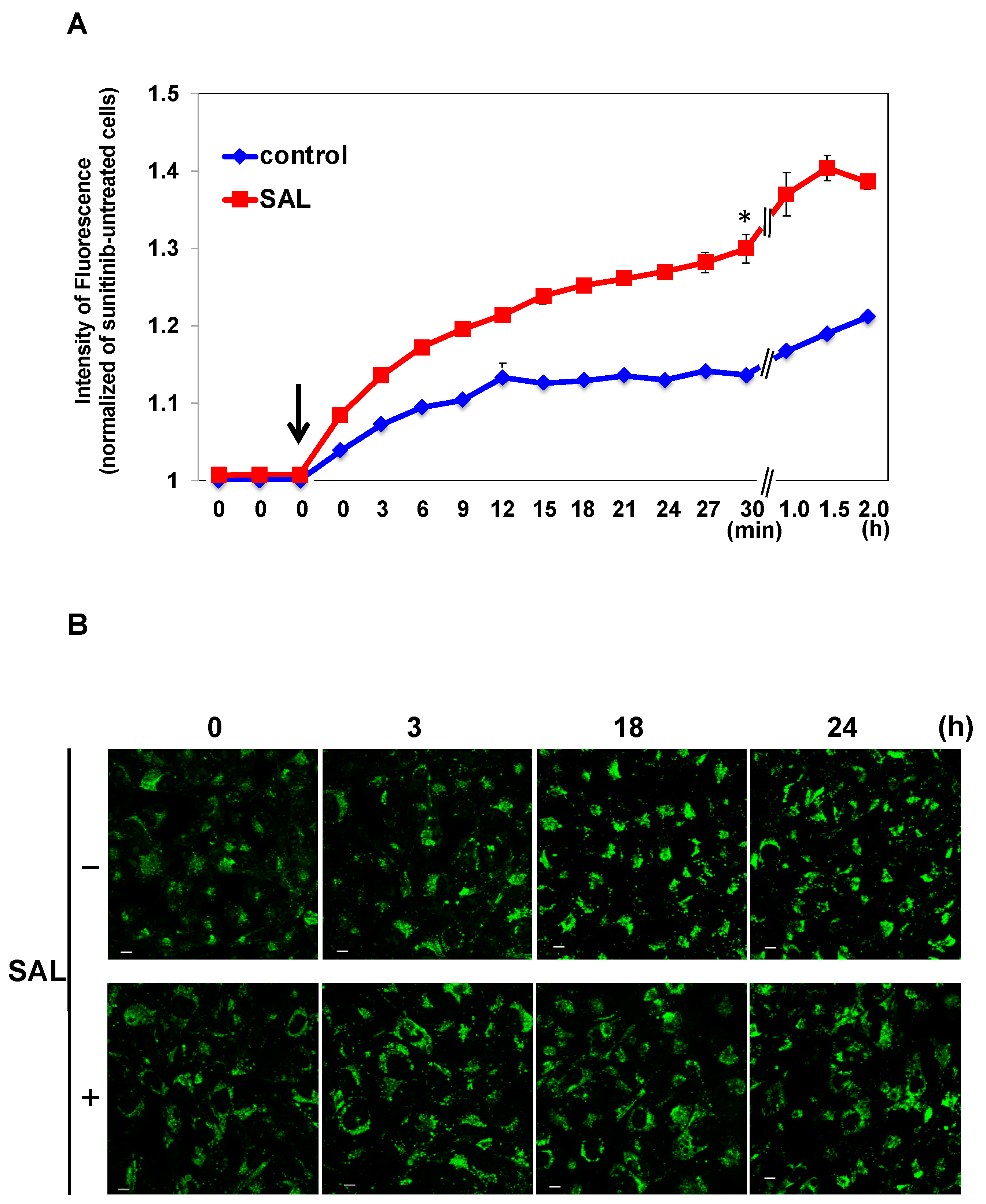
3.4. Effects of SAL on Intracellular Uptake and Extracellular Excretion of SU
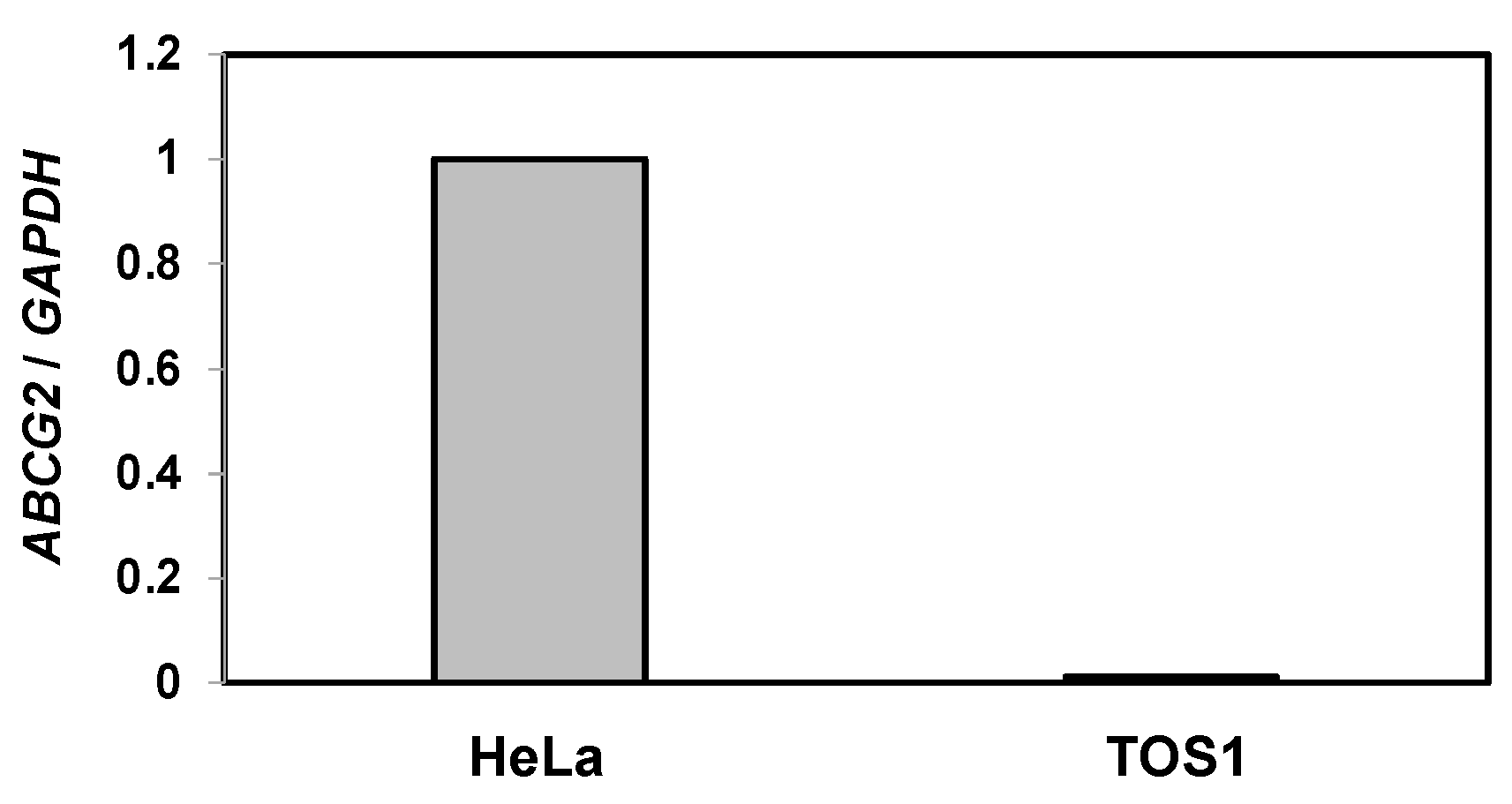
3.5. Expression of ATP-Binding Cassette (ABC) Subfamily G (ABCG2) on TOS1 Cells
3.6. Effect of SAL on Normal Human Renal Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almassi, N.; Gill, B.C.; Rini, B.; Fareed, K. Management of the small renal mass. Transl. Androl. Urol. 2017, 6, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Vida, A.; Hutson, T.E.; Bellmunt, J.; Strijbos, M.H. New treatment options for metastatic renal cell carcinoma. ESMO Open 2017, 2, e000185. [Google Scholar] [CrossRef]
- Singh, D. Current updates and future perspectives on the management of renal cell carcinoma. Life Sci. 2021, 264, 118632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bai, L.; Wu, X.Q.; Tian, X.; Feng, J.; Wu, X.; Shi, G.H.; Pei, X.; Lyu, J.; Yang, G.; et al. Proteogenomics of clear cell renal cell carcinoma response to tyrosine kinase inhibitor. Nat. Commun. 2023, 14, 4274. [Google Scholar] [CrossRef]
- Cheung, R.C.; Wong, J.H.; Pan, W.; Chan, Y.S.; Yin, C.; Dan, X.; Ng, T.B. Marine lectins and their medicinal applications. Appl. Microbiol. Biotechnol. 2015, 99, 3755–3773. [Google Scholar] [CrossRef]
- Sugawara, S.; Im, C.; Kawano, T.; Tatsuta, T.; Koide, Y.; Yamamoto, D.; Ozeki, Y.; Nitta, K.; Hosono, M. Catfish rhamnose-binding lectin induces G(0/1) cell cycle arrest in Burkitt’s lymphoma cells via membrane surface Gb3. Glycoconj J. 2017, 34, 127–138. [Google Scholar] [CrossRef]
- Sugawara, S.; Sasaki, S.; Ogawa, Y.; Hosono, M.; Nitta, K. 2005b. Catfish (Silurus asotus) lectin enhances the cytotoxic effects of doxorubicin. Yakugaku Zasshi 2005, 125, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, S.; Takayanagi, M.; Honda, S.; Tatsuta, T.; Fujii, Y.; Ozeki, Y.; Ito, J.; Sato, M.; Hosono, A.M. Catfish egg lectin affects influx and efflux rates of sunitinib in human cervical carcinoma HeLa cells. Glycobiology 2020, 30, 802–816. [Google Scholar] [CrossRef]
- Satoh, M.; Nejad, F.M.; Nakano, O.; Ito, A.; Kawamura, S.; Ohyama, C.; Saito, S.; Orikasa, S. Four new human renal cell carcinoma cell lines expressing globo-series gangliosides. Tohoku J. Exp. Med. 1999, 189, 95–105. [Google Scholar] [CrossRef]
- Brodaczewska, K.K.; Szczylik, C.; Fiedorowicz, M.; Porta, C.; Czarnecka, A.M. Choosing the right cell line for renal cell cancer research. Mol. Cancer 2016, 15, 83. [Google Scholar] [CrossRef]
- Gotink, K.J.; Broxterman, H.J.; Labots, M.; de Haas, R.R.; Dekker, H.; Honeywell, R.J.; Rudek, M.A.; Beerepoot, L.V.; Musters, R.J.; Jansen, G.; et al. Lysosomal sequestration of sunitinib: A novel mechanism of drug resistance. Clin. Cancer Res. 2011, 17, 7337–7346. [Google Scholar] [CrossRef]
- Fadok, V.A.; Voelker, D.R.; Campbell, P.A.; Cohen, J.J.; Bratton, D.L.; Henson, P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992, 148, 2207–2216. [Google Scholar] [CrossRef] [PubMed]
- Geyer, P.E.; Maak, M.; Nitsche, U.; Perl, M.; Novotny, A.; Slotta-Huspenina, J.; Dransart, E.; Holtorf, A.; Johannes, L.; Janssen, K.P. Gastric adenocarcinomas express the glycosphingolipid Gb3/CD77: Targeting of castric cancer cells with shiga toxin B-subunit. Mol. Cancer Ther. 2016, 15, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Demetri, G.D.; van Oosterom, A.T.; Garrett, C.R.; Blackstein, M.E.; Shah, M.H.; Verweij, J.; McArthur, G.; Judson, I.R.; Heinrich, M.C.; Morgan, J.A.; et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 2006, 368, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Dahan, L.; Raoul, J.L.; Bang, Y.J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef]
- Boegemann, M.; Hubbe, M.; Thomaidou, D.; Blackburn, S.; Bent-Ennakhil, N.; Wood, R.; Bargo, D. Sunitinib treatment modification in first-line metastatic renal cell carcinoma: Analysis of the STAR-TOR registry. Anticancer Res. 2018, 38, 6413–6422. [Google Scholar] [CrossRef]
- Młynarczyk, G.; Mikłosz, A.; Suchański, J.; Reza, S.; Romanowicz, L.; Sobolewski, K.; Chabowski, A.; Baranowski, M. Grade-dependent changes in sphingolipid metabolism in clear cell renal cell carcinoma. J. Cell Biochem. 2022, 123, 819–829. [Google Scholar] [CrossRef]
- Patwardhan, G.A.; Liu, Y.Y. Sphingolipids and expression regulation of genes in cancer. Prog. Lipid Res. 2011, 50, 104–114. [Google Scholar] [CrossRef]
- Johansson, D.; Kosovac, E.; Moharer, J.; Ljuslinder, I.; Brannstrom, T.; Johansson, A.; Behnam-Motlagh, P. Expression of verotoxin-1 receptor Gb3 in breast cancer tissue and verotoxin-1 signal transduction to apoptosis. BMC Cancer 2009, 9, 67. [Google Scholar] [CrossRef]
- Kovbasnjuk, O.; Mourtazina, R.; Baibakov, B.; Wang, T.; Elowsky, C.; Choti, M.A.; Kane, A.; Donowitz, M. The glycosphingolipid globotriaosylceramide in the metastatic transformation of colon cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 19087–19092. [Google Scholar] [CrossRef]
- Kang, J.L.; Rajpert-De Meyts, E.; Wiels, J.; Skakkebaek, N.E. Expression of the glycolipid globotriaosylceramide (Gb3) in testicular carcinoma in situ. Virchows Arch. 1995, 426, 369–374. [Google Scholar] [CrossRef]
- Kawamura, S.; Ohyama, C.; Watanabe, R.; Satoh, M.; Saito, S.; Hoshi, S.; Gasa, S.; Orikasa, S. Glycolipid composition in bladder tumor: A crucial role of GM3 ganglioside in tumor invasion. Int. J. Cancer 2001, 94, 343–347. [Google Scholar] [CrossRef]
- Tyler, A.; Johansson, A.; Karlsson, T.; Gudey, S.K.; Brannstrom, T.; Grankvist, K.; Behnam-Motlagh, P. Targeting glucosylceramide synthase induction of cell surface globotriaosylceramide (Gb3) in acquired cisplatin-resistance of lung cancer and malignant pleural mesothelioma cells. Exp. Cell Res. 2015, 336, 23–32. [Google Scholar] [CrossRef]
- Gabius, H.J. Animal lectins. Eur. J. Biochem. 1997, 243, 543–576. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tateno, H.; Nakamura-Tsuruta, S.; Kominami, J.; Hirabayashi, J.; Nakamura, O.; Watanabe, T.; Kamiya, H.; Naganuma, T.; Ogawa, T.; et al. The function of rhamnose-binding lectin in innate immunity by restricted binding to Gb3. Dev. Comp. Immunol. 2009, 33, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Watanabe, Y.; Lee, M.S.; Ogawa, T.; Muramoto, K. Structure of rhamnose-binding lectin CSL3: Unique pseudo-tetrameric architecture of a pattern recognition protein. J. Mol. Biol. 2009, 391, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.H.; Chien, C.T.; Wu, H.Y.; Huang, K.F.; Wang, I.; Ho, M.R.; Tu, I.F.; Lee, I.M.; Li, W.; Shih, Y.L.; et al. A multivalent marine lectin from Crenomytilus grayanus possesses anticancer activity through recognizing globotriose Gb3. J. Am. Chem. Soc. 2016, 138, 4787–4795. [Google Scholar] [CrossRef] [PubMed]
| Cell Line | Disease | Species | Source Organ | Sex | Established in | |
|---|---|---|---|---|---|---|
| TOS1 * | Clear cell RCC | Human | Soft tissue | Metastasis | Male | 1999 |
| TOS2 * | Clear cell RCC | Human | Soft tissue | Metastasis | Male | 1999 |
| TOS3 * | Clear cell RCC | Human | Kidney | Primary | Male | 1999 |
| TOS3LN * | Clear cell RCC | Human | Lymph node | Metastasis | Male | 1999 |
| ACHN † | Papillary RCC | Human | Pleural effusion | Metastasis | Male | 1979 |
| caki-1 † | Clear cell RCC | Human | Skin | Metastasis | Male | 1971 |
| caki-2 † | Clear cell RCC | Human | Kidney | Primary | Male | 1971 |
| 786-O † | Clear cell RCC | Human | Kidney | Primary | Male | 1976 |
| 769-P † | Clear cell RCC | Human | Kidney | Primary | Female | 1976 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, J.; Sugawara, S.; Tatsuta, T.; Hosono, M.; Sato, M. Catfish Egg Lectin Enhances the Cytotoxicity of Sunitinib on Gb3-Expressing Renal Cancer Cells. Biomedicines 2023, 11, 2317. https://doi.org/10.3390/biomedicines11082317
Ito J, Sugawara S, Tatsuta T, Hosono M, Sato M. Catfish Egg Lectin Enhances the Cytotoxicity of Sunitinib on Gb3-Expressing Renal Cancer Cells. Biomedicines. 2023; 11(8):2317. https://doi.org/10.3390/biomedicines11082317
Chicago/Turabian StyleIto, Jun, Shigeki Sugawara, Takeo Tatsuta, Masahiro Hosono, and Makoto Sato. 2023. "Catfish Egg Lectin Enhances the Cytotoxicity of Sunitinib on Gb3-Expressing Renal Cancer Cells" Biomedicines 11, no. 8: 2317. https://doi.org/10.3390/biomedicines11082317





